Iron-Catalyzed Cross-Coupling of Bis-(aryl)manganese Nucleophiles with Alkenyl Halides: Optimization and Mechanistic Investigations
Abstract
1. Introduction
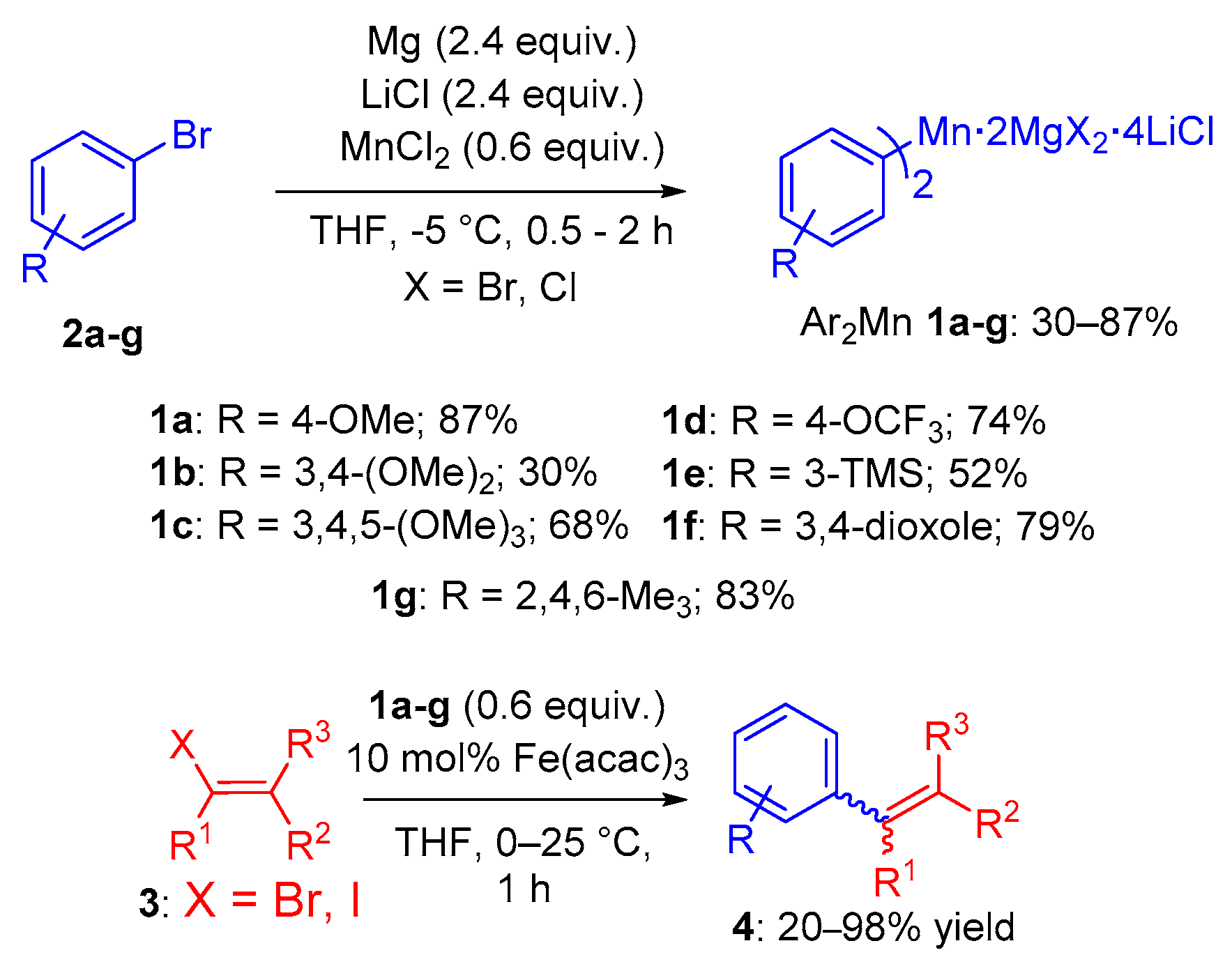
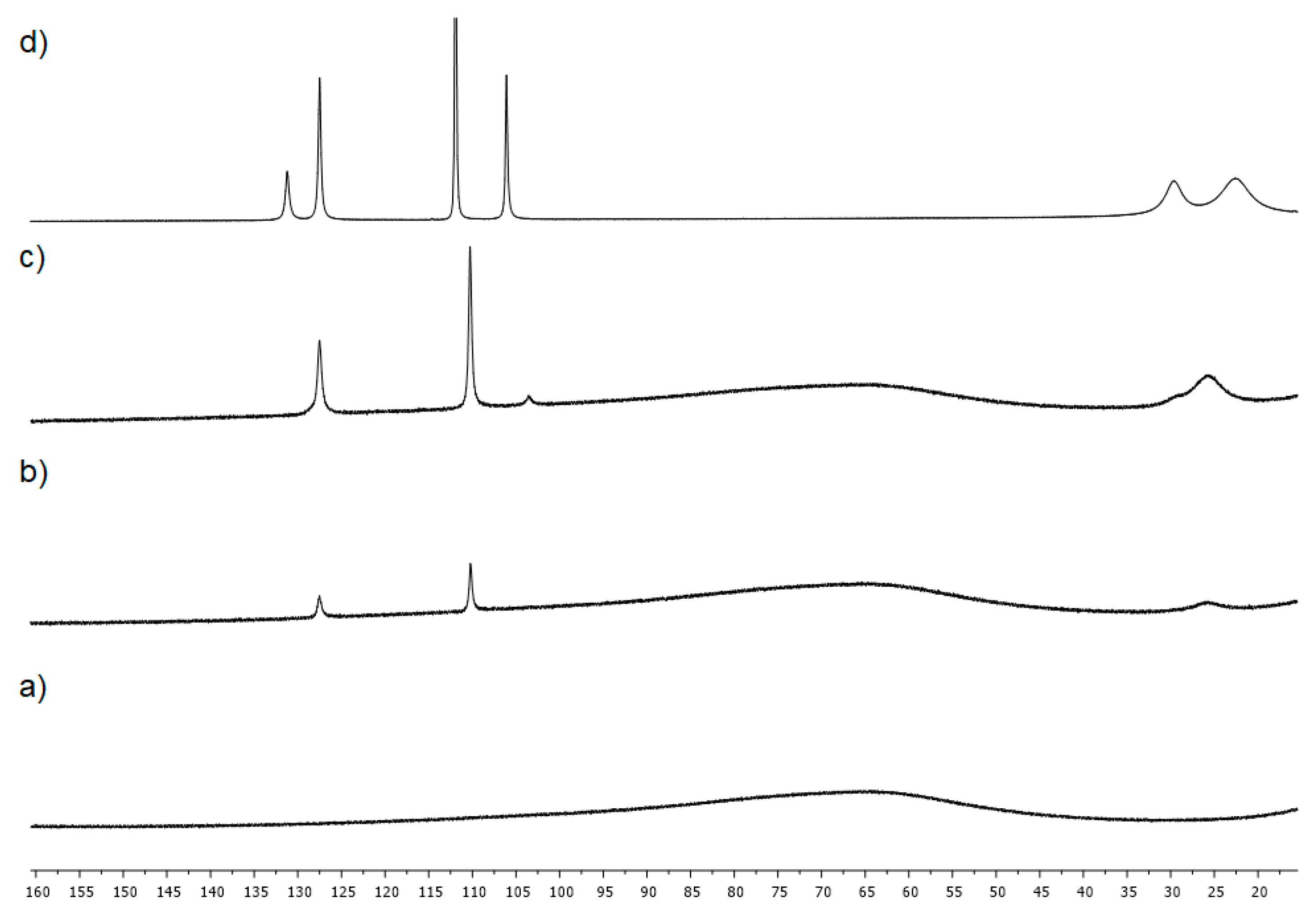
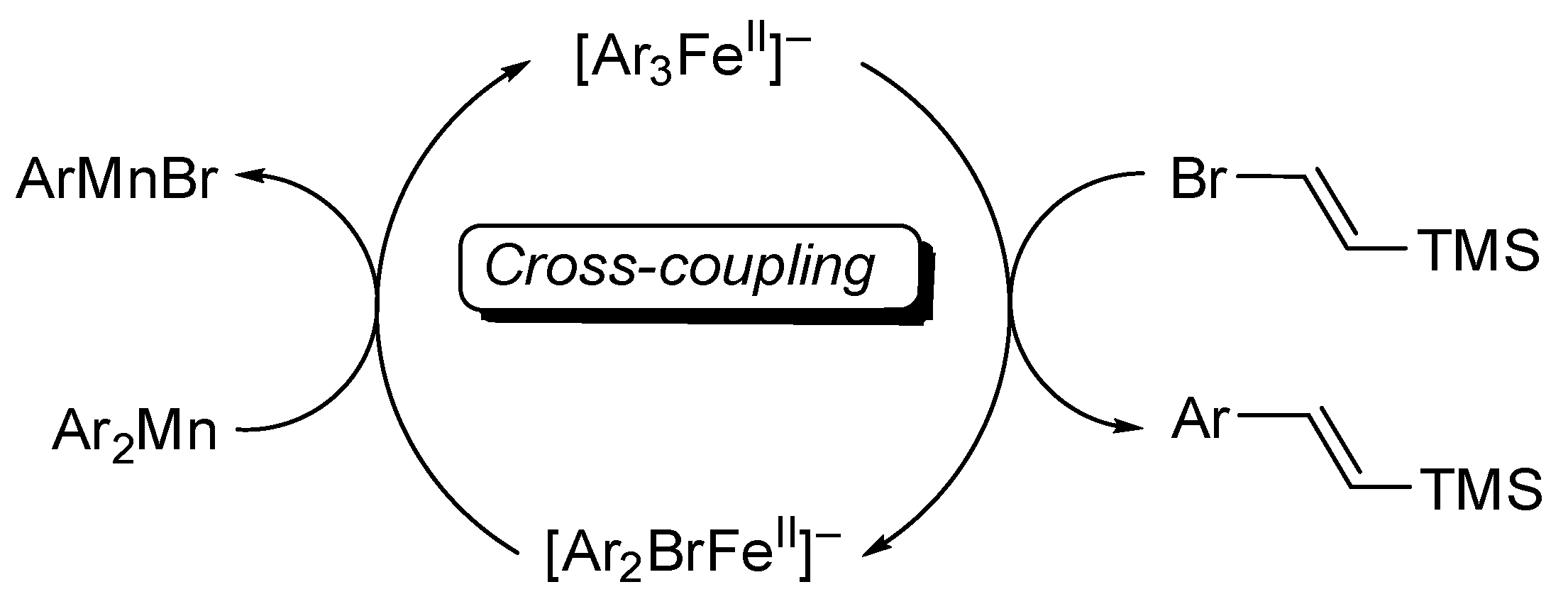
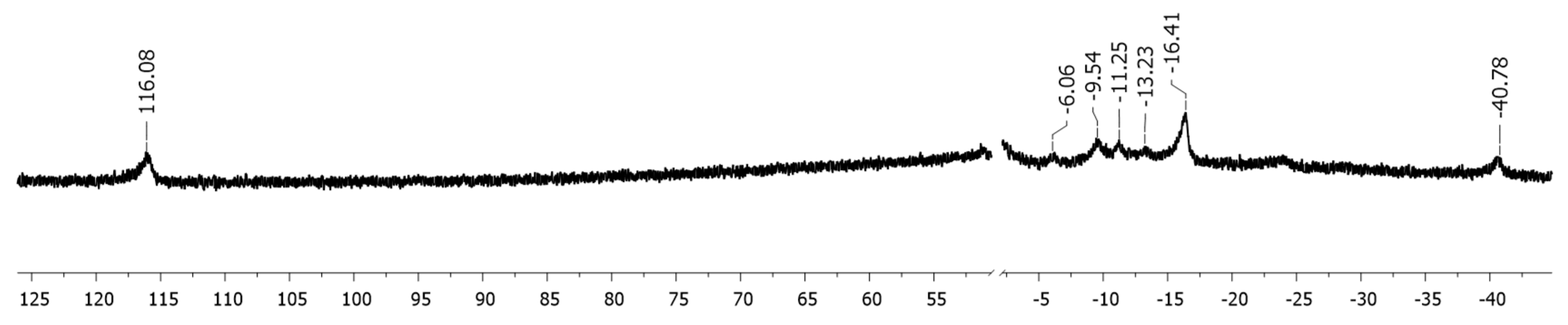
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Chemicals, Solvents, and Typical Procedures
4.2.1. Typical Procedure for the One-Pot Preparation of Bis-(aryl)manganese Reagents 1a–g
4.2.2. Typical Procedure for the Cross-Coupling Reactions of Bis-(aryl)manganese Reagents 1a–g with Different Electrophiles 3a–e
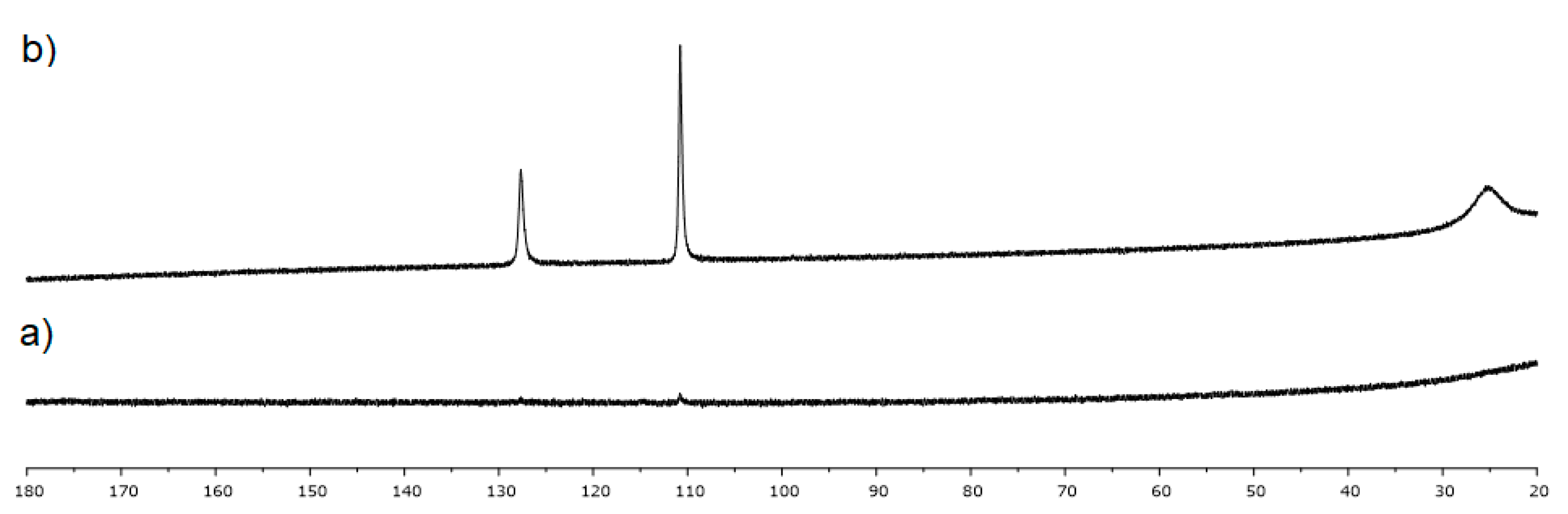
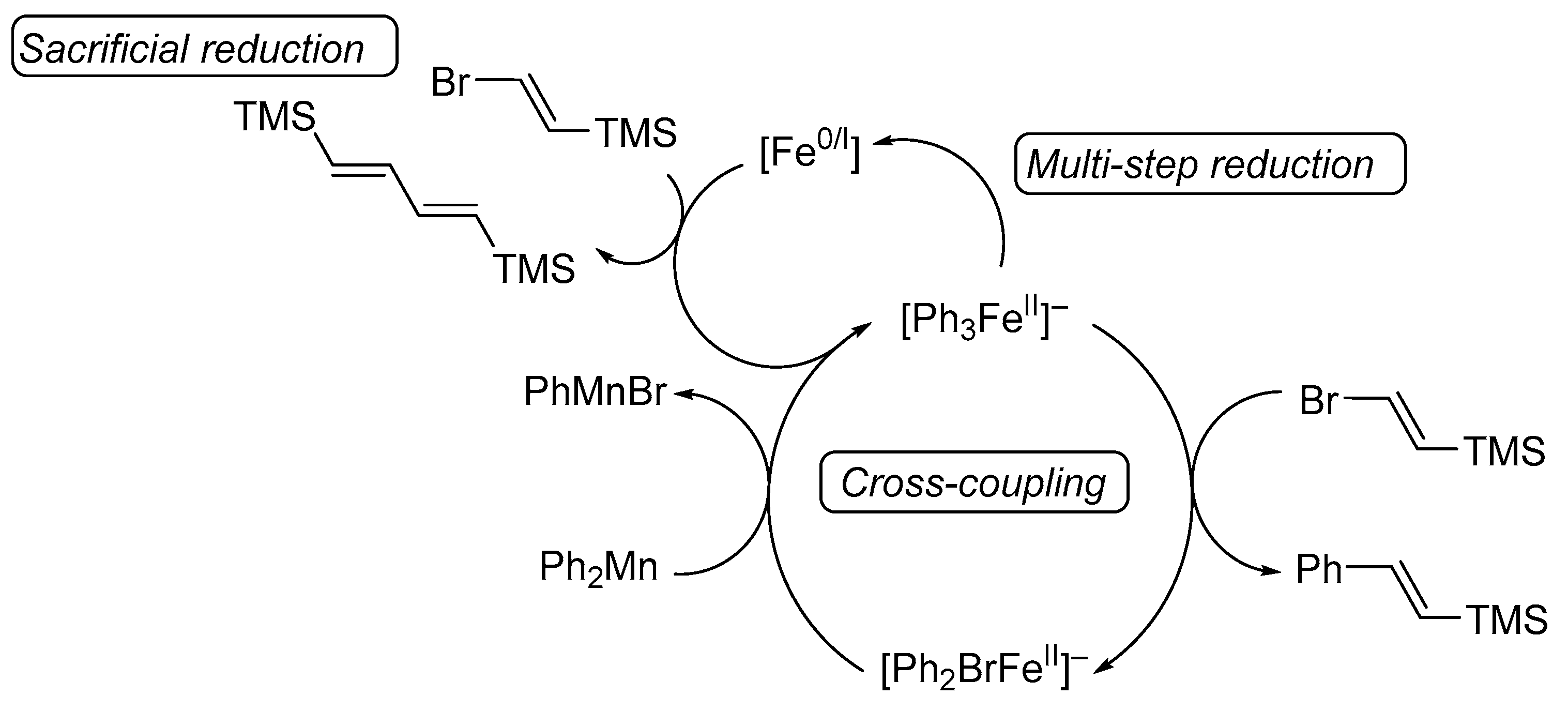
4.3. Studies on the Catalytically Active Species and Catalytic Cycle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Crawley, M.L.; Trost, B.M. Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Miyaura, N. Cross-Coupling Reactions. A Practical Guide. Org. Process Res. Dev. 2003, 7, 1084. [Google Scholar] [CrossRef]
- Phapale, V.B.; Cárdenas, D.J. Nickel-Catalysed Negishi cross-coupling reactions: Scope and mechanisms. Chem. Soc. Rev. 2009, 38, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Organotransition Metal Chemistry. From Bonding to Catalysis. Angew. Chem. Int. Ed. 2010, 49, 7622. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef] [PubMed]
- LD50(FeCl2, rat oral) = 900 mg/kg; LD50(NiCl2, rat oral) = 186 mg/kg.
- Egorova, K.S.; Ananikov, V.P. Which Metals are Green for Catalysis? Comparison of the Toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au Salts. Angew. Chem. Int. Ed. 2016, 55, 12150–12162. [Google Scholar] [CrossRef] [PubMed]
- FeCl2 ca. 332 €/mol, PdCl2 ca. 6164 €/mol; prices retrieved from Alfa Aesar in August 2019.
- Thapa, S.; Shrestha, B.; Gurung, S.K.; Giri, R. Copper-catalysed cross-coupling: An untapped potential. Org. Biomol. Chem. 2015, 13, 4816–4827. [Google Scholar] [CrossRef]
- Fürstner, A.; Leitner, A.; Méndez, M.; Krause, H. Iron-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc. 2002, 124, 13856–13863. [Google Scholar] [CrossRef]
- Bedford, R.B.; Brenner, P.B. Iron Catalysis II; Bauer, E., Ed.; Springer: Berlin, Germany, 2015. [Google Scholar]
- Cahiez, G.; Moyeux, A.; Cossy, J. Grignard Reagents and Non-Precious Metals: Cheap and Eco-Friendly Reagents for Developing Industrial Cross-Couplings. A Personal Account. Adv. Synth. Catal. 2015, 357, 1983–1989. [Google Scholar] [CrossRef]
- Bauer, I.; Knölker, H.-J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef]
- Cahiez, G.; Moyeux, A. Cobalt-Catalyzed Cross-Coupling Reactions. Chem. Rev. 2010, 110, 1435–1462. [Google Scholar] [CrossRef]
- Cahiez, G.; Duplais, C.; Buendia, J. Chemistry of Organomanganese(II) Compounds. Chem. Rev. 2009, 109, 1434–1476. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, N.; Sun, X.; Wang, F.; Xu, L.; Jiang, C.; Song, L.; Yan, Z.-F. The transition-metal-catalyst-free oxidative homocoupling of organomanganese reagents prepared by the insertion of magnesium into organic halides in the presence of MnCl2·2LiCl. Org. Biomol. Chem. 2014, 12, 7800–7809. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Hammann, J.M.; Moyeux, A.; Cahiez, G.; Knochel, P. Oxidative Homocoupling of Diheteroaryl- or Diarylmanganese Reagents Generated via Directed Manganation Using TMP2Mn. Synlett 2015, 26, 1515. [Google Scholar] [CrossRef]
- Cahiez, G.; Masuda, A.; Bernard, D.; Normant, J.F. Reactivite des derives organo-manganeux.I-action sur les chlorures d’acides. synthese de cetones. Tetrahedron Lett. 1976, 36, 3155–3156. [Google Scholar] [CrossRef]
- Friour, G.; Alexakis, A.; Cahiez, G.; Normant, J.F. Reactivite des derives organomanganeux—VIII: Préparation de cétones par acylation d’organomanganeux. Influence de la nature de l’agent acylant, des solvants et des ligands. Tetrahedron 1984, 40, 683–693. [Google Scholar] [CrossRef]
- Kauffmann, T.; Bisling, M. Zwei neue reaktionsweisen von alkyl-Mn(II)-verbindungen: 1,4-addition an cyclohex-2-enon(1) und desoxygenierung von oxiranen (1). Tetrahedron Lett. 1984, 25, 293–296. [Google Scholar] [CrossRef]
- Cahiez, G.; Alami, M. Organomanganese (II) reagents XI.: A study of their reactions with cyclic conjugated enones: Conjugate addition and reductive dimerization. Tetrahedron Lett. 1986, 27, 569–572. [Google Scholar] [CrossRef]
- Cahiez, G.; Alami, M. Organomanganese (II) reagents XVI1: Copper-catalyzed 1,4-addition of organomanganese chlorides to conjugated enones. Tetrahedron Lett. 1989, 30, 3541–3544. [Google Scholar] [CrossRef]
- Cahiez, G.; Alami, M. Composés organomanganeux XXI. Réaction d’un composé organomanganeux avec une énone conjuguée: Influence d’un acide de Lewis, d’un sel de fer ou d’un sel de nickel. J. Organomet. Chem. 1990, 397, 291–298. [Google Scholar] [CrossRef]
- Quinio, P.; Benischke, A.D.; Moyeux, A.; Cahiez, G.; Knochel, P. New Preparation of Benzylic Manganese Chlorides by the Direct Insertion of Magnesium into Benzylic Chlorides in the Presence of MnCl2·2LiCl. Synlett 2015, 26, 514. [Google Scholar] [CrossRef]
- Cahiez, G.; Marquais, S. Highly Chemo- and Stereoselective Fe-Catalyzed Alkenylation of Organomanganese Reagents. Tetrahedron Lett. 1996, 37, 1773–1776. [Google Scholar] [CrossRef]
- Desaintjean, A.; Belrhomari, S.; Rousseau, L.; Lefèvre, G.; Knochel, P. Iron-Catalyzed Cross-Coupling of Functionalized Benzylmanganese Halides with Alkenyl Iodides, Bromides, and Triflates. Org. Lett. 2019, 21, 8684–8688. [Google Scholar] [CrossRef] [PubMed]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Haas, D.; Hammann, J.F.; Greiner, R.; Knochel, P. Recent Developments in Negishi Cross-Coupling Reactions. ACS Catal. 2016, 6, 1540–1552. [Google Scholar] [CrossRef]
- O’Donovan, M.R.; Mee, C.D.; Fenner, S.; Teasdale, A.; Phillips, D.H. Boronic acids-a novel class of bacterial mutagen. Mutat. Res. 2011, 724, 1–6. [Google Scholar] [CrossRef]
- Hansen, M.M.; Jolly, R.A.; Linder, R.J. Boronic Acids and Derivatives-Probing the Structure-Activity Relationships for Mutagenicity. Org. Process Res. Dev. 2015, 19, 1507–1516. [Google Scholar] [CrossRef]
- Benischke, A.D.; Desaintjean, A.; Juli, T.; Cahiez, G.; Knochel, P. Nickel-Catalyzed Cross-Coupling of Functionalized Organo-manganese Reagents with Aryl and Heteroaryl Halides Promoted by 4-Fluorostyrene. Synthesis 2017, 49, 5396. [Google Scholar] [CrossRef]
- Cahiez, G.; Gager, O.; Lecomte, F. Manganese-Catalyzed Cross-Coupling Reaction between Aryl Grignard Reagents and Alkenyl Halides. Org. Lett. 2008, 10, 5255–5256. [Google Scholar] [CrossRef]
- Additionally, 55Mn nucleus is quadrupolar (100 % natural abundance, I = 5/2).
- Morris, R.J.; Girolami, G.S. High-valent organomanganese chemistry. 2. Synthesis and characterization of manganese(III) aryls. Organometallics 1991, 10, 799–804. [Google Scholar] [CrossRef]
- Krasovskiy, A.; Knochel, P. Convenient Titration Method for Organometallic Zinc, Magnesium, and Lanthanide Reagents. Synthesis 2006, 5, 890. [Google Scholar] [CrossRef]
- Fischer, R.; Görls, H.; Friedrich, M.; Westerhausen, M. Reinvestigation of arylmanganese chemistry—Synthesis and molecular structures of [(thf)4Mg(μ-Cl)2Mn(Br)Mes], [Mes(thf)Mn(μ-Mes)]2, and (MnPh2)∞ (Ph=C6H5; Mes= mesityl, 2,4,6-Me3C6H2). J. Organomet. Chem. 2009, 694, 1107–1111. [Google Scholar] [CrossRef]
- Meyer, R.M.; Hanusa, T.P. Structural organomanganese chemistry. In The Chemistry of Organomanganese Compounds; Rappoport, Z., Marek, I., Eds.; Wiley: Hoboken, NJ, USA, 2011; p. 45. [Google Scholar]
- Bedford, R.B.; Brenner, P.B.; Carter, E.; Cogswell, P.M.; Haddow, M.F.; Harvey, J.N.; Murphy, D.M.; Nunn, J.; Woodall, C.H. TMEDA in Iron-Catalyzed Kumada Coupling: Amine Adduct versus Homoleptic “ate” Complex Formation. Angew. Chem. Int. Ed. 2014, 53, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.B.; Daifuku, S.L.; Sears, J.D.; Baker, T.M.; Carpenter, S.H.; Brennessel, W.W.; Neidig, M.L. The N-Methylpyrrolidone (NMP) Effect in Iron-Catalyzed Cross-Coupling with Simple Ferric Salts and MeMgBr. Angew. Chem. Int. Ed. 2018, 57, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Clémancey, M.; Cantat, T.; Blondin, G.; Latour, J.-M.; Dorlet, P.; Lefèvre, G. Structural insights into the nature of Fe0 and FeI low-valent species obtained upon reduction of Iron salts by Aryl Grignard reagents. Inorg. Chem. 2017, 56, 3834–3848. [Google Scholar] [CrossRef]
- Rousseau, L.; Herrero, C.; Clémancey, M.; Imberdis, A.; Blondin, G.; Lefèvre, G. Evolution of ate organoiron(II) species towards lower oxidation states: Role of the steric and electronic factors. Chem. Eur. J. 2019. [Google Scholar] [CrossRef]
- The mechanistic studies were pursued using only aryl Grignard nucleophiles, as the transmetalation of the latter with iron was proceeding in the same way than that of manganese, and as the NMR spectra allowed a much more precise and accurate interpretation thanks to the absence of highly paramagnetic Mn-containing species.
- Cheng, J.; Chen, Q.; Leng, X.; Ye, S.; Deng, L. Three-Coordinate Iron(0) complexes with N-Heterocyclic Carbene and Vinyltrimethylsilane Ligation: Synthesis, Characterization, and Ligand Substitution Reactions. Inorg. Chem. 2019, 58, 13129–13141. [Google Scholar] [CrossRef]
- Mo, Z.; Deng, L. Open-shell iron hydrocarbyls. Coord. Chem. Rev. 2017, 350, 285–299. [Google Scholar] [CrossRef]
- Mayer, M.; Welther, A.; Jacobi von Wangelin, A. Iron-Catalyzed Isomerizations of Olefins. ChemCatChem 2011, 3, 1567–1571. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds described in this article are available from the authors. |
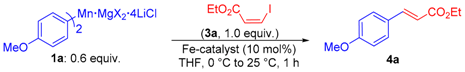
| Entry | Catalyst (10 mol%) | Yield (%) a |
|---|---|---|
| 1 | none | 60 b |
| 2 | FeBr2 | 42 |
| 3 | FeCl3 | 54 |
| 4 | FeBr3 | 57 |
| 5 | FeCl2 | 64 |
| 6 | Fe(acac)2 | 67 |
| 7 | Fe(acac)3 (>99% purity) | 79 |

| Entry | Ar2Mn | Electrophile | Yield (%) b | Entry | Ar2Mn | Electrophile | Yield (%) b |
|---|---|---|---|---|---|---|---|
| 1 | 1a |  3b: Z/E = 10:90 | 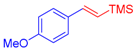 4b: 98, Z/E = 1:99 (24, Z/E = 20:80) c | 8 | 1e | 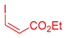 3a | 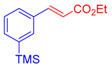 4i: 64, Z/E = 1:99 (51, Z/E = 57:43) c |
| 2 | 1b |  3a | 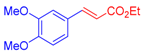 4c: 69, Z/E = 1:99 (58, Z/E = 69:31) c | 9 | 1f |  3b: Z/E = 10:90 | 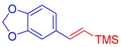 4j: 78, Z/E = 1:99 (39, Z/E = 20:80) c |
| 3 | 1c |  3b: Z/E = 10:90 | 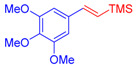 4d: 80, Z/E = 1:99 (8, Z/E = 1:99) c | 10 | 1f |  3a | 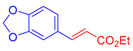 4k: 84, Z/E = 1:99 (48, Z/E = 99:1) c |
| 4 | 1c |  3a | 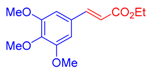 4e: 57, Z/E = 1:99 (66, Z/E = 72:28) c | 11 | 1g |  3b: Z/E = 10:90 | 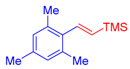 4l: d 20, Z/E = 21:79 91, Z/E = 5:95 e (traces, Z/E = 50:50) c |
| 5 | 1c |  3c: Z/E = 18:82 | 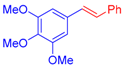 4f: 82, Z/E = 1:99 (80, Z/E = 53:47) c | 12 | 1d | 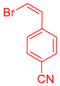 3e: Z/E = 98:2 | 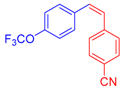 4m: 74, Z/E = 98:2 (19, Z/E = 73:27) c |
| 6 | 1c |  3d |  4g: 87, Z/E = 9:91 (77, Z/E = 4:96) c | 13 | 1e | 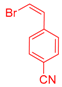 3e: Z/E = 98:2 | 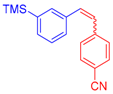 4n: 66, Z/E = 70:30 (41, Z/E = 72:28)c |
| 7 | 1d |  3a |  4h: 77, Z/E = 1:99 (74, Z/E = 81:19) c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau, L.; Desaintjean, A.; Knochel, P.; Lefèvre, G. Iron-Catalyzed Cross-Coupling of Bis-(aryl)manganese Nucleophiles with Alkenyl Halides: Optimization and Mechanistic Investigations. Molecules 2020, 25, 723. https://doi.org/10.3390/molecules25030723
Rousseau L, Desaintjean A, Knochel P, Lefèvre G. Iron-Catalyzed Cross-Coupling of Bis-(aryl)manganese Nucleophiles with Alkenyl Halides: Optimization and Mechanistic Investigations. Molecules. 2020; 25(3):723. https://doi.org/10.3390/molecules25030723
Chicago/Turabian StyleRousseau, Lidie, Alexandre Desaintjean, Paul Knochel, and Guillaume Lefèvre. 2020. "Iron-Catalyzed Cross-Coupling of Bis-(aryl)manganese Nucleophiles with Alkenyl Halides: Optimization and Mechanistic Investigations" Molecules 25, no. 3: 723. https://doi.org/10.3390/molecules25030723
APA StyleRousseau, L., Desaintjean, A., Knochel, P., & Lefèvre, G. (2020). Iron-Catalyzed Cross-Coupling of Bis-(aryl)manganese Nucleophiles with Alkenyl Halides: Optimization and Mechanistic Investigations. Molecules, 25(3), 723. https://doi.org/10.3390/molecules25030723




