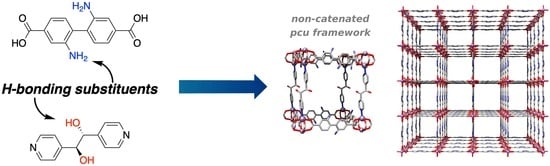
Hydrogen-Bonding Linkers Yield a Large-Pore, Non-Catenated, Metal-Organic Framework with pcu Topology
Abstract
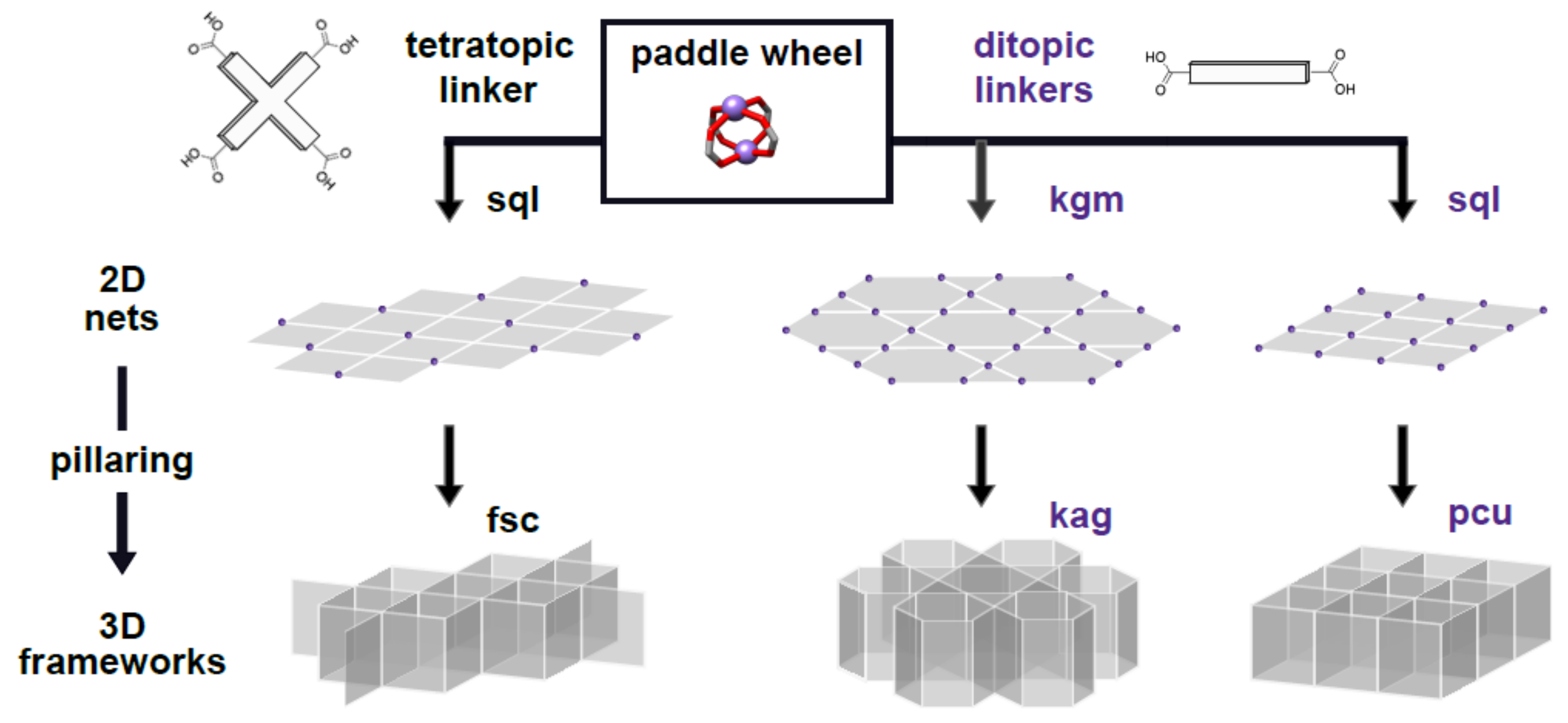
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, J.-S.; Yuan, S.; Wang, Q.; Alsalme, A.; Zhou, H.-C. Mixed-linker strategy for the construction of multifunctional metal–organic frameworks. J. Mater. Chem. A 2017, 5, 4280–4291. [Google Scholar] [CrossRef]
- Hashemi, L.; Morsali, A. Pillared Metal-Organic Frameworks: Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 978-1-119-46024-4. [Google Scholar]
- ZareKarizi, F.; Joharian, M.; Morsali, A. Pillar-layered MOFs: Functionality, interpenetration, flexibility and applications. J. Mater. Chem. A 2018, 6, 19288–19329. [Google Scholar] [CrossRef]
- Burtch, N.C.; Walton, K.S. Modulating adsorption and stability properties in pillared metal–organic frameworks: A model system for understanding ligand effects. Acc. Chem. Res. 2015, 48, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.; Bury, W.; Karagiaridi, O.; Brown, Z.; Hupp, J.T.; Farha, O.K. Transmetalation: Routes to metal exchange within metal–organic frameworks. J. Mater. Chem. A 2013, 1, 5453–5468. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Bury, W.; Fairen-Jimenez, D.; Wilmer, C.E.; Sarjeant, A.A.; Hupp, J.T.; Farha, O.K. Enhanced gas sorption properties and unique behavior toward liquid water in a pillared-paddlewheel metal–organic framework transmetalated with Ni(II). Inorg. Chem. 2014, 53, 10432–10436. [Google Scholar] [CrossRef]
- Xu, Y.; Howarth, A.J.; Islamoglu, T.; da Silva, C.T.; Hupp, J.T.; Farha, O.K. Combining solvent-assisted linker exchange and transmetallation strategies to obtain a new non-catenated nickel (II) pillared-paddlewheel MOF. Inorg. Chem. Commun. 2016, 67, 60–63. [Google Scholar] [CrossRef]
- Farha, O.K.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T. Control over catenation in metal−organic frameworks via rational design of the organic building block. J. Am. Chem. Soc. 2010, 132, 950–952. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Bury, W.; Tylianakis, E.; Sarjeant, A.A.; Hupp, J.T.; Farha, O.K. Opening metal–organic frameworks Vol. 2: Inserting longer pillars into pillared-paddlewheel structures through solvent-assisted linker exchange. Chem. Mater. 2013, 25, 3499–3503. [Google Scholar] [CrossRef]
- Samarakoon, K.P.; Satterfield, C.S.; McCoy, M.C.; Pivaral-Urbina, D.A.; Islamoglu, T.; Day, V.W.; Gadzikwa, T. Uniform, binary functionalization of a metal–organic framework material. Inorg. Chem. 2019, 58, 8906–8909. [Google Scholar] [CrossRef]
- Samarakoon, K.; Yazdanparast, M.; Day, V.W.; Gadzikwa, T. Uniform and simultaneous orthogonal functionalization of a metal-organic framework material. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Kondo, M.; Takashima, Y.; Seo, J.; Kitagawa, S.; Furukawa, S. Control over the nucleation process determines the framework topology of porous coordination polymers. CrystEngComm 2010, 12, 2350–2353. [Google Scholar] [CrossRef]
- Zhou, K.; Chaemchuen, S.; Wu, Z.; Verpoort, F. Rapid room temperature synthesis forming pillared metal-organic frameworks with Kagomé net topology. Microporous Mesoporous Mater. 2017, 239, 28–33. [Google Scholar] [CrossRef]
- Ko, N.; Hong, J.; Sung, S.; Cordova, K.E.; Park, H.J.; Yang, J.K.; Kim, J. A significant enhancement of water vapour uptake at low pressure by amine-functionalization of UiO-67. Dalton Trans. 2015, 44, 2047–2051. [Google Scholar] [CrossRef]
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Soo Lah, M.; Eddaoudi, M. A supermolecular building approach for the design and construction of metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef]
- Chun, H.; Moon, J. Discovery, synthesis, and characterization of an isomeric coordination polymer with pillared kagome net topology. Inorg. Chem. 2007, 46, 4371–4373. [Google Scholar] [CrossRef]
- Hungerford, J.; Walton, K.S. Room-Temperature synthesis of metal–organic framework isomers in the tetragonal and kagome crystal structure. Inorg. Chem. 2019, 58, 7690–7697. [Google Scholar] [CrossRef] [PubMed]
- Barron, P.M.; Son, H.-T.; Hu, C.; Choe, W. Highly tunable heterometallic frameworks constructed from paddle-wheel units and metalloporphyrins. Cryst. Growth Des. 2009, 9, 1960–1965. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-Harvesting metal–organic frameworks (MOFs): Efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef]
- Park, J.; Feng, D.; Yuan, S.; Zhou, H.-C. Photochromic metal–organic frameworks: Reversible control of singlet oxygen generation. Angew. Chem. Int. Ed. 2015, 54, 430–435. [Google Scholar] [CrossRef]
- Danowski, W.; van Leeuwen, T.; Abdolahzadeh, S.; Roke, D.; Browne, W.R.; Wezenberg, S.J.; Feringa, B.L. Unidirectional rotary motion in a metal–organic framework. Nat. Nanotechnol. 2019, 14, 488–494. [Google Scholar] [CrossRef]
- Mulfort, K.L.; Farha, O.K.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T. An Interpenetrated Framework Material with Hysteretic CO2 Uptake. Chem. Eur. J. 2010, 16, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Bury, W.; Fairen-Jimenez, D.; Lalonde, M.B.; Snurr, R.Q.; Farha, O.K.; Hupp, J.T. Control over catenation in pillared paddlewheel metal–organic framework materials via solvent-assisted linker exchange. Chem. Mater. 2013, 25, 739–744. [Google Scholar] [CrossRef]
- Gadzikwa, T.; Farha, O.K.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T.; Nguyen, S.T. Selective bifunctional modification of a non-catenated metal−organic framework material via “Click” chemistry. J. Am. Chem. Soc. 2009, 131, 13613–13615. [Google Scholar] [CrossRef] [PubMed]
- Shultz, A.M.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. A catalytically active, permanently microporous MOF with metalloporphyrin struts. J. Am. Chem. Soc. 2009, 131, 4204–4205. [Google Scholar] [CrossRef] [PubMed]
- Shultz, A.M.; Farha, O.K.; Adhikari, D.; Sarjeant, A.A.; Hupp, J.T.; Nguyen, S.T. Selective Surface and Near-Surface Modification of a Noncatenated, Catalytically Active Metal-Organic Framework Material Based on Mn(salen) Struts. Inorg. Chem. 2011, 50, 3174–3176. [Google Scholar] [CrossRef]
- Mulfort, K.L.; Farha, O.K.; Stern, C.L.; Sarjeant, A.A.; Hupp, J.T. Post-Synthesis alkoxide formation within metal–organic framework materials: A strategy for incorporating highly coordinatively unsaturated metal ions. J. Am. Chem. Soc. 2009, 131, 3866–3868. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Mulfort, K.L.; Hupp, J.T. Microporous pillared paddle-wheel frameworks based on mixed-ligand coordination of zinc ions. Inorg. Chem. 2005, 44, 4912–4914. [Google Scholar] [CrossRef]
- Dau, P.V.; Cohen, S.M. The influence of nitro groups on the topology and gas sorption property of extended Zn(II)-paddlewheel MOFs. CrystEngComm 2013, 15, 9304–9307. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A microporous metal–organic framework for gas-chromatographic separation of alkanes. Angew. Chem. Int. Ed. 2006, 45, 1390–1393. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Z.-H.; Wang, X.-G.; Tang, L.-F.; Zhao, X.-J. Structural modulation of polythreading and interpenetrating coordination networks with an elongated dipyridyl building block and various anionic co-ligands. CrystEngComm 2008, 10, 1855–1865. [Google Scholar] [CrossRef]
- Seo, J.; Bonneau, C.; Matsuda, R.; Takata, M.; Kitagawa, S. Soft secondary building unit: Dynamic bond rearrangement on multinuclear core of porous coordination polymers in gas media. J. Am. Chem. Soc. 2011, 133, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Xie, L.-H.; Lin, J.-B.; Lin, R.-B.; Zhang, J.-P.; Chen, X.-M. Flexible porous coordination polymers constructed from 1,2-bis(4-pyridyl)hydrazinevia solvothermal in situreduction of 4,4′-azopyridine. Dalton Trans. 2011, 40, 8549–8554. [Google Scholar] [CrossRef] [PubMed]
- Safarifard, V.; Morsali, A. Influence of an amine group on the highly efficient reversible adsorption of iodine in two novel isoreticular interpenetrated pillared-layer microporous metal–organic frameworks. CrystEngComm 2014, 16, 8660–8663. [Google Scholar] [CrossRef]
- Jiang, X.; Duan, H.-B.; Khan, S.I.; Garcia-Garibay, M.A. Diffusion-Controlled rotation of triptycene in a metal–organic framework (MOF) sheds light on the viscosity of mof-confined solvent. ACS Cent. Sci. 2016, 2, 608–613. [Google Scholar] [CrossRef]
- Chun, H.; Dybtsev, D.N.; Kim, H.; Kim, K. Synthesis, X-ray Crystal Structures, and Gas Sorption Properties of Pillared Square Grid Nets Based on Paddle-Wheel Motifs: Implications for Hydrogen Storage in Porous Materials. Chem. Eur. J. 2005, 11, 3521–3529. [Google Scholar] [CrossRef]
- Gadzikwa, T.; Lu, G.; Stern, C.L.; Wilson, S.R.; Hupp, J.T.; Nguyen, S.T. Covalent surface modification of a metal–organic framework: Selective surface engineering via CuI-catalyzed Huisgen cycloaddition. Chem. Commun. 2008, 5493–5495. [Google Scholar] [CrossRef]
- Chen, B.; Ma, S.; Zapata, F.; Fronczek, F.R.; Lobkovsky, E.B.; Zhou, H.-C. Rationally designed micropores within a metal–organic framework for selective sorption of gas molecules. Inorg. Chem. 2007, 46, 1233–1236. [Google Scholar] [CrossRef]
- Dau, P.V.; Kim, M.; Garibay, S.J.; Münch, F.H.L.; Moore, C.E.; Cohen, S.M. Single-Atom ligand changes affect breathing in an extended metal–organic framework. Inorg. Chem. 2012, 51, 5671–5676. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, H.; Li, X.; Tu, B.; Pang, Q.; Lin, X.; Ning, E.; Li, Q. Structure transformation of a luminescent pillared-layer metal–organic framework caused by point defects accumulation. Chem. Mater. 2018, 30, 5478–5484. [Google Scholar] [CrossRef]
- Zhu, K.; Vukotic, V.N.; O’Keefe, C.A.; Schurko, R.W.; Loeb, S.J. Metal–Organic frameworks with mechanically interlocked pillars: Controlling ring dynamics in the solid-state via a reversible phase change. J. Am. Chem. Soc. 2014, 136, 7403–7409. [Google Scholar] [CrossRef]
- Servati-Gargari, M.; Mahmoudi, G.; Batten, S.R.; Stilinović, V.; Butler, D.; Beauvais, L.; Kassel, W.S.; Dougherty, W.G.; VanDerveer, D. Control of interpenetration in two-dimensional metal–organic frameworks by modification of hydrogen bonding capability of the organic bridging subunits. Cryst. Growth Des. 2015, 15, 1336–1343. [Google Scholar] [CrossRef]
- Goswami, R.; Mandal, S.C.; Pathak, B.; Neogi, S. Guest-Induced Ultrasensitive detection of multiple toxic organics and Fe3+ Ions in a strategically designed and regenerative smart fluorescent metal–organic framework. ACS Appl. Mater. Interfaces 2019, 11, 9042–9053. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdanparast, M.S.; Day, V.W.; Gadzikwa, T. Hydrogen-Bonding Linkers Yield a Large-Pore, Non-Catenated, Metal-Organic Framework with pcu Topology. Molecules 2020, 25, 697. https://doi.org/10.3390/molecules25030697
Yazdanparast MS, Day VW, Gadzikwa T. Hydrogen-Bonding Linkers Yield a Large-Pore, Non-Catenated, Metal-Organic Framework with pcu Topology. Molecules. 2020; 25(3):697. https://doi.org/10.3390/molecules25030697
Chicago/Turabian StyleYazdanparast, Mohammad S., Victor W. Day, and Tendai Gadzikwa. 2020. "Hydrogen-Bonding Linkers Yield a Large-Pore, Non-Catenated, Metal-Organic Framework with pcu Topology" Molecules 25, no. 3: 697. https://doi.org/10.3390/molecules25030697
APA StyleYazdanparast, M. S., Day, V. W., & Gadzikwa, T. (2020). Hydrogen-Bonding Linkers Yield a Large-Pore, Non-Catenated, Metal-Organic Framework with pcu Topology. Molecules, 25(3), 697. https://doi.org/10.3390/molecules25030697