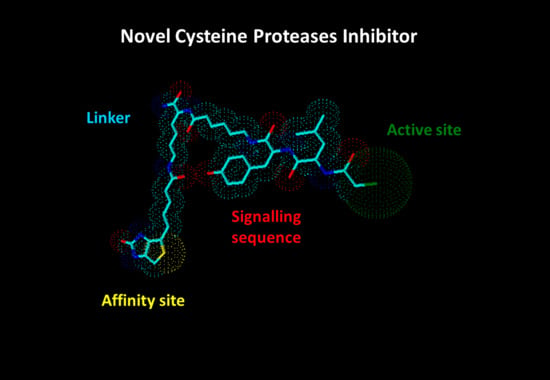
Synthesis of the Novel Covalent Cysteine Proteases Inhibitor with Iodoacetic Functional Group
Abstract
Share and Cite
Hartman, K.; Mielczarek, P.; Silberring, J. Synthesis of the Novel Covalent Cysteine Proteases Inhibitor with Iodoacetic Functional Group. Molecules 2020, 25, 813. https://doi.org/10.3390/molecules25040813
Hartman K, Mielczarek P, Silberring J. Synthesis of the Novel Covalent Cysteine Proteases Inhibitor with Iodoacetic Functional Group. Molecules. 2020; 25(4):813. https://doi.org/10.3390/molecules25040813
Chicago/Turabian StyleHartman, Kinga, Przemyslaw Mielczarek, and Jerzy Silberring. 2020. "Synthesis of the Novel Covalent Cysteine Proteases Inhibitor with Iodoacetic Functional Group" Molecules 25, no. 4: 813. https://doi.org/10.3390/molecules25040813
APA StyleHartman, K., Mielczarek, P., & Silberring, J. (2020). Synthesis of the Novel Covalent Cysteine Proteases Inhibitor with Iodoacetic Functional Group. Molecules, 25(4), 813. https://doi.org/10.3390/molecules25040813