Temperature Effect on the Adsorption and Volumetric Properties of Aqueous Solutions of Kolliphor®ELP
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Otter, M.; Oswald, S.; Siegmund, W.; Keiser, M. Effects of frequently used pharmaceutical excipients on the organic cation transporters 1–3 and peptide transporters 1/2 stably expressed in MDCKII cells. Europ. J. Pharm. Biopharm. 2017, 112, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Shi, Y.; Porter, W.; Merdan, T.; Li, L.C. Recent advances in intravenous delivery of poorly water-soluble compounds. Expert Opin. Drug Deliv. 2009, 6, 1261–1282. [Google Scholar] [CrossRef] [PubMed]
- Hanke, U.; May, K.; Rozehnal, V.; Nagel, S.; Siegmund, W.; Weitschies, W. Commonly used nonionic surfactants interact differently with the human efflux transporters ABCB1 (p-glycoprotein) and ABCC2 (MRP2). Eur. J. Pharm. Biopharm. 2010, 76, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.; Backensfeld, T.; Denner, K.; Weitschies, W. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. Eur. J. Pharm. Biopharm. 2011, 78, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Berthelsen, R.; Holm, R.; Jacobsen, J.; Kristensen, J.; Abrahamsson, B.; Müllertz, A. Kolliphor surfactants affect solubilization and bioavailability of fenofibrate. Studies of in vitro digestion and absorption in rats. Mol. Pharma. 2015, 12, 1062–1071. [Google Scholar] [CrossRef]
- Christiansen, A.; Backensfeld, T.; Weitschies, W. Effects of nonionic surfactants on in vitro triglyceride digestion and their susceptibility to digestion by pancreatic enzymes. Eur. J. Pharm. Sci. 2010, 41, 376–382. [Google Scholar] [CrossRef]
- Bakonyia, M.; Berkóa, S.; Kovácsa, A.; Budai-Szűcsa, M.; Kisa, N.; Erős, G.; Csókaa, I.; Csányi, E. Application of quality by design principles in the development and evaluation of semisolid drug carrier systems for the transdermal delivery of lidocaine. J. Drug Deliv. Sci. Technol. 2018, 44, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Buggins, T.R.; Dickinson, P.A.; Taylor, G. The effects of pharmaceutical excipients on drug disposition. Adv. Drug Deliv. Rev. 2007, 59, 1482–1503. [Google Scholar] [CrossRef] [Green Version]
- Ganetsky, M.; Böhlke, M.; Pereira, L.; Williams, D.; LeDuc, B.; Guatam, S.; Salhanick, S.D. Effect of excipients on acetaminophen metabolism and its implications for prevention of liver injury. J. Clin. Pharmacol. 2013, 53, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Rosen, J.M. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley Interscience: New York, NY, USA, 2004; pp. 59–167. [Google Scholar]
- Szymczyk, K. The properties of binary mixtures of ethoxylatedoctyl phenols with ethoxylated fluorinated alkanols at the water/air interface. J. Surf. Deterg. 2011, 14, 415–423. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1997; pp. 71–77. [Google Scholar]
- Xie, Y.; Li, J.; Sun, T.; Han, Y.; Qu, G.; Niu, R. Synthesis, surface activity, and corrosion inhibition of dentritic quaternary ammonium salt-type tetrameric surfactants. J. Disper Sci. Technol. 2018, 39, 1153–1159. [Google Scholar] [CrossRef]
- Szymczyk, K. Behaviour of the fluorocarbon surfactants in the monolayer at the water-air interface and in the bulk phase. J. Fluorine Chem. 2013, 150, 109–116. [Google Scholar] [CrossRef]
- Wu, C.; Li, N.J.; Chen, K.C.; Hsu, H.-F. Determination of critical micelle concentrations of ionic and nonionic surfactants based on relative viscosity measurements by capillary electrophoresis. Res. Chem. Int. 2014, 40, 2371–2379. [Google Scholar] [CrossRef]
- Queimada, A.J.; Marrucho, I.M.; Stenby, E.H.; Coutinho, J.A.P. Generalized relation between surface tension and viscosity: a study on pure and mixed n-alkanes. Fluid Phase. Equilib. 2004, 222–223, 161–168. [Google Scholar] [CrossRef]
- Pelofsky, A.H. Surface tension-viscosity relation for liquids. J. Chem. Eng. Data 1966, 11, 394–397. [Google Scholar] [CrossRef]
- Subrahmanyam, M.S.R.; Vedanayagam, H.S.; Venkateshwar, R.D.; Rajaiah, A.; Venkatacharyulu, P. Estimation of Sharma constant and thermoacoustic properties of fatty acids. J. Am. Oil Chem. Soc. 1995, 1537–1540. [Google Scholar] [CrossRef]
- Sharma, B.K. Volume dependence of thermodynamic Grüneisen parameter of fluorocarbon fluids. Phys. Lett. 1983, 99, 227–229. [Google Scholar] [CrossRef]
- Mukherjee, I.; Manna, K.; Dinda, G.; Ghosh, S.; Moulik, S.P. Shear- and temperature-dependent viscosity behavior of two phosphonium-based ionic liquids and surfactant triton X-100 and their biocidal activities. J. Chem. Eng. Data 2012, 57, 1376–1386. [Google Scholar] [CrossRef]
- Szymczyk, K.; Taraba, A. Aggregation behavior of Triton X-114 and Tween 80 at various temperatures and concentrations studied by density and viscosity measurements. J. Therm. Anal. Calorim. 2016, 126, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Szymczyk, K.; Taraba, A. Properties of aqueous solutions of nonionic surfactants, Triton X-114 and Tween 80, at temperatures from 293 to 318K: spectroscopic and ultrasonic studies. Chem. Phys. 2017, 483–484, 96–102. [Google Scholar] [CrossRef]
- El Eini, D.I.; Barry, B.W.; Rhodes, J. Micellar size, shape, and hydration of long-chain polyoxyethylene nonionic surfactants. J. Colloid Inter. Sci. 1976, 54, 348–351. [Google Scholar] [CrossRef]
- Desai, T.R.; Dixit, S.G. Interaction and viscous properties of aqueous solutions of mixed cationic and nonionic surfactants. J. Colloid Inter. Sci. 1966, 177, 471–477. [Google Scholar] [CrossRef]
- Jones, G.; Dole, M. The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am.Chem. Soc. 1929, 51, 2950–2964. [Google Scholar] [CrossRef]
- Ali, A.; Hyder, S.; Akhtar, Y. Viscometric studies of a-amino acid in aqueous NaCl and MgCl2 at 303 K. Indian J. Phys. 2005, 79, 157–160. [Google Scholar]
- Huque, M.; Siddiquey, I.A.; Uddin, N. Volumetric and viscometric studies on dodecyltrimethylammonium bromide in aqueous and in aqueous amino acid solutions in premicellar region. J. Chem. Thermodyn. 2006, 38, 1474–1478. [Google Scholar] [CrossRef]
- Gautam, P.K.; Gautam, R.K.; Rai, R.; Pandey, J.D. Thermodynamic and transport properties of sodium dodecylbenzenesulphonate (SDBS) in aqueous medium over the temperature range 298.15 K to 333.15 K. J. Mol. Liq. 2014, 191, 107–110. [Google Scholar] [CrossRef]
- Szymczyk, K.; Jańczuk, B. The adsorption at solution-air interface and volumetric properties of mixtures of cationic and nonionic surfactants. Coll. Surf. A 2007, 293, 39–50. [Google Scholar] [CrossRef]
- Hepler, L.G. Thermal expansion and structure in water and aqueous solutions. Can. J. Chem. 1969, 47, 4613–4617. [Google Scholar] [CrossRef]
- SunilBabu, K.; VenkateswaraRao, A.; MadhaviLatha, D.; Pardhasaradhi, P.; Pisipati, V.G.K.M. Estimation of thermodynamic parameters in 4-(hexyloxybenzylidene)-4′alkoxy anilines, 6O·Om liquid crystalline compounds– A densitystudy. J. Mol. Liq. 2016, 220, 999–1003. [Google Scholar]
- VenkataRao, D.; Pardhasaradhi, P.; Pisipati, V.G.K.M.; Madhavi Latha, D.; Datta Prasad, P.V. Estimation of thermodynamic parameters in 3.Om and 3O.Om liquid crystalline compounds. J. Mol. Liq. 2015, 211, 90–95. [Google Scholar] [CrossRef]
- Reddy, R.R.; Rama Gopal, K.; Narasimhulu, K.; Siva Sankara Reddy, L.; Raghavendra Kumar, K.; Venkatesulu, A.; Krishna Reddy, C.V. Correlations between Moelwyn–Hughes parameter, available volume and intermolecular free-lengths in liquid systems. J. Mol. Liq. 2008, 140, 48–53. [Google Scholar] [CrossRef]
- Sannaningannavar, F.M.; Patil, S.N.; Navati, B.S.; Melavanki, R.M.; Ayachit, N.H. Studies on thermodynamic properties of purepoly(ethyleneglycol)-400 in the temperature range 299–363 K using volume expansivities. Polym. Bull. 2013, 70, 3171–3183. [Google Scholar] [CrossRef]
- Sharma, B.K.; Reddy, R.R. Sharmaconstant and some more thermo-acousticproperties of ionic liquids. Ind. J. Pure. Appl. Phys. 1985, 23, 396–399. [Google Scholar]
- Fakruddin, K.; Jeevan Kumar, R.; Pisipati, V.G.K.M.; MadhaviLatha, D.; Madhav, B.T.P.; Datta Prasad, P.V. Phase transitions and thermodynamic parameters of N-(p-n-octyloxybenzylidene)-p-nalkoxyanilines—A dilatometric study. Mol. Cryst. Liq. Cryst. 2010, 524, 102–118. [Google Scholar] [CrossRef]
- Dong, D.C.; Winnik, M.A. The Py scale of solvent polarities. Can. J. Chem. 1984, 62, 2560–2565. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

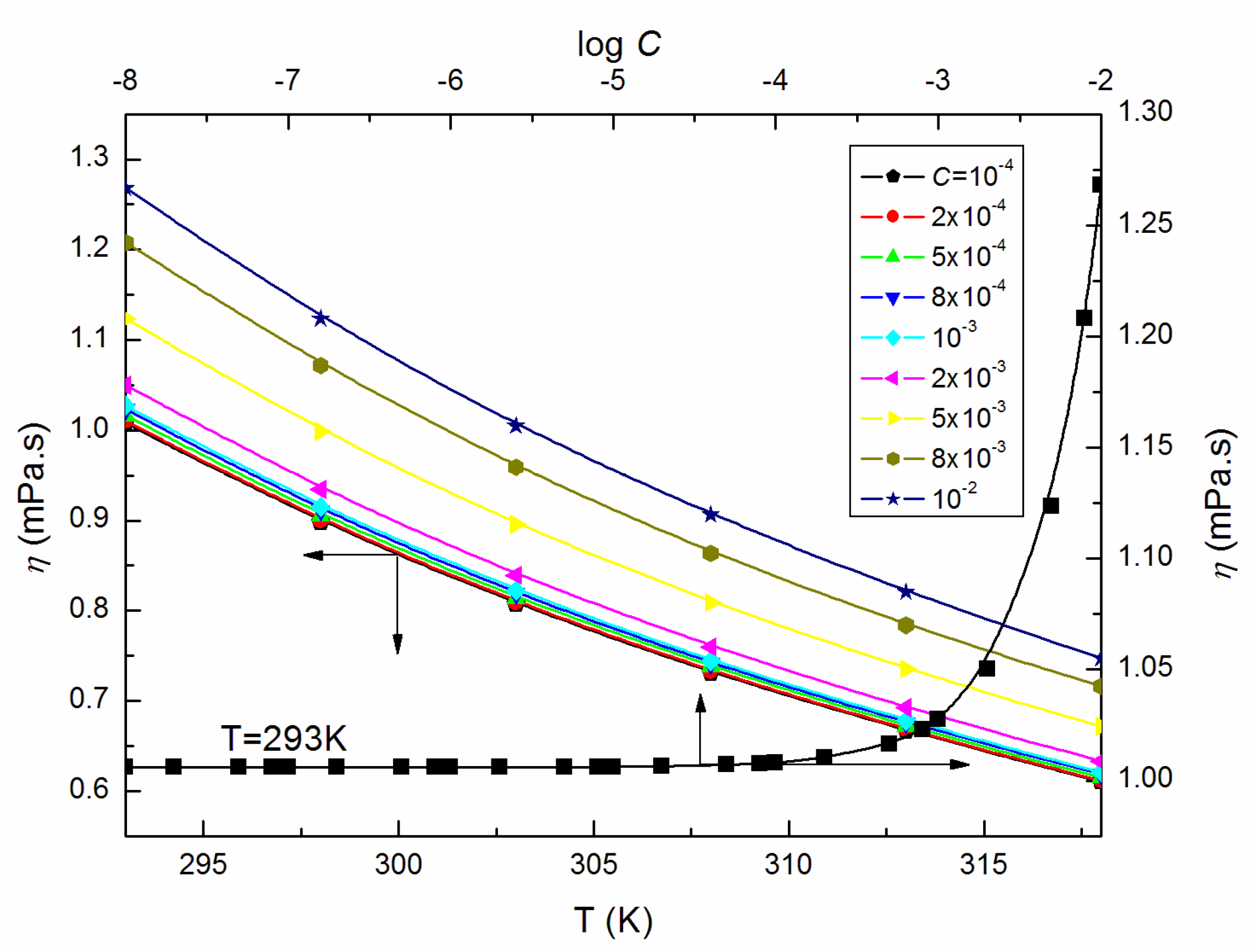



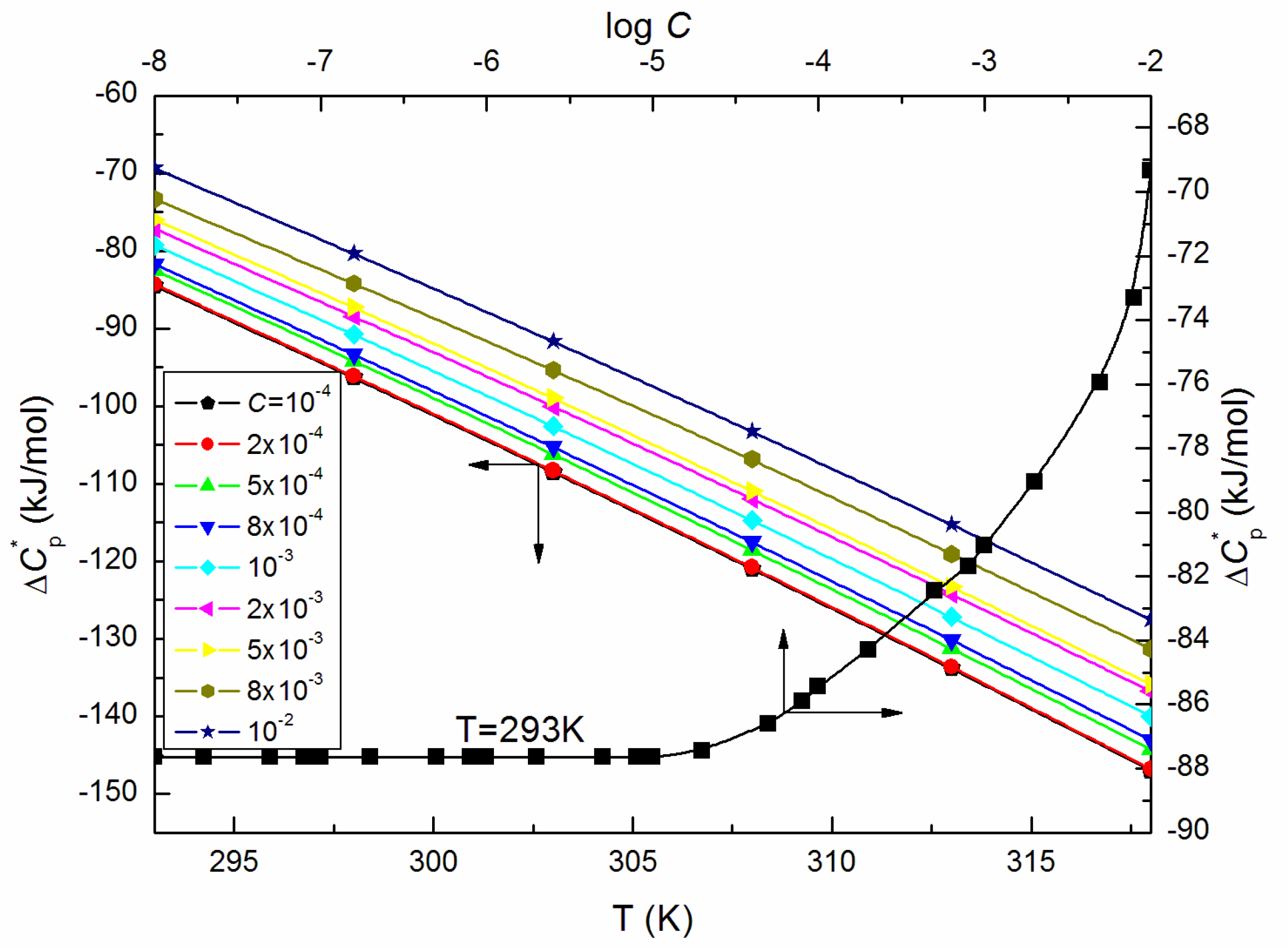


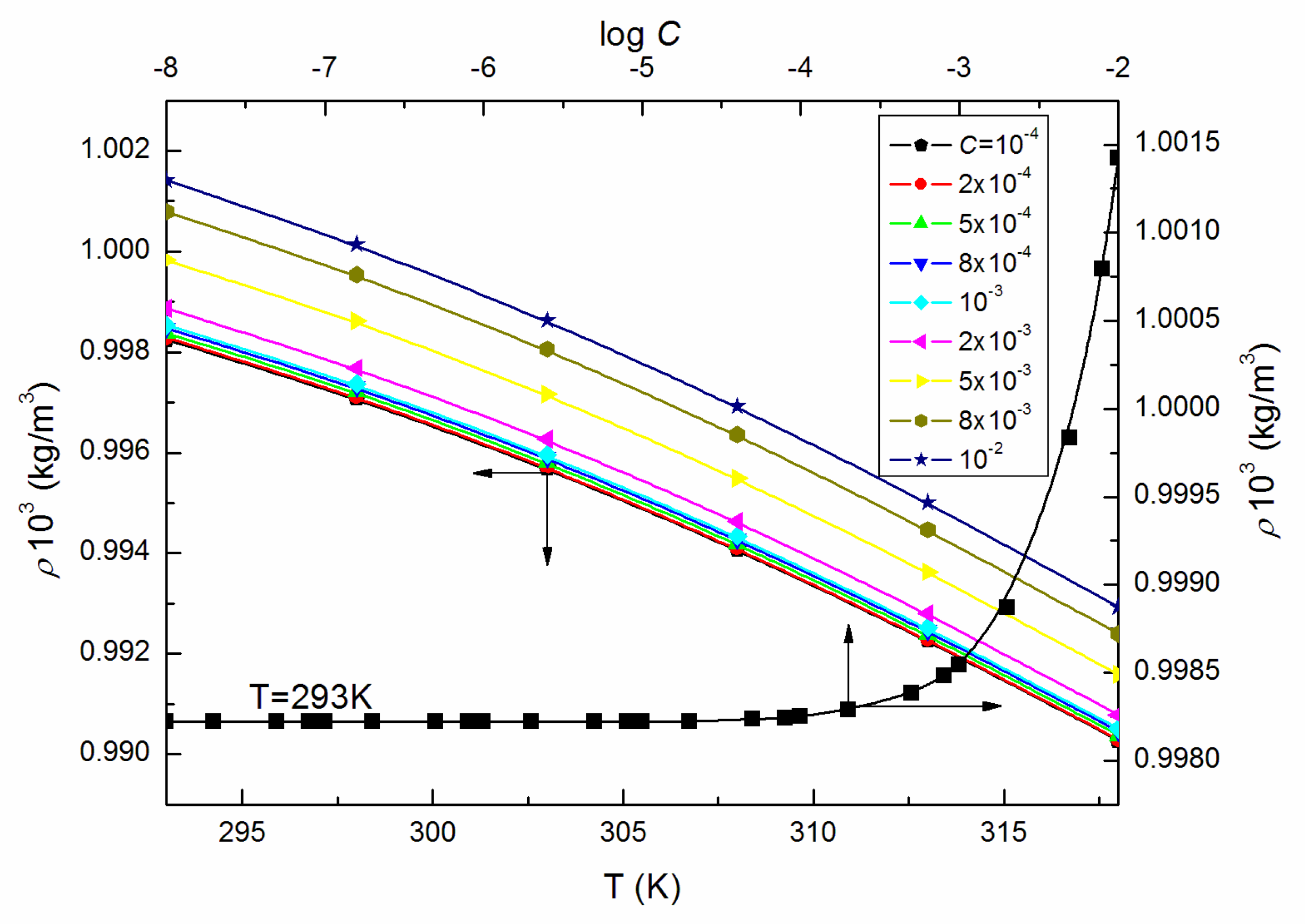



| T = 293K | 298K | 303K | 308K | 313K | 318K | |
|---|---|---|---|---|---|---|
| (mol/dm3) | 2.14 × 10−5 | 2.09 × 10−5 | 2.03 × 10−5 | 1.97 × 10−5 | 1.91 × 10−5 | 1.85 × 10−5 |
| (mol/dm3) | 3.83 × 10−6 | 3.82 × 10−6 | 3.79 × 10−6 | 3.74 × 10−6 | 3.65 × 10−6 | 3.53 × 10−6 |
| 5.59 | 5.47 | 5.35 | 5.26 | 5.23 | 5.24 | |
| (mN/m) | 32.91 | 32.82 | 32.72 | 32.59 | 32.43 | 32.29 |
| (kJ/mol) | −26.19 | −26.70 | −27.22 | −27.75 | -28.28 | −28.81 |
| (kJ/mol K) | 0.105 | |||||
| (kJ/mol) | 4.58 | 4.59 | 4.59 | 4.59 | 4.59 | 4.58 |
| (mol/m2) | 3.17 × 10−6 | 3.11 × 10−6 | 3.06 × 10−6 | 3.01 × 10-6 | 2.95 × 10-6 | 2.90 × 10-6 |
| (nm2) | 0.524 | 0.533 | 0.542 | 0.552 | 0.563 | 0.572 |
| (kJ/mol) | −36.58 | −37.23 | −37.91 | −38.58 | −39.27 | −39.93 |
| (kJ/mol K) | 0.135 | |||||
| (kJ/mol) | 2.98 | 3.00 | 3.00 | 3.00 | 2.99 | 3.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczyk, K.; Szaniawska, M.; Krawczyk, J. Temperature Effect on the Adsorption and Volumetric Properties of Aqueous Solutions of Kolliphor®ELP. Molecules 2020, 25, 743. https://doi.org/10.3390/molecules25030743
Szymczyk K, Szaniawska M, Krawczyk J. Temperature Effect on the Adsorption and Volumetric Properties of Aqueous Solutions of Kolliphor®ELP. Molecules. 2020; 25(3):743. https://doi.org/10.3390/molecules25030743
Chicago/Turabian StyleSzymczyk, Katarzyna, Magdalena Szaniawska, and Joanna Krawczyk. 2020. "Temperature Effect on the Adsorption and Volumetric Properties of Aqueous Solutions of Kolliphor®ELP" Molecules 25, no. 3: 743. https://doi.org/10.3390/molecules25030743
APA StyleSzymczyk, K., Szaniawska, M., & Krawczyk, J. (2020). Temperature Effect on the Adsorption and Volumetric Properties of Aqueous Solutions of Kolliphor®ELP. Molecules, 25(3), 743. https://doi.org/10.3390/molecules25030743






