Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of UPLC and MS Conditions
2.2. Optimization of Sample Extraction Procedure
2.3. Method Validation
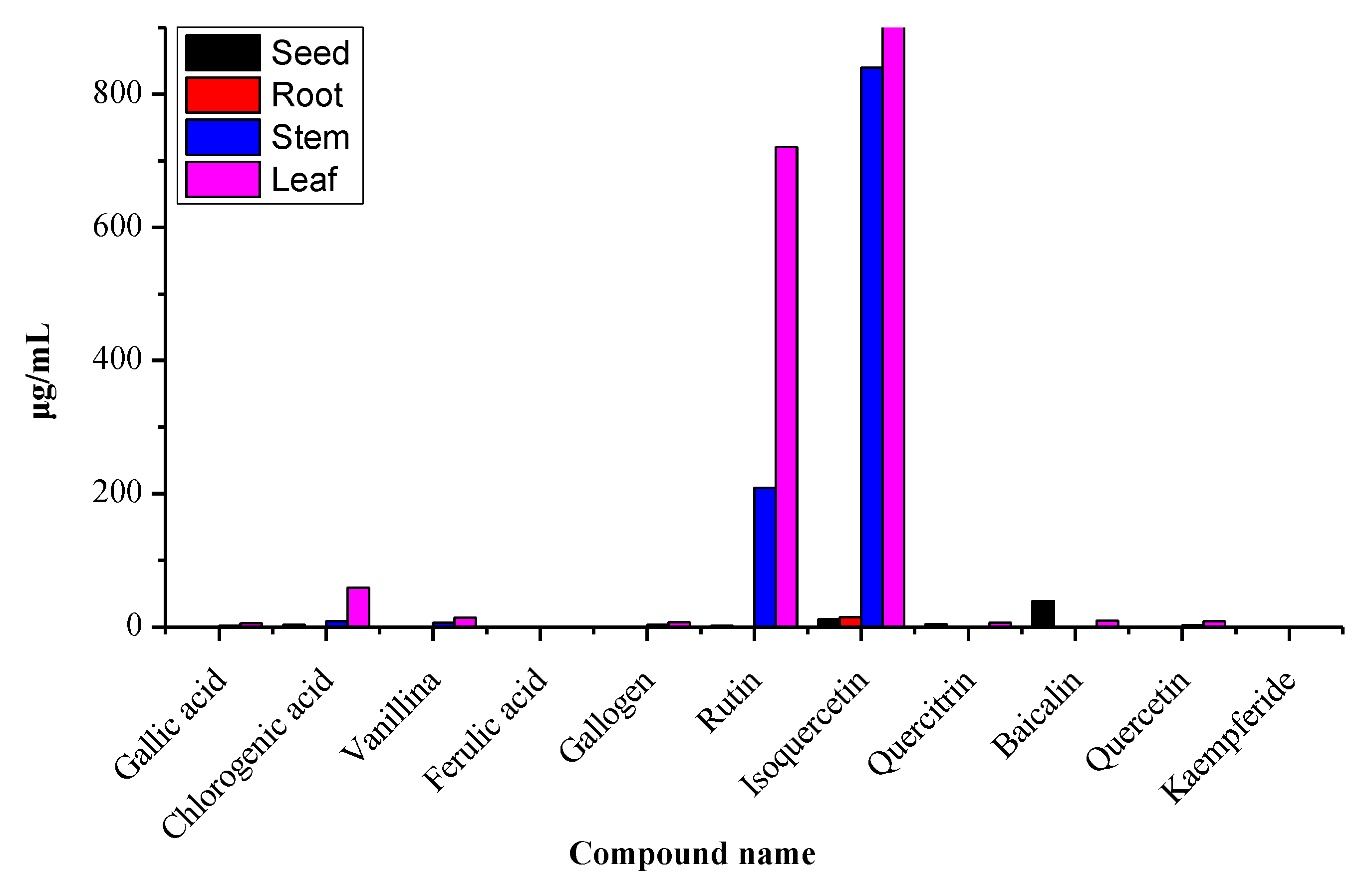
2.4. Distribution of Polyphenols in Different Parts of Moringa oleifera
2.5. Sample Analysis
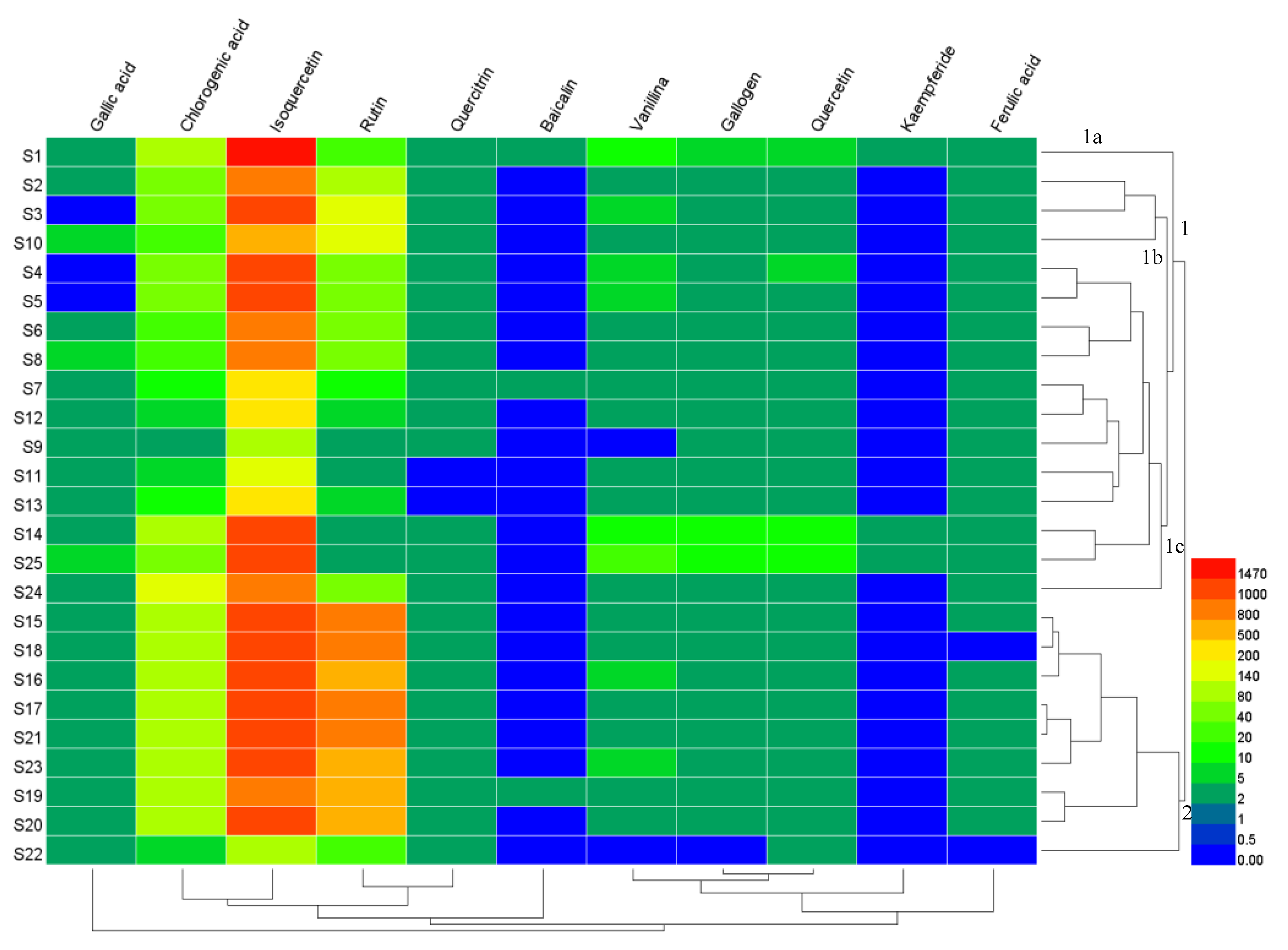
2.6. Hierarchical Cluster Analysis (HCA)
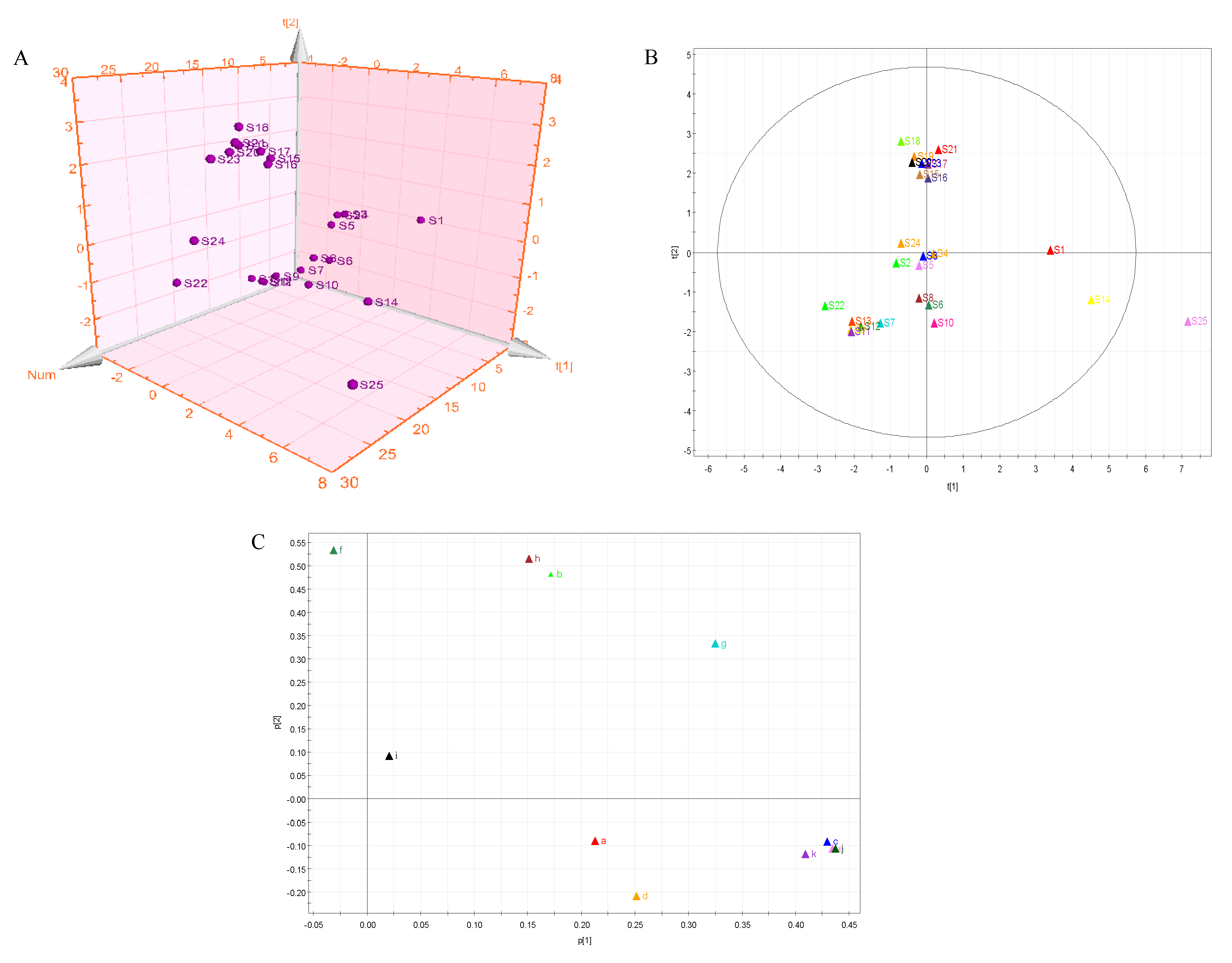
2.7. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Standard Solutions
3.3. Liquid Chromatographic Conditions
3.4. MS/MS Conditions
3.5. Sample Preparation
3.6. Data Processing and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933. [Google Scholar]
- Fakurazi, S.; Sharifudin, S.A.; Arulselvan, P. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen induced hepatotoxicity in experimental rats through their antioxidant nature. Molecules 2012, 17, 8334–8350. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Sharma, V. A review on horse radish tree (Moringa oleifera): A multipurpose tree with high economic and commercial importance. Asian J. Biotechnol. 2011, 3, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839. [Google Scholar]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Martin, G.; Garcia, A.; Fernandez, T.; Hernandez, E.; Puls, L. Potential applications of Moringa oleifera. A critical review. Pastosy Forrajes 2013, 36, 150–158. [Google Scholar]
- Stohs, S.; Hartman, M.J. Review of the safety and efficacy of Moringa oleífera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Farias, D.F.; Oliveira, J.T.D.A.; Carvalho, A.D.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008, 21, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Efiong, E.E.; Igile, G.O.; Mgbeje, B.I.A.; Out, E.A.; Ebong, P.E. Hepatoprotective and anti-diabetic effect of combined extracts of Moringa oleifera and Vernoniaamygdalina in streptozotocin-induced diabetic albino Wistar rats. J. Diabetes Endocrinol. 2013, 4, 45–50. [Google Scholar]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Pande, A.; Tewari, S.K.; Prakash, D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutr. 2005, 56, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Bhatta, R.; Saravanan, M.; Baruah, L.; Sampath, K.T. Nutrient content, in vitro ruminal fermentation characteristics and methane reduction potential of tropical tannin-containing leaves. J. Sci. Food Agric. 2012, 92, 2929–2935. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef]
- Khatun, S.; Absar, N.; Ashraduzzaman, M. Changes in physico-chemical compositions and activities of some hydrolytic and oxidative enzymes in the two types of sajna (Moringa oleifera Lam.) leaves at different maturity levels. Indian J. Plant Physiol. 2013, 8, 6–11. [Google Scholar]
- Manguro, L.O.; Lemmen, P. Phenolics of Moringa oleifera leaves. Nat. Prod. Res. 2007, 21, 56–68. [Google Scholar] [CrossRef]
- Coppin, J.P.; Xu, Y.P.; Chen, H.; Pan, M.H.; Ho, C.T.; Juliani, R.; Simon, J.E.; Wu, Q.L. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J. Funct. Foods 2013, 5, 1892–1899. [Google Scholar] [CrossRef]
- Hamed, H.S.; El-Sayed, Y.S. Antioxidant activities of Moringa oleifera leaf extract against pendimethalin-induced oxidative stress and genotoxicity in Nile tilapia, Oreochromis niloticus (L.). Fish Physiol. Biochem. 2019, 45, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Sharifudin, S.A.; Fakurazi, S.; Hidayat, M.T.; Hairuszah, I.; Moklas, M.A.; Arulselvan, P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm. Biol. 2013, 51, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halaby, M.S.; Metwally, E.M.; Omar, A.A. Effect of Moringa oleifera on serum lipids and kidney function of hyperlipidemic rats. J. Appl. Sci. Res. 2013, 9, 5189–5198. [Google Scholar]
- Okwari, O.; Dasofunjo, K.; Asuk, A.; Alagwu, E.; Mokwe, C. Anti-hypercholesterolemic and hepatoprotective effect of aqueous leaf extract of Moringa oleifera in rats fed with thermoxidized palm oil diet. J. Pharm. Biol. Sci. 2013, 8, 57–62. [Google Scholar]
- Walter, A.; Samuel, W.; Peter, A.; Joseph, O. Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and N-hexane seed extracts on bacteria implicated in water borne diseases. Afr. J. Microbiol. Res. 2011, 5, 153–157. [Google Scholar]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Hamza, A.A. Ameliorative effects of Moringa oleifera Lam. seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010, 48, 345–355. [Google Scholar] [CrossRef]
- Pari, L.; Kumar, N.A. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J. Med. Food 2002, 5, 171–177. [Google Scholar] [CrossRef]
- Sivasankari, B.; Anandharaj, M.; Gunasekaran, P. An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J. Ethnopharmacol. 2014, 153, 408–423. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Phatsimo, G.M.; Ewa, C.; Luke, C. Development of pressurised hot water extraction (PHWE) for essential compounds from Moringa oleifera leaf extracts. Food Chem. 2015, 172, 423–427. [Google Scholar]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringa oleifera lam. J. Chromatogr. Sci. 2014, 52, 641–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, V.C.; Krishna, B.G.; Viswanatha, G.L. Simultaneous determination of quercetin, rutin and kaempferol in the leaf extracts of Moringa oleifera Lam. and Raphinus sativus Linn. by liquid chromatography-tandem mass spectrometry. Chin. J. Integr. Med. 2011, 9, 1022–1030. [Google Scholar] [CrossRef]
- Makita, C.; Chimuka, L.; Cukrowska, E.; Steenkamp, P.A.; Kandawa-Schutz, M.; Ndhlala, A.R.; Madala, N.E. UPLC-qTOF-MS profiling of pharmacologically important chlorogenic acids and associated glycosides in Moringa ovalifolia leaf extracts. S. Afr. J. Bot. 2017, 108, 193–199. [Google Scholar] [CrossRef]
- Niessen, W.M.A.; Manini, P.; Andreoli, R. Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2006, 25, 881–899. [Google Scholar] [CrossRef]
- Niranjan, A.; Ngpoore, N.K.; Anis, N.; Kumar, A.; Lehri, A.; Shirke, P.A.; Tewari, S.K. Simultaneous quantification of six phenolic compounds in various parts of Moringa oleifera Lam. using high-performance thin-layer chromatography. J. Planar Chromat. 2017, 30, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Ademiluyi, A.O.; Aladeselu, O.H.; Oboh, G.; Boligon, A.A. Drying alters the phenolic constituents, antioxidant properties, α-amylase, and α-glucosidase inhibitory properties of Moringa (Moringa oleifera) leaf. Food Sci. Nutr. 2018, 6, 2123–2133. [Google Scholar] [CrossRef] [Green Version]
- Forster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R.; Mewis, I. Ecotype variability in growth and secondary metabolite profile in Moringa oleifera: Impact of sulfur and water availability. J. Agric. Food Chem. 2015, 63, 2852–2861. [Google Scholar] [CrossRef]
- Leone, A.F.G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; Lello, S.; et al. Nutritional characterization and phenolic profiling of Moringa Oleifera leaves grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, N.; Ledin, S.; Ledin, I. Biomass production and chemical composition of Moringa Oleifera under different management regimes in Nicaragua. Agroforest. Syst. 2006, 66, 231–242. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Compound Name | Precursor (m/z) | Product (m/z) | Cone (V) | Collision (eV) | Retention Time (min) | Remark |
|---|---|---|---|---|---|---|
| Gallic acid | 168.96 | 125.04 | 36 | 14 | 1.37 | Quantifier |
| 168.96 | 79.07 | 36 | 22 | 1.37 | Qualifier | |
| Chlorogenic acid | 353.09 | 191.11 | 34 | 20 | 1.72 | Quantifier |
| 353.09 | 85.12 | 34 | 46 | 1.72 | Qualifier | |
| Vanillin | 150.99 | 106.99 | 60 | 8 | 2.61 | Quantifier |
| 150.99 | 83.06 | 60 | 14 | 2.61 | Qualifier | |
| Ferulic acid | 193.03 | 134.09 | 36 | 16 | 2.24 | Quantifier |
| 193.03 | 178.12 | 36 | 14 | 2.24 | Qualifier | |
| Gallogen | 301.06 | 151.11 | 42 | 24 | 2.61 | Quantifier |
| 301.06 | 179.07 | 42 | 16 | 2.61 | Qualifier | |
| Rutin | 609.10 | 300.24 | 66 | 36 | 1.94 | Quantifier |
| 609.10 | 271.11 | 66 | 62 | 1.94 | Qualifier | |
| Isoquercetin | 463.03 | 300.24 | 44 | 26 | 2.03 | Quantifier |
| 463.03 | 271.12 | 44 | 46 | 2.03 | Qualifier | |
| Quercitrin | 447.03 | 300.42 | 46 | 24 | 2.16 | Quantifier |
| 447.03 | 271.11 | 46 | 44 | 2.16 | Qualifier | |
| Baicalin | 445.08 | 269.10 | 28 | 20 | 2.35 | Quantifier |
| 445.08 | 275.00 | 28 | 42 | 2.35 | Qualifier | |
| Quercetin | 301.00 | 151.05 | 40 | 24 | 2.61 | Quantifier |
| 301.00 | 179.08 | 40 | 16 | 2.61 | Qualifier | |
| Kaempferide | 298.97 | 284.13 | 56 | 22 | 3.46 | Quantifier |
| 298.97 | 151.04 | 56 | 30 | 3.46 | Qualifier |
| Compound Name | Regression Equation | Calibration Range (µg/mL) | R2 | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| Gallic acid | Y = 246.765X − 5.02768 | 0.12–4.81 | 0.9952 | 15.29 | 50.98 |
| Chlorogenic acid | Y = 269.863X + 6.49917 | 0.09–3.64 | 0.9921 | 15.21 | 50.69 |
| Vanillin | Y = 97.6209X − 2.37704 | 0.13–5.14 | 0.9954 | 103.13 | 343.77 |
| Ferulic acid | Y = 233.118X − 10.8833 | 0.13–5.06 | 0.9952 | 0.87 | 2.90 |
| Gallogen | Y = 492.921X − 10.2403 | 0.10–4.00 | 0.9968 | 0.84 | 2.81 |
| Rutin | Y = 296.566X − 8.78358 | 0.13–5.33 | 0.9984 | 0.65 | 2.18 |
| Isoquercetin | Y = 210.1X − 3.09922 | 0.11–4.31 | 0.9988 | 0.86 | 2.86 |
| Quercitrin | Y = 286.726X − 9.29514 | 0.10–3.77 | 0.9926 | 0.61 | 2.05 |
| Baicalin | Y = 73.2853X − 3.93154 | 0.12–4.74 | 0.9916 | 3.00 | 9.99 |
| Quercetin | Y = 420.472X − 5.82231 | 0.11–4.20 | 0.9942 | 5.21 | 17.36 |
| Kaempferide | Y = 2221.11X + 11.1101 | 0.08–3.34 | 0.9995 | 6.78 | 22.59 |
| Compound Name | Precision (n = 6) RSD (%) | Ruggedness (RSD%) | Recovery (n = 6) | ||
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Mean (%) | RSD (%) | ||
| Gallic acid | 5.5 | 3.8 | 4.3 | 95.5 | 1.0 |
| Chlorogenic acid | 5.5 | 6.4 | 3.8 | 95.5 | 3.0 |
| Vanillin | 5.3 | 7.9 | 4.6 | 96.8 | 3.5 |
| Ferulic acid | 5.7 | 6.2 | 3.4 | 89.2 | 3.7 |
| Gallogen | 6.9 | 7.2 | 2.7 | 95.7 | 3.0 |
| Rutin | 6.5 | 6.6 | 3.2 | 94.9 | 2.4 |
| Isoquercetin | 6.2 | 6.4 | 1.3 | 93.7 | 1.4 |
| Quercitrin | 6.6 | 5.0 | 4.3 | 98.2 | 3.2 |
| Baicalin | 5.4 | 4.5 | 3.0 | 95.4 | 2.2 |
| Quercetin | 6.1 | 6.7 | 2.3 | 92.6 | 3.4 |
| Kaempferide | 1.4 | 1.8 | 1.9 | 94.5 | 1.1 |
| No. | Collecting Area | Sample Source | Altitude (m) | Annual Mean Temperature (°C) | Annual Precipitation (mm) | Part | Collection Time (yy/mm/dd) |
|---|---|---|---|---|---|---|---|
| S1 | Baoshan | Lujiang farm, Baoshan city | 758 | 21 | 850 | Leaf | 2018/2/1 |
| S2 | Baoshan | Xincheng farm, Baoshan city | 758 | 21 | 850 | Leaf | 2018/2/2 |
| S3 | Baoshan | Baihua village, Longyang district | 758 | 21 | 850 | Leaf | 2018/2/2 |
| S4 | Dehong | Zhina township, Yingjiang county | 820 | 19 | 1464 | Leaf | 2018/2/3 |
| S5 | Dehong | Mengnong township, Yingjiang county | 820 | 19 | 1464 | Leaf | 2018/2/4 |
| S6 | Dehong | Mangshi Lamu division | 807 | 20 | 1655 | Leaf | 2018/2/5 |
| S7 | Dehong | Mangshi Zhefang town | 807 | 20 | 1655 | Leaf | 2018/2/2 |
| S8 | Dehong | Mangshi Laman village | 807 | 20 | 1655 | Leaf | 2018/2/3 |
| S9 | Dehong | Mangshi Menghuan load | 908 | 20 | 1655 | Leaf | 2018/2/5 |
| S10 | Dehong | Mangshi Fapa town | 884 | 20 | 1655 | Leaf | 2018/2/4 |
| S11 | Dehong | Nongdao town, Ruili city | 748 | 21 | 1395 | Leaf | 2018/2/5 |
| S12 | Dehong | Mengxiu township, Ruili city | 778 | 21 | 1395 | Leaf | 2018/2/5 |
| S13 | Dehong | Ruili farm, Ruili city | 778 | 21 | 1395 | Leaf | 2018/2/5 |
| S14 | Dehong | Huguo township, Longchuan county | 965 | 19 | 1595 | Leaf | 2018/2/5 |
| S15 | Nujiang | Liuku town, Lushui county | 869 | 21 | 747 | Leaf | 2018/2/6 |
| S16 | Dehong | Padi industrial district, Mangshi | 874 | 20 | 1655 | Leaf | 2018/2/6 |
| S17 | Dehong | Xishan township, Mangshi | 933 | 20 | 1655 | Leaf | 2018/2/6 |
| S18 | Dehong | Mangzhang township, Yingjiang county | 820 | 19 | 1464 | Leaf | 2018/2/6 |
| S19 | Dehong | Mangjiu reservoir, Mangshi | 888 | 20 | 1655 | Leaf | 2018/3/2 |
| S20 | Dehong | Mangshi town, Mangshi | 911 | 20 | 1655 | Leaf | 2018/3/2 |
| S21 | Dehong | South-sky gate, Mangshi | 1203 | 20 | 1655 | Leaf | 2018/3/2 |
| S22 | Dehong | Menghuan load B, Mangshi | 908 | 20 | 1655 | Leaf | 2018/3/2 |
| S23 | Dehong | Menghuan load A, Mangshi | 908 | 20 | 1655 | Leaf | 2018/3/3 |
| S24 | Xishuangbanna | Menghan town, Jinghong city | 527 | 22 | 1068 | Leaf | 2018/3/3 |
| S25 | Kunming | Chenggong district, Kunming city | 1903 | 15 | 1035 | Leaf | 2018/3/3 |
| No. | Content (μg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid | Chlorogenic Acid | Vanillin | Ferulic Acid | Gallogen | Rutin | Isoquercetin | Quercitrin | Baicalin | Quercetin | Kaempferide | |
| S1 | 2.00 ± 0.02 | 110 ± 2 | 11.7 ± 0.4 | 1.20 ± 0.06 | 5.58 ± 0.28 | 32.64 ± 3.59 | 1575 ± 40 | 1.61 ± 0.08 | 0.82 ± 0.02 | 6.69 ± 0.33 | 0.01 ± 0.001 |
| S2 | 0.20 ± 0.02 | 45.5 ± 0.7 | 1.72 ± 0.05 | 0.53 ± 0.01 | 3.00 ± 0.15 | 133 ± 14 | 996 ± 35 | 0.93 ± 0.05 | ND | 2.62 ± 0.13 | ND |
| S3 | ND | 65.9 ± 1.0 | 5.92 ± 0.20 | 0.69 ± 0.02 | 3.91 ± 0.20 | 187 ± 21 | 1134 ± 28 | 0.86 ± 0.04 | ND | 3.21 ± 0.16 | ND |
| S4 | ND | 69.3 ± 0.2 | 7.46 ± 0.09 | 0.72 ± 0.02 | 3.21 ± 0.16 | 58.2 ± 6.4 | 1172 ± 42 | 1.34 ± 0.07 | ND | 4.12 ± 0.21 | ND |
| S5 | ND | 63.6 ± 1.3 | 5.87 ± 0.12 | 0.54 ± 0.01 | 3.59 ± 0.18 | 42.6 ± 4.7 | 1057 ± 16 | 0.84 ± 0.04 | ND | 3.90 ± 0.20 | ND |
| S6 | 3.88 ± 0.09 | 36.3 ± 0.2 | 3.36 ± 0.05 | 1.41 ± 0.06 | 2.35 ± 0.12 | 73.0 ± 8.0 | 975 ± 24 | 0.54 ± 0.03 | ND | 1.43 ± 0.07 | ND |
| S7 | 1.59 ± 0.06 | 10.8 ± 0.3 | 2.39 ± 0.08 | 1.26 ± 0.06 | 0.54 ± 0.03 | 13.4 ± 1.5 | 341 ± 9 | 0.34 ± 0.02 | 0.91 ± 0.03 | 0.56 ± 0.03 | ND |
| S8 | 4.24 ± 0.10 | 30.4 ± 0.7 | 3.07 ± 0.07 | 1.08 ± 0.05 | 1.61 ± 0.08 | 53.8 ± 5.9 | 860 ± 22 | 0.89 ± 0.04 | ND | 1.96 ± 0.10 | ND |
| S9 | 0.74 ± 0.04 | 2.91 ± 0.09 | ND | 0.96 ± 0.05 | 0.26 ± 0.01 | 2.52 ± 0.28 | 115 ± 3 | 0.40 ± 0.02 | ND | 0.14 ± 0.01 | ND |
| S10 | 6.26 ± 0.13 | 25.4 ± 0.8 | 3.63 ± 0.06 | 1.59 ± 0.07 | 1.55 ± 0.08 | 143 ± 16 | 670 ± 17 | 0.58 ± 0.03 | ND | 1.93 ± 0.10 | ND |
| S11 | 0.55 ± 0.01 | 4.66 ± 0.05 | 1.71 ± 0.02 | 0.75 ± 0.02 | 0.36 ± 0.02 | 1.51 ± 0.17 | 191 ± 5 | ND | ND | 0.23 ± 0.01 | ND |
| S12 | 1.12 ± 0.07 | 5.41 ± 0.13 | 0.93 ± 0.04 | 0.91 ± 0.05 | 0.44 ± 0.02 | 7.53 ± 0.83 | 241 ± 6 | 0.38 ± 0.02 | ND | 0.66 ± 0.03 | ND |
| S13 | 0.40 ± 0.02 | 11.0 ± 0.2 | 1.57 ± 0.05 | 0.45 ± 0.01 | 1.03 ± 0.05 | 5.34 ± 0.59 | 263 ± 7 | ND | ND | 1.07 ± 0.05 | ND |
| S14 | 2.10 ± 0.09 | 83.3 ± 2.7 | 10.0 ± 0.4 | 1.34 ± 0.06 | 13.0 ± 0.65 | 1.94 ± 0.21 | 1302 ± 33 | 1.36 ± 0.07 | ND | 13.5 ± 0.7 | 0.01 ± 0.002 |
| S15 | 1.14 ± 0.03 | 121 ± 3 | 2.29 ± 0.11 | 0.93 ± 0.05 | 1.21 ± 0.06 | 839 ± 92 | 1180 ± 29 | 1.87 ± 0.09 | ND | 1.80 ± 0.09 | ND |
| S16 | 1.51 ± 0.04 | 103 ± 4 | 4.21 ± 0.15 | 0.98 ± 0.05 | 1.77 ± 0.09 | 750 ± 83 | 1037 ± 26 | 2.69 ± 0.13 | ND | 1.55 ± 0.08 | ND |
| S17 | 1.83 ± 0.08 | 118 ± 1 | 3.43 ± 0.09 | 0.79 ± 0.04 | 1.75 ± 0.09 | 817 ± 90 | 1178 ± 42 | 2.51 ± 0.13 | ND | 1.55 ± 0.08 | ND |
| S18 | 1.37 ± 0.06 | 115 ± 1 | 0.72 ± 0.01 | ND | 1.38 ± 0.07 | 826 ± 91 | 1154 ± 27 | 2.76 ± 0.14 | ND | 2.16 ± 0.11 | ND |
| S19 | 1.85 ± 0.05 | 114 ± 3 | 1.76 ± 0.04 | 0.57 ± 0.01 | 1.79 ± 0.09 | 753 ± 83 | 992 ± 25 | 2.36 ± 0.12 | 1.86 ± 0.05 | 1.35 ± 0.07 | ND |
| S20 | 1.49 ± 0.02 | 117 ± 4 | 2.52 ± 0.17 | 0.57 ± 0.01 | 1.32 ± 0.07 | 791 ± 87 | 1056 ± 26 | 2.53 ± 0.13 | ND | 1.15 ± 0.06 | ND |
| S21 | 1.76 ± 0.10 | 122 ± 3 | 3.24 ± 0.22 | 0.96 ± 0.05 | 1.84 ± 0.09 | 859 ± 54 | 1242 ± 31 | 3.08 ± 0.15 | ND | 2.05 ± 0.10 | ND |
| S22 | 0.20 ± 0.07 | 6.56 ± 0.27 | ND | ND | ND | 36.0 ± 4.0 | 103 ± 6 | 0.37 ± 0.02 | ND | 0.14 ± 0.02 | ND |
| S23 | 1.61 ± 0.06 | 115 ± 2 | 4.38 ± 0.30 | 0.67 ± 0.02 | 1.51 ± 0.08 | 764 ± 84 | 1087 ± 27 | 2.73 ± 0.14 | ND | 0.98 ± 0.05 | ND |
| S24 | 0.56 ± 0.04 | 153 ± 5 | 0.72 ± 0.08 | 1.04 ± 0.05 | 0.70 ± 0.04 | 51.2 ± 5.6 | 846 ± 21 | 0.63 ± 0.03 | ND | 1.28 ± 0.06 | ND |
| S25 | 4.24 ± 0.16 | 77.2 ± 2.4 | 26.2 ± 2.5 | 1.43 ± 0.06 | 17.3 ± 0.9 | 2.95 ± 0.32 | 1319 ± 33 | 1.73 ± 0.09 | ND | 18.3 ± 0.9 | 0.01 ± 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. https://doi.org/10.3390/molecules25030676
Zhu Y, Yin Q, Yang Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules. 2020; 25(3):676. https://doi.org/10.3390/molecules25030676
Chicago/Turabian StyleZhu, Yanqin, Qinhong Yin, and Yaling Yang. 2020. "Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS" Molecules 25, no. 3: 676. https://doi.org/10.3390/molecules25030676
APA StyleZhu, Y., Yin, Q., & Yang, Y. (2020). Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules, 25(3), 676. https://doi.org/10.3390/molecules25030676




