Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protein Content and Enzymatic Hydrolysis
2.2. Biostimulant Activity
2.2.1. Gibberellin-Like Effect. Germination Index
2.2.2. Auxin-like Effect. Root Formation of Mung Bean and Cucumber
2.2.3. Cytokinin-like Effect. Cucumber Cotyledon Root Expansion
3. Material and Methods
3.1. Microalgae, Enzymes and Chemicals
3.2. Microalgae Cultivation
3.3. Downstream Processing of Microalgal Biomass
3.4. Cell Disruption by Ultrasounds
3.5. Enzymatic Hydrolysis
3.6. Centrifugation
3.7. Protein Content and Hydrolysis Degree
3.8. Bioassays to Determine the Biostimulant Potential of the Microalgae Biomass
3.8.1. Germination Index of Watercress seeds (Lepidium sativum) for Gibberellins-like Effect
3.8.2. Adventitious Mung Bean (Vigna radiata) Root Induction for Auxin-like Effect
3.8.3. Excised Cucumber (Cucumis sativus L.) Cotyledon Root Induction for Auxin-like Effect
3.8.4. Excised Cucumber (Cucumis sativus, L.) Expansion Test for Cytokinin-like Effect
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García, D.; Posadas, E.; Blanco, S.; Acién, G.; García-Encina, P.; Bolado, S.; Muñoz, R. Evaluation of the dynamics of microalgae population structure and process performance during piggery wastewater treatment in algal-bacterial photobioreactors. Bioresour. Technol. 2018, 248, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. Microalgae cultivation in wastewater. Microalgae-Based Biofuels Bioprod. 2017, 67–91. [Google Scholar]
- Ferreira, A.; Ribeiro, B.; Marques, P.A.S.S.; Ferreira, A.F.; Dias, A.P.; Pinheiro, H.M.; Reis, A.; Gouveia, L. Scenedesmus obliquus mediated brewery wastewater remediation and CO2 biofixation for green energy purposes. J. Clean. Prod. 2017, 165, 1316–1327. [Google Scholar] [CrossRef]
- Ferreira, A.; Marques, P.; Ribeiro, B.; Assemany, P.; de Mendonça, H.V.; Barata, A.; Oliveira, A.C.; Reis, A.; Pinheiro, H.M.; Gouveia, L. Combining biotechnology with circular bioeconomy: From poultry, swine, cattle, brewery, dairy and urban wastewaters to biohydrogen. Environ. Res. 2018, 164, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Ribeiro, B.; Tavares, M.L.A.; Vladic, J.; Vidovic, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; Gouveia, L. Scenedesmus obliquus microalga-based biorefinery—from brewery effluent to bioactive compounds, biofuels and biofertilizers—aiming a circular bioeconomy. Biofuels, Bioprod. Biorefining 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Batista, A.P.; Ambrosano, L.; Graça, S.; Sousa, C.; Marques, P.A.S.S.; Ribeiro, B.; Botrel, E.P.; Castro Neto, P.; Gouveia, L. Combining urban wastewater treatment with biohydrogen production—An integrated microalgae-based approach. Bioresour. Technol. 2015, 184, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Posadas, E.; del Mar Morales, M.; Gomez, C.; Acién, F.G.; Muñoz, R. Influence of pH and CO 2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem. Eng. 2015, 265, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Cabanelas, I.T.D.; Ruiz, J.; Arbib, Z.; Chinalia, F.A.; Garrido-Pérez, C.; Rogalla, F.; Nascimento, I.A.; Perales, J.A.; Garrido-Perez, C.; Rogalla, F.; et al. Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour. Technol. 2013, 131, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losasso, C.; Cibin, V.; Cappa, V.; Roccato, A.; Vanzo, A.; Andrighetto, I.; Ricci, A. Food safety and nutrition: Improving consumer behaviour. Food Control 2012, 26, 252–258. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, K.; Michalak, I.; Dmytryk, A.; Wilk, R.; Gorecki, H. Innovative Natural Plant Growth Biostimulants. In Advances in Fertilizer Technology II Biofertilizers; Studium Press LLC: Houston, TX, USA, 2014; pp. 451–489. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A Systematic Approach to Discover and Characterize Natural Plant Biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. (Amsterdam) 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Chojnacka, K. The potential usefulness of a new generation of agro-products based on raw materials of biological origin. Acta Sci. Pol. Hortorum Cultus 2016, 15, 97–120. [Google Scholar]
- Michalak, I.; Chojnacka, K.; Saeid, A. Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts. Molecules 2017, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains 1. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; Chojnacka, K. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar]
- Kim, S.K.; Chojnacka, K. Marine Algae Extracts: Processes, Products, and Applications; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- European Union Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 relating to fertilizers. Off. J. Eur. Union 2003, 123, 194.
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Romero García, J.M.; Acién Fernández, F.G.; Fernández Sevilla, J.M. Development of a process for the production of l-amino-acids concentrates from microalgae by enzymatic hydrolysis. Bioresour. Technol. 2012, 112, 164–170. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant. Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Kumar, G.; Sahoo, D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J. Appl. Phycol. 2011, 23, 251–255. [Google Scholar] [CrossRef]
- De Carvalho, M.E.A.; de Camargo, P.R.; Gallo, L.A.; Junior, M.V.C.F. Seaweed extracts provides development and production of wheat. Dourados 2014, 7, 166–170. [Google Scholar]
- Davies, P.J. Plant Hormones. Biosynthesis, Signal Transduction, Action! Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Ennis, C.J.; Clarke, J.; Neate, K.; Cerejeira, J.; Tull, L. Hydrothermal extraction of microalgae fatty acid Influences hydrochar phytotoxicity. Front. Environ. Sci. 2017, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Amin, G.H.; Al-Gendy, A.A.; Yassin, M.E.-A.; Abdel-Motteleb, A. Effect of Spirulina Platensis extract on growth, phenolic compounds and antioxidant activities of Sisymbrium Irio callus and cell suspension cultures. Aust. J. Basic Appl. Sci. 2009, 3, 2097–2110. [Google Scholar]
- Niemann, D.I.; Dörffling, K. Growth-inhibitors and growth-promoters in enteromorpha compress a (chlorophyta)1. J. Phycol. 1980, 16, 383–389. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Vambe, M.; Lovász, C.; Molnár, Z.; van Staden, J.; Ördög, V. Effect of cell disruption methods on the extraction of bioactive metabolites from microalgal biomass. J. Biotechnol. 2020, 307, 35–43. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Evidence for rooting factors in a seaweed concentrate prepared from Ecklonia maxima. J. Plant. Physiol. 1991, 137, 319–322. [Google Scholar] [CrossRef]
- Bumandalai, O.; Tserennadmid, R. Effect of chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int. J. Aquat. Biol. 2019, 7, 95–99. [Google Scholar]
- Stirk, W.A.; Ördög, V.; Van Staden, J.; Jäger, K. Cytokinin- and auxin-like activity in Cyanophyta and microalgae. J. Appl. Phycol. 2002, 14, 215–221. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Lo Piero, A.R.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- González López, C.V.; García, M.D.C.C.; Fernández, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Zucconi, F.; Forte, M.; Monaco, A.; De Bertoldi, M. Biological evaluation of compost maturity. BioCycle 1981, 22, 27–29. [Google Scholar]
- Zhao, Z.R.; Wu, Z.L.; Huang, G.Q.; Li, G.R. An improved disk bioassay for determining activities of plant growth regulators. J. Plant. Growth Regul. 1992, 11, 209. [Google Scholar] [CrossRef]


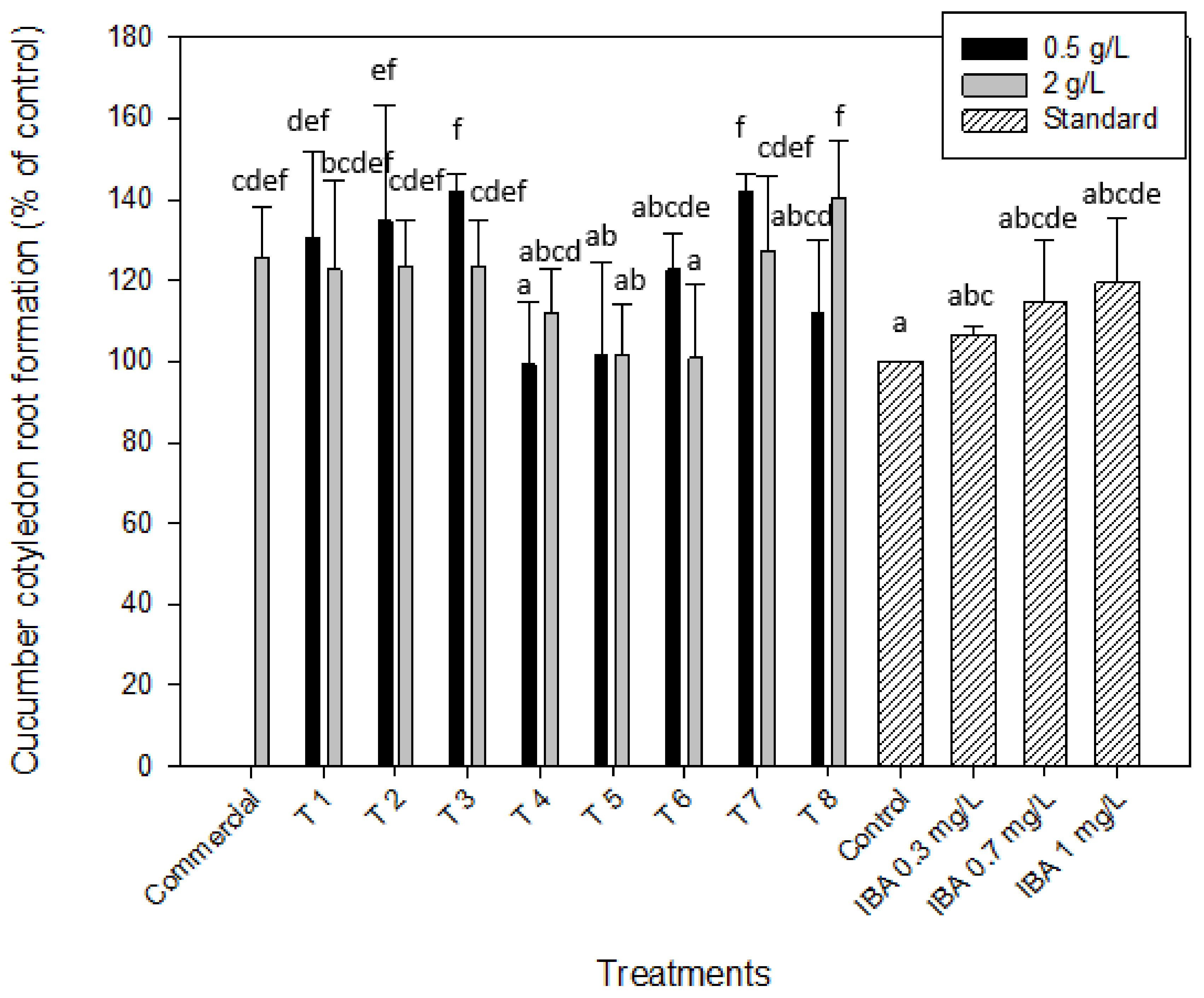
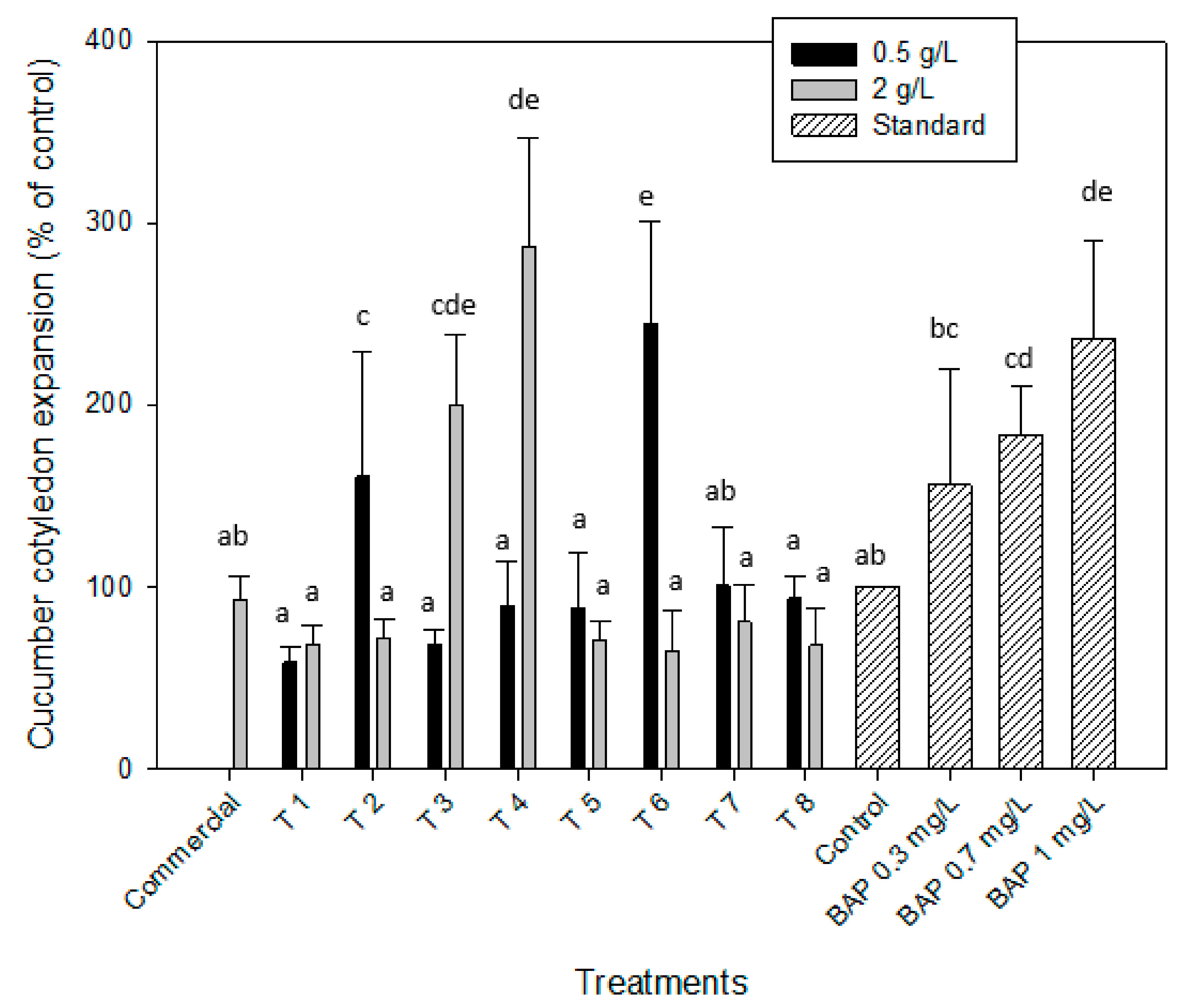

| Gibberellin-Like Effect | Auxin-Like Effect | Cytokinin-Like Effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Germination Index (Watercress Seeds) | Mung bean Bioassay (Root Formation) | Cucumber Bioassay (Root Formation) | Cucumber Expansion Bioassay (Mass of Cotyledons) | |||||
| Treatments | ||||||||
| 0.1 g/L | 0.5 g/L | 0.5 g/L | 2 g/L | 0.5 g/L | 2 g/L | 0.5 g/L | 2 g/L | |
| Commercial | 93.1 ± 2.8 | 102.1 ± 3.2 | 94.3 ± 13.1 | 125.8 ± 12.1 | 92.9 ± 13.1 | |||
| T 1 | 139.1 ± 1.9 | 118.9 ± 3.2 | 137.7 ± 3.3 | 109.4 ± 26.7 | 130.6 ± 21.3 | 122.6 ± 21.9 | 58.8 ± 8.9 | 68.7 ± 9.9 |
| T 2 | 118.8 ± 3.1 | 108.2 ± 4.5 | 137.7 ± 3.3 | 147.2 ± 27.9 | 134.7 ± 28.4 | 123.4 ± 11.6 | 160.9 ± 68.7 | 71.9 ± 11.1 |
| T 3 | 86.5 ± 5.2 | 88.1 ± 5.8 | 126.4 ± 4.3 | 37.7 ± 10.1 | 141.9 ± 4.6 | 123.4 ± 11.6 | 68.5 ± 7.3 | 200.3 ± 38.8 |
| T 4 | 81.1 ± 4.9 | 91.1 ± 6.2 | 113.2 ± 22.6 | 122.6 ± 15.1 | 99.2 ± 15.4 | 112.1 ± 10.6 | 89.6 ± 24.3 | 287.5 ± 59.2 |
| T 5 | 109.2 ± 3.2 | 113.1 ± 4.9 | 145.3 ± 11.8 | 164.2 ± 8.1 | 101.6 ± 22.7 | 101.6 ± 12.5 | 88.7 ± 29.9 | 70.8 ± 11.1 |
| T 6 | 121.8 ± 5.8 | 100.2 ± 2.3 | 150.9 ± 8.7 | 158.5 ± 9.8 | 122.6 ± 8.7 | 100.8 ± 18.3 | 245.1 ± 55.9 | 65.1 ± 21.8 |
| T 7 | 97.9 ± 6.1 | 84.6 ± 3.8 | 118.9 ± 16.9 | 75.5 ± 7.8 | 141.9 ± 4.6 | 127.4 ± 18.3 | 100.99 ± 32.5 | 80.8 ± 20.4 |
| T 8 | 79.3 ± 5.4 | 82.8 ± 4.1 | 167.9 ± 14.2 | 135.8 ± 16.9 | 112.1 ± 17.7 | 140.3 ± 14.3 | 93.9 ± 12.5 | 68.3 ± 20.1 |
| Response | Statistics | Algal Concentration | Cell Disruption | Enzymatic Hydrolysis | Centrifugation | Concentration-Enzymatic Hydrolysis |
|---|---|---|---|---|---|---|
| Germination index | Variation (%) | 0.91 | 0.99 | 59.9 | 1.6 | 36.58 |
| (Lepidium sativum) | p-value | 0.7214 | 0.5528 | 0.0001 | 0.455 | 0.0003 |
| Root development | Variation (%) | 39.94 | 27.12 | 25.1 | 7.8 | 14.19 |
| (Vigna radiata) | p-value | 0.027 | 0.1236 | 0.138 | 0.3395 | 0.2974 |
| Cotyledon root formation | Variation (%) | 42.54 | 37.9 | 6.45 | 6.65 | 6.45 |
| (Cucumis sativus) | p-value | 0.1213 | 0.18 | 0.5746 | 0.5672 | 0.8102 |
| Cotyledon root expansion | Variation (%) | 0.78 | 0.52 | 25.33 | 9.66 | 63.7 |
| (Cucumis sativus) | p-value | 0.9917 | 0.8966 | 0.3319 | 0.5454 | 0.0894 |
| Pollutant | pH | NH3 (mg·L−1) | TKN (mg·L−1) | PO43- (mg·L−1) | P-PO43- (mg·L−1) | COD (mg·O2·L−1) |
|---|---|---|---|---|---|---|
| Composition | 7.2 | 29.4 ± 1.4 | 47.6 ± 2.8 | 44.0 ± 0.1 | 14.22 ± 0.08 | 295.7 ± 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664. https://doi.org/10.3390/molecules25030664
Navarro-López E, Ruíz-Nieto A, Ferreira A, Acién FG, Gouveia L. Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules. 2020; 25(3):664. https://doi.org/10.3390/molecules25030664
Chicago/Turabian StyleNavarro-López, Elvira, Angela Ruíz-Nieto, Alice Ferreira, F. Gabriel Acién, and Luisa Gouveia. 2020. "Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater" Molecules 25, no. 3: 664. https://doi.org/10.3390/molecules25030664
APA StyleNavarro-López, E., Ruíz-Nieto, A., Ferreira, A., Acién, F. G., & Gouveia, L. (2020). Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules, 25(3), 664. https://doi.org/10.3390/molecules25030664








