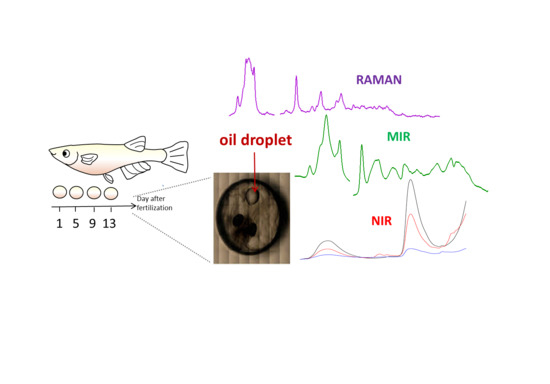
Lipid Droplet Composition Varies Based on Medaka Fish Eggs Development as Revealed by NIR-, MIR-, and Raman Imaging
Abstract
:1. Introduction
2. Results and Discussion
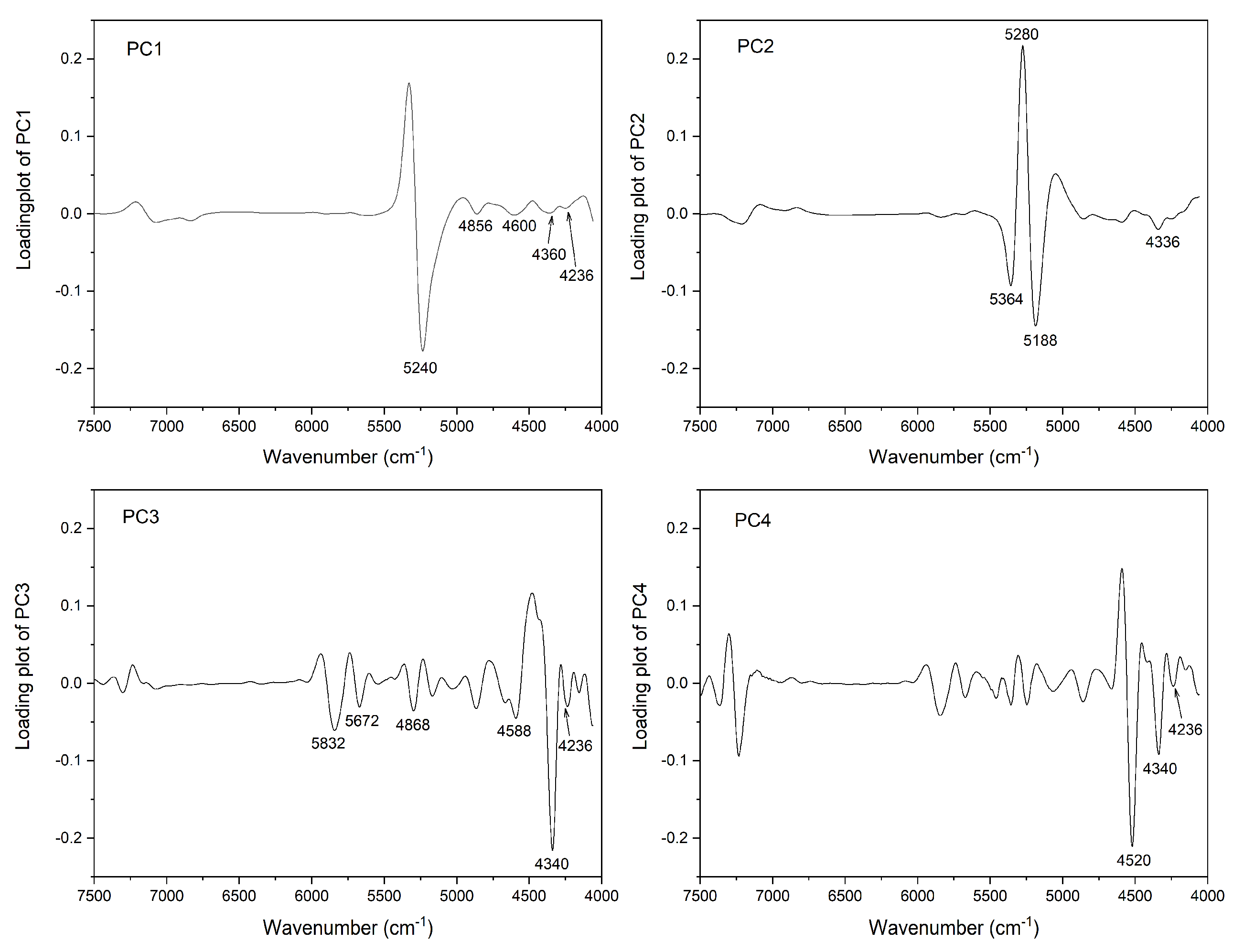
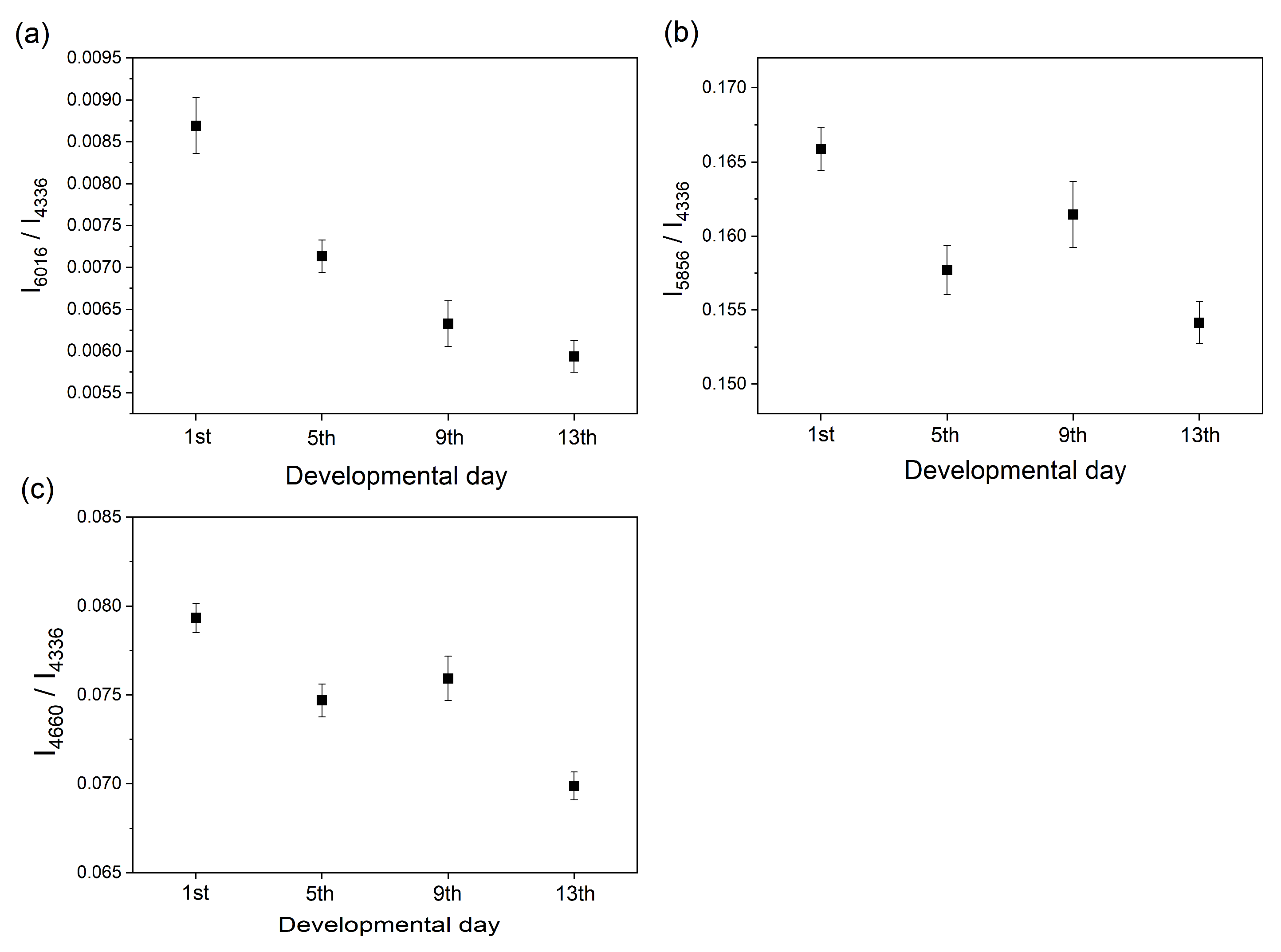
2.1. Near-Infrared (NIR) Imaging
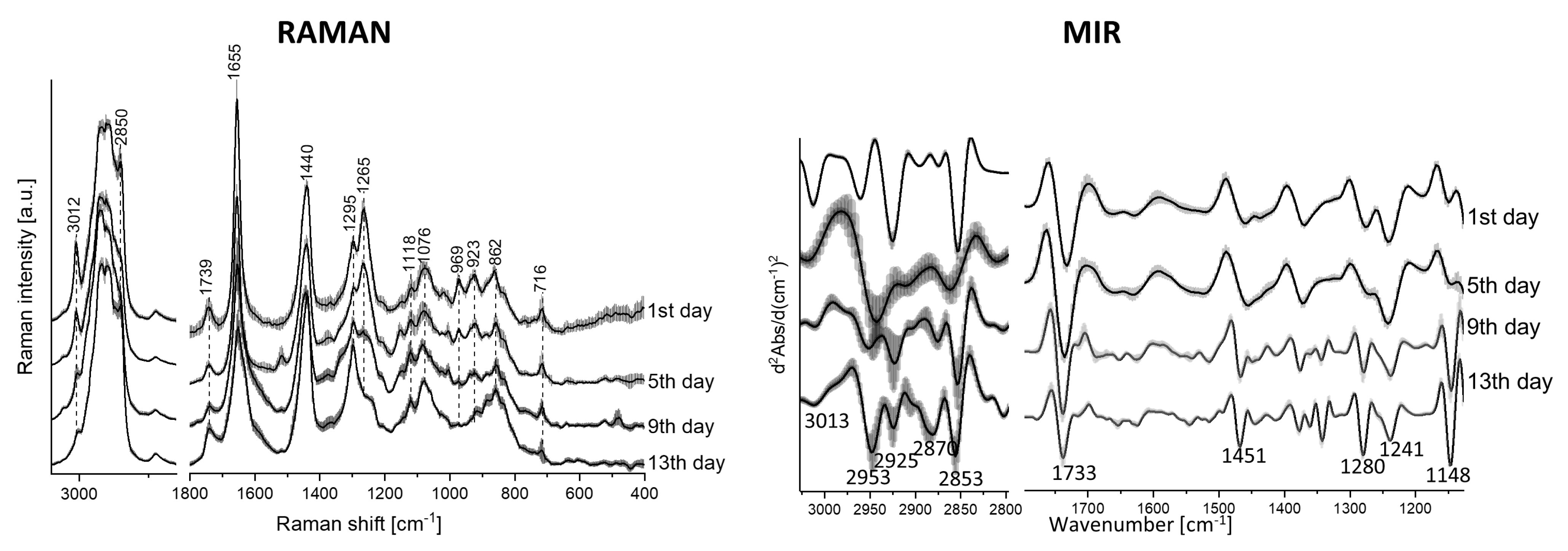
2.2. Raman and Mid-Infrared (MIR ) Imaging
3. Materials and Methods
3.1. Sample Preparation
- Separate embryos by rolling them gently in the petri dish or on a sheet of Whatman-paper.
- Transfer separated embryos to a 25 mL glass vial and fix for 4 h at room temperature in 4% paraformaldehyde with 0.1% Tween20 in phosphate-buffered saline by vigorously rocking on a shaker.
- Transfer embryos to a small petri dish.
- Transfer embryos back to glass vial and wash 4 × 5 min in with 0.1% Tween20 in phosphate-buffered saline.
- Wash 5 min at room temperature in 100% methanol.
- Replace methanol and store embryos at least over night at −20 °C Comments: methanol treatment enhances probe penetration, a longer storage of embryos at −20 °C in methanol is advantageous. Set up fixed embryo stocks during periods of good egg laying.
3.2. NIR Imaging
3.3. MIR Imaging
3.4. Raman Imaging
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schie, I.W.; Nolte, L.; Pedersen, T.L.; Smith, Z.; Wu, J.; Yahiatène, I.; Newman, J.W.; Huser, T. Direct comparison of fatty acid ratios in single cellular lipid droplets as determined by comparative Raman spectroscopy and gas chromatography. Analyst 2013, 138, 6662–6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, T.C.; Farese, R.V. Lipid Droplets and Cellular Lipid Metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamatsu, T.; Muramatsu, T.; Kobayashi, H. Oil droplets and yolk spheres during development of Medaka embryos. Ichthyol. Res. 2008, 55, 344–348. [Google Scholar] [CrossRef]
- Iwamatsu, T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004, 121, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R. Effect of a Docosahexaenoic Acid Devoid Diet on Medaka Oryzias latipes. Ph.D. Thesis, University of California, Davis, CA, USA, 2013. [Google Scholar]
- Majzner, K.; Kochan, K.; Kachamakova-Trojanowska, N.; Maslak, E.; Chlopicki, S.; Baranska, M. Raman imaging providing insights into chemical composition of lipid droplets of different size and origin: In hepatocytes and endothelium. Anal. Chem. 2014, 86, 6666–6674. [Google Scholar] [CrossRef]
- Liu, K.S. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. J. Am. Oil Chem. Soc. 1994, 71, 1179–1187. [Google Scholar] [CrossRef]
- Kochan, K.; Maslak, E.; Krafft, C.; Kostogrys, R.; Chlopicki, S.; Baranska, M. Raman spectroscopy analysis of lipid droplets content, distribution and saturation level in non-Alcoholic Fatty Liver Disease in mice. J. Biophotonics 2015, 8, 597–609. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Matthäus, C.; Bergner, G.; Krafft, C.; Dietzek, B.; Lorkowski, S.; Popp, J. Monitoring intra-cellular lipid metabolism in macrophages by Raman- and CARS-microscopy. Biophotonics Photonic Solut. Better Heal. Care II 2010, 7715, 771511. [Google Scholar]
- Gazi, E.; Gardner, P.; Lockyer, N.P.; Hart, C.A.; Brown, M.D.; Clarke, N.W. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J. Lipid Res. 2007, 48, 1846–1856. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.D.; Zhang, F.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Evaluation of FT-IR and Nile Red methods for microalgal lipid characterization and biomass composition determination. Bioresour. Technol. 2013, 128, 107–112. [Google Scholar] [CrossRef]
- Ishigaki, M.; Kawasaki, S.; Ishikawa, D.; Ozaki, Y. Near-infrared spectroscopy and imaging studies of fertilized fish eggs: In vivo monitoring of egg growth at the molecular level. Sci. Rep. 2016, 6, 20066. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, M.; Nishii, T.; Puangchit, P.; Yasui, Y.; Huck, C.W.; Ozaki, Y. Noninvasive, high-speed, near-infrared imaging of the biomolecular distribution and molecular mechanism of embryonic development in fertilized fish eggs. J. Biophotonics 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, M.; Yasui, Y.; Puangchit, P.; Kawasaki, S.; Ozaki, Y. In vivo monitoring of the growth of fertilized eggs of medaka fish (Oryzias latipes) by near-infrared spectroscopy and Near-Infrared imaging—A marked change in the relative content of weakly hydrogen-bonded water in egg yolk just before hatching. Molecules 2016, 21, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Ozaki, Y.; McClure, W.F.; Christy, A.A. Near-Infrared Spectroscopy in Food Science and Technology; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Workman, J., Jr.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Sato, T.; Kawano, S.; Iwamoto, M.. Near infrared spectral patterns of fatty acid analysis from fats and oils. J. Am. Oil Chem. Soc. 1991, 68, 827–833. [Google Scholar]
- Hug, W.; Chalmers, J.M.; Griffith, P.R. Handbook of Vibrational Spectroscopy; John Wiley and Son Ltd.: Chichester, UK, 2002. [Google Scholar]
- Holly, S.; Egyed, O.; Jalsovszky, G. Assignment problems of amino acids, di-and tripeptides and proteins in the near infrared region. Spectrochim. Acta A Mol. Spectrosc. 1992, 48, 101–109. [Google Scholar] [CrossRef]
- Yang, W.Y.; Larios, E.; Gruebele, M. On the extended β-conformation propensity of polypeptides at high temperature. J. Am. Chem. Soc. 2003, 125, 16220–16227. [Google Scholar] [CrossRef]
- Robert, P.; Devaux, M.F.; Mouhous, N.; Dufour, E. Monitoring the secondary structure of proteins by near-infrared spectroscopy. Appl. Spectrosc. 1999, 53, 226–232. [Google Scholar] [CrossRef]
- Izutsu, K.I.; Fujimaki, Y.; Kuwabara, A.; Hiyama, Y.; Yomota, C.; Aoyagi, N. Near-infrared analysis of protein secondary structure in aqueous solutions and freeze-dried solids. J. Pharm. Sci. 2006, 95, 781–789. [Google Scholar] [CrossRef]
- Grabska, J.; Ishigaki, M.; Beć, K.B.; Wójcik, M.J.; Ozaki, Y. Correlations between Structure and Near-Infrared Spectra of Saturated and Unsaturated Carboxylic Acids. Insight from Anharmonic Density Functional Theory Calculations. J. Phys. Chem. A 2017, 121, 3437–3451. [Google Scholar] [CrossRef]
- Morais, S.; Mourente, G.; Ortega, A.; Tocher, J.A.; Tocher, D.R. Expression of fatty acyl desaturase and elongase genes, and evolution of DHA: EPA ratio during development of unfed larvae of Atlantic bluefin tuna (Thunnus thynnus L.). Aquaculture 2011, 313, 129–139. [Google Scholar] [CrossRef]
- Sato, T. Application of principal-component analysis on near-infrared spectroscopic data of vegetable oils for their classification. J. Am. Oil Chem. Soc. 1994, 71, 293–298. [Google Scholar] [CrossRef]
- Grabska, J.; Beć, K.B.; Ishigaki, M.; Huck, C.W.; Ozaki, Y. NIR spectra simulations by anharmonic DFT-saturated and unsaturated long-chain fatty acids. J. Phys. Chem. B 2018, 122, 6931–6944. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef]
- Ishigaki, M.; Taketani, A.; Sato, H. Discrimination of fish egg quality and viability by Raman spectroscopy. Anal. Methods 2014, 6, 9206–9211. [Google Scholar] [CrossRef]
- Wrobel, T.P.; Mateuszuk, L.; Chlopicki, S.; Malek, K.; Baranska, M. Imaging of lipids in atherosclerotic lesion in aorta from ApoE/LDLR -/- mice by FT-IR spectroscopy and Hierarchical Cluster Analysis. Analyst 2011, 136, 5247–5255. [Google Scholar] [CrossRef]
- Blat, A.; Dybas, J.; Chrabaszcz, K.; Bulat, K.; Jasztal, A.; Kaczmarska, M.; Pulyk, R.; Popiela, T.; Slowik, A.; Malek, K.; et al. FTIR, Raman and AFM characterization of the clinically valid biochemical parameters of the thrombi in acute ischemic stroke. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Blat, A.; Dybas, J.; Kaczmarska, M.; Chrabaszcz, K.; Bulat, K.; Kostogrys, R.B.; Cernescu, A.; Malek, K.; Marzec, K.M. An analysis of isolated and intact rbc membranes—A comparison of a semiquantitative approach by means of FTIR, Nano-FTIR, and Raman Spectroscopies. Anal. Chem. 2019, 91, 9867–9874. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Bally, L.; Carl, M.; Driever, W.; Furutani-Seiki, M.; Grabher, C.; Henrich, T.; Ho, R.; Hong, Y.; Kane, D.; Krone, A.; et al. Molecular and Genetic Tools for the Analysis of Medaka and Zebrafish Development; EMBL: Heidelberg, Germany, 2002. [Google Scholar]
- Lasch, P.; Cytospec, T.M. A Matlab Based Application for Infrared Imaging. 2001. Available online: http://www.cytospec.com (accessed on 3 January 2020).
Sample Availability: Spectral data are available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bik, E.; Ishigaki, M.; Blat, A.; Jasztal, A.; Ozaki, Y.; Malek, K.; Baranska, M. Lipid Droplet Composition Varies Based on Medaka Fish Eggs Development as Revealed by NIR-, MIR-, and Raman Imaging. Molecules 2020, 25, 817. https://doi.org/10.3390/molecules25040817
Bik E, Ishigaki M, Blat A, Jasztal A, Ozaki Y, Malek K, Baranska M. Lipid Droplet Composition Varies Based on Medaka Fish Eggs Development as Revealed by NIR-, MIR-, and Raman Imaging. Molecules. 2020; 25(4):817. https://doi.org/10.3390/molecules25040817
Chicago/Turabian StyleBik, Ewelina, Mika Ishigaki, Aneta Blat, Agnieszka Jasztal, Yukihiro Ozaki, Kamilla Malek, and Malgorzata Baranska. 2020. "Lipid Droplet Composition Varies Based on Medaka Fish Eggs Development as Revealed by NIR-, MIR-, and Raman Imaging" Molecules 25, no. 4: 817. https://doi.org/10.3390/molecules25040817