Green Synthesis of Ag-MnO2 Nanoparticles using Chelidonium majus and Vinca minor Extracts and Their In Vitro Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussions
2.1. Plant Extracts
2.2. Nanoparticles
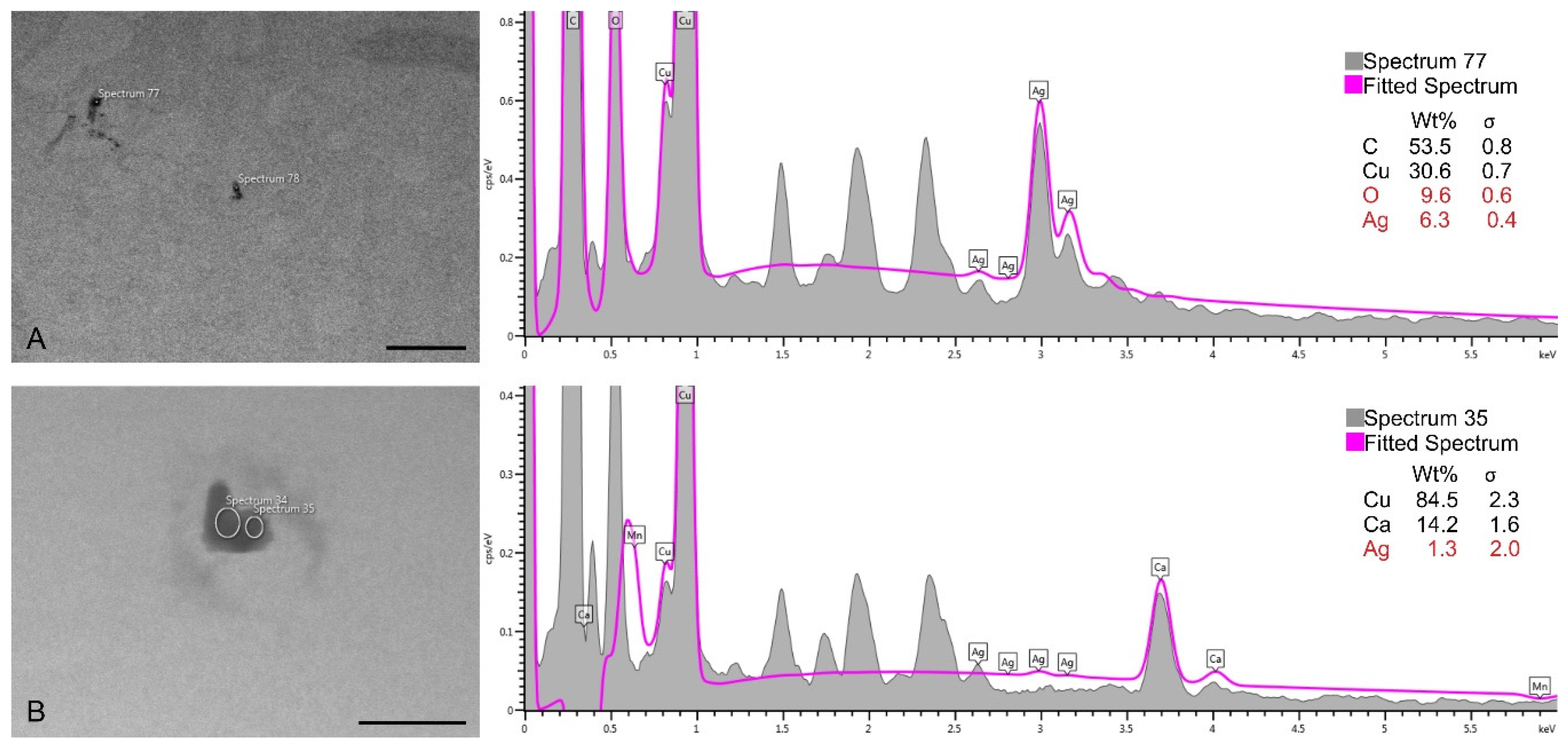
2.2.1. S/TEM Analysis
2.2.2. XRD Analysis
2.2.3. FTIR Analysis
2.3. Cell Toxicity
2.3.1. MTT Assay
2.3.2. LDH Assay
2.3.3. NO Assay
2.4. Nanoparticle Uptake by HaCaT and A375 Cells
3. Materials and Methods
3.1. Plant Material
3.2. Hydroalcoholic Extract Preparation
3.3. Chemical Composition of Plant Extracts
3.4. Synthesis of Ag-MnO2 Nanoparticles
- (1)
- CmNPs—prepared using only C. majus extract;
- (2)
- VmNPs—prepared using only V. minor extract;
- (3)
- MNPs—prepared using a mix of the two extracts in 1:1 ratio.
3.5. Characterization of Ag-MnO2 Nanoparticles
3.6. Cell Toxicity
3.6.1. Established Cell Lines
3.6.2. Cell Viability Assay
3.6.3. Membrane Integrity Assay
3.6.4. Nitric Oxide Assay
3.7. Nanoparticle Uptake by HaCaT and A375 Cells
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alavi, M.; Karimi, N. Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artif. Cells Nanomed Biotechnol. 2018, 46, 2066–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, R.; Singh, A.K.; Kumar, R.; Gupta, A.; Pandey, A.K.; Pandey, A.K. Nanoparticles as modulators of oxidative stress. In Nanotechnology in Modern Animal Biotechnology: Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–35. [Google Scholar]
- Anzabi, Y. Biosynthesis of ZnO nanoparticles using barberry (Berberis vulgaris) extract and assessment of their physico-chemical properties and antibacterial activities. Green Process. Synth. 2018, 7, 114–121. [Google Scholar] [CrossRef]
- Hajebi, S.; Tabrizi, M.H.; Moghaddam, M.N.; Shahraki, F.; Yadamani, S. Rapeseed flower pollen biogreen synthesized silver nanoparticles: A promising antioxidant, anticancer and antiangiogenic compound. J. Biol. Inorg. Chem. 2019, 24, 395–404. [Google Scholar] [CrossRef] [PubMed]
- National Nanotechnology Initiative. Available online: https://www.nano.gov (accessed on 16 January 2020).
- European Commission. Available online: https://ec.europa.eu (accessed on 16 January 2020).
- Grewal, D.S. Funding nanotechnology-A comparative study of global and national funding. J. Nanom. Nanos. Tech. 2019, 105. [Google Scholar]
- European Commission Decision C. Horizon 2020 Work-Programme 2018–2020. 2019, p. 94. Available online: https://ec.europa.eu/research/participants/data/ref/h2020/wp/2018-2020/main/h2020-wp1820-infrastructures_en.pdf (accessed on 16 January 2020).
- Web of Science. Available online: https://webofknowledge.com (accessed on 16 January 2020).
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms. Regul. Toxicol. Pharmacol. 2018, 98, 231–239. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Thoonen, K.; Osch, L.V.; Vries, H.D.; Jongen, S.; Schneider, F. Are Environmental interventions targeting skin cancer prevention among children and adolescents effective? A Systematic Review. Int. J. Environ. Res. Pub. Health 2020, 17, 529. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund International. Available online: https://www.wcrf.org (accessed on 31 January 2020).
- Skin Cancer Foundation. Available online: https://www.skincancer.org (accessed on 31 January 2020).
- Shukla, S.; Mehta, A. Anticancer potential of medicinal plants and their phytochemicals: A review. Braz. J. Bot. 2015, 38, 199–210. [Google Scholar] [CrossRef]
- Petruczynik, A.; Tuzimski, T.; Plech, T.; Misiurek, J.; Szalast, K.; Szymczak, G.Z. Comparison of anticancer activity and HPLC-DAD determination of selected isoquinoline alkaloids from Thalictrum foetidum, Berberis sp. and Chelidonium majus extracts. Molecules 2019, 24, 3417. [Google Scholar] [CrossRef] [Green Version]
- Soejima, T.; Nishizawa, K.; Isoda, R. Monodisperse manganese oxide nanoparticles: Synthesis, characterization, and chemical reactivity. J. Colloid Interf. Sci. 2018, 510, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M.; Arefian, Z.; Dastjerdi, H.A.; Ajdary, M. Toxic effects of Mn2O3 nanoparticles on rat testis and sex hormone. J. Nat. Sci. Biol. Med. 2019, 6, 335–339. [Google Scholar]
- Asaikkutti, A.; Bhavan, P.S.; Vimala, K.; Karthik, M.; Cheruparambath, P. Dietary supplementation of green synthesized manganese-oxide nanoparticles and its effect on growth performance, muscle composition and digestive enzyme activities of the giant freshwater prawn Macrobrachium rosenbergii. J. Trace Elem. Med. Biol. 2016, 35, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.A.; Salunke, B.K.; Alkotaini, B.; Sathiyamoorthi, E.; Kim, B.S. Biological synthesis of manganese dioxide nanoparticles by Kalopanax pictus plant extract. IET Nanobiotechnol. 2014, 9, 220–225. [Google Scholar] [CrossRef]
- Nair Sreekala, G.; Abdullakutty, F.; Beena, B. Green synthesis, characterization and photo catalytic degradation efficiency of trimanganese tetroxide nanoparticle. Int. J. Nano. Dimens. 2019, 10, 400–409. [Google Scholar]
- Noghabi, M.P.; Parizadeh, M.R.; Ghayour-Mobarhan, M.; Taherzadeh, D.; Hosseini, H.A.; Darroudi, M. Green synthesis of silver nanoparticles and investigation of their colorimetric sensing and cytotoxicity effects. J. Mol. Struct. 2017, 1146, 499–503. [Google Scholar] [CrossRef]
- Salaheldin, T.A.; El-Chaghaby, G.A.; El-Sherbiny, M.A. Green synthesis of silver nanoparticles using Portulacaria afra plant extract: Characterization and evaluation of its antibacterial, anticancer activities. Novel Res. Microbiol. J. 2019, 3, 215–222. [Google Scholar] [CrossRef]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Prabu, H.G. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photoch. Photobio. B. 2017, 170, 225–234. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017, 10, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, B.; Tafvizi, F.; Bostanabad, S.Z. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif. Cells Nanomed. Biotechnol. 2018, 46, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, B.; Qiao, Q.; Arandiyan, H.; Li, J.; Hao, J. Three-dimensional ordered mesoporous MnO2 supported Ag nanoparticles for catalytic removal of formaldehyde. Environ. Sci. Technol. 2016, 50, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Guo, Z.; Che, H.; Mu, J.; Zhang, X.; Zhang, Z.; Wang, G.; Bai, Y.; Xie, H. Core/shell nanorods of MnO2/carbon embedded with Ag nanoparticles as high-performance electrode materials for supercapacitors. Chem. Eng. J. 2018, 331, 23–30. [Google Scholar] [CrossRef]
- Kunkalekar, R.K.; Naik, M.M.; Dubey, S.K.; Salker, A.V. Antibacterial activity of silver-doped manganese dioxide nanoparticles on multidrug-resistant bacteria. J Chem. Technol. Biotechnol. 2012, 88, 873–877. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- Yan-yu, R.; Hui, Y.; Tao, W.; Chuang, W. Green synthesis and antimicrobial activity of monodisperse silver nanoparticles synthesized using Ginkgo biloba leaf extract. Phys. Lett. A. 2016, 380, 3773–3777. [Google Scholar]
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium majus–an integrative review: Traditional knowledge versus modern findings. Forsch. Komplementmed. 2010, 17, 241–248. [Google Scholar] [CrossRef]
- Parvu, M.; Parvu, A.E.; Craciun, C.; Barbu-Tudoran, L.; Tamas, M. Antifungal activities of Chelidonium majus extract on Botrytis cinerea in vitro and ultrastructural changes in its conidia. J. Phytopathol. 2008, 156, 550–552. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Eid, S.; Ashour, M.L.; Tahrani, A.; Wink, M. Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids. Phytomedicine 2013, 20, 282–294. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Guo, X.; Guo, Q.; Li, D. Metabolomics Characterization of Two Apocynaceae Plants, Catharanthus roseus and Vinca minor, Using GC-MS and LC-MS Methods in Combination. Molecules 2017, 22, 997. [Google Scholar] [CrossRef] [Green Version]
- Khanavi, M.; Pourmoslemi, S.; Farahanikia, B.; Hadjiakhoondi, A.; Ostad, S.N. Cytotoxicity of Vinca minor. Pharm. Biol. 2010, 48, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Deljanin, M.; Nikolic, M.; Baskic, D.; Todorovic, D.; Djurdjevic, P.; Zaric, M.; Stankovic, M.; Todorovic, M.; Avramovic, D.; Popovic, S. Chelidonium majus crude extract inhibits migration and induces cell cycle arrest and apoptosis in tumor cell lines. J. Ethnopharmacol. 2016, 190, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Capistrano, R.I.; Wouters, A.; Lardon, F.; Gravekamp, C.; Apers, S.; Pieters, L. In vitro and in vivo investigations on the antitumour activity of Chelidonium majus. Phytomedicine 2015, 22, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, H.; Pannert, L.; Pfeiffer, S.; Wachter, F.; Amtmann, E.; Jeremias, I. Enhanced anti-tumour effects of Vinca alkaloids given separately from cytostatic therapies. Br. J. Pharmacol. 2013, 168, 1558–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, M.A.; Thrower, D.; Wilson, L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991, 51, 2212–2222. [Google Scholar] [PubMed]
- Moudi, M.; Go, R.; Seok Yien, C.Y.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 41, 1231–1235. [Google Scholar]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices. 2017, 20. [Google Scholar] [CrossRef] [Green Version]
- Parvu, M.; Vlase, L.; Fodorpataki, L.; Parvu, O.; Bartha, C.; Rosca-Casian, O.; Barbu-Tudoran, L.; Parvu, A.E. Chemical composition of celandine (Chelidonium majus L.) extract and its effects on Botrytis tulipae (Lib.) lind fungus and the tulip. Not. Bot. Horti. Agrobot. 2013, 41, 414–426. [Google Scholar] [CrossRef] [Green Version]
- AlSalhi, M.S.; Elangovan, K.; Ranjitsingh, A.J.A.; Murali, P.; Devanesan, S. Synthesis of silver nanoparticles using plant derived 4-N-methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci. 2019, 26, 970–978. [Google Scholar] [CrossRef]
- Dehghanizadea, S.; Arasteha, J.; Mirzaie, A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: Characterization and in vitro biological activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Krishnaraj, C.; Ji, B.-J.; Harper, S.L.; Yun, S.-I. Plant extract-mediated biogenic synthesis of silver, manganese dioxide, silver-doped manganese dioxide nanoparticles and their antibacterial activity against food- and water-borne pathogens. Bioprocess Biosyst. Eng. 2016, 39, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.A.; Al-Thabaiti, S.A.; Al-Arjan, W.S.; Malik, M.A.; Khan, Z. Preparation of ultra long a-MnO2 and Ag@MnO2 nanoparticles by seedless approach and their photocatalytic performance. J. Mol. Struct. 2017, 1137, 495–505. [Google Scholar] [CrossRef]
- Ramalingam, J.R.; Vaali-Mohammed, M.-A.; Al-Lohedan, H.A.; Appaturi, J.N. Synthesis and bio-physical characterization of silver nanoparticle and Ag-mesoporous MnO2 nanocomposite for anti-microbial and anti-cancer activity. J. Mol. Liq. 2017, 243, 348–357. [Google Scholar]
- Zavoi, S.; Fetea, F.; Ranga, F.; Pop, R.M.; Baciu, A.; Socaciu, C. Comparative fingerprint and extraction yield of medicinal herb phenolics with hepatoprotective potential, as determined by UV-VIS and FT-MIR spectroscopy. Not. Bot. Horti. Agrobot. 2011, 39, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Baciu, A.; Ranga, F.; Fetea, F.; Zavoi, S.; Socaciu, C. Fingerprinting food dupplements and their botanical ingredients by coupled UV/Vis/FTIR Spectrometry. Bull. UASVM Food Sci. Technol. 2013, 70, 8–15. [Google Scholar]
- Farahanikia, B.; Akbarzadeh, T.; Jahangirzadeh, A.; Yassa, N.; Shams Ardekani, M.R.; Mirnezami, T.; Hadjiakhoondi, A.; Khanavi, M. Phytochemical investigation of Vinca minor cultivated in Iran. Iran. J. Pharm. Res. 2011, 10, 777–785. [Google Scholar]
- Poojary, M.M.; Vishnumurthy, K.A.; Adhikari, A.V. Extraction, characterization and biological studies of phytochemicals from Mammea suriga. J. Pharm. Anal. 2015, 5, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-J.; Li, W.-S. Preparation of manganese dioxide for oxygen reduction in zinc air battery by hydro thermal method. J. Inorg. Mater. 2013, 28, 341–346. [Google Scholar] [CrossRef]
- Jaganyi, D.; Altaf, M.; Wekesa, I. Synthesis and characterization of whisker-shaped MnO2 nanostructure at room temperature. Appl. Nanosci. 2013, 3, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Structural and optical properties of Ag nanoparticles synthesized by thermal treatment method. Materials 2017, 10, 402. [Google Scholar] [CrossRef]
- Spectral Database for Organic Compounds, SDBS. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_disp.cgi?sdbsno=10560& (accessed on 31 January 2020).
- Leona, M.; Lombardi, J.R. Identification of berberine in ancient and historical textiles by surfaceenhanced Raman scattering. J. Raman Spectrosc. 2007, 38, 853–858. [Google Scholar] [CrossRef]
- Macavei, S.G.; Suciu, M.; Craciunescu, I.; Barbu-Tudoran, L.; Tripon, S.C.; Balan, R. Hyperthermia effects on normal and tumor skin cells. Annals of R.S.C.B. 2016, 21, 11–21. [Google Scholar]
- Moulton, M.C.; Braydich-Stolle, L.K.; Nadagouda, M.N.; Kunzelman, S.; Hussain, S.M.; Varma, R.S. Synthesis, characterization and biocompatibility of ‘‘green’’ synthesized silver nanoparticles using tea polyphenols. Nanoscale 2010, 2, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Eo, S.-H.; Seo, S.-Y.; Kim, S.J.; Hong, S.-K.; Choi, C.-S.; Lee, K.H. A potential mediator for photodynamic therapy based on silver nanoparticles functionalized with porphyrin. J. Photochem. Photobiol. A. 2019, 377, 26–35. [Google Scholar] [CrossRef]
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Kokura, S.; Handa, O.; Takagi, T.; Ishikawa, T.; Naito, Y.; Yoshikawa, T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomed. Nanotech. Biol. Med. 2010, 6, 570–574. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Baldea, I.; Clichici, S.; Filip, G.A. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mater. Sci. Eng. C 2017, 79, 720–727. [Google Scholar] [CrossRef]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Microwave assisted green synthesis of silver nanoparticles using leaf extract of Elephantopus scaber and its environmental and biological applications. Artif. Cells Nanomed. Biotechnol. 2017, 46, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Phull, A.R.; Kim, S.J.; Lee, K.H. Gallotannin mediated silver colloidal nanoparticles as multifunctional nano platform: Rapid colorimetric and turn-on fluorescent sensor for Hg2+, catalytic and in vitro anticancer activities. J. Lumin. 2019, 206, 624–633. [Google Scholar] [CrossRef]
- Garvey, M.; Cruz-Romero, M.; Padmanabhan, S.C.; Kerry, J.P.; Pillai, S.C.; Morris, M.A. In vitro cytotoxicity of water soluble silver (Ag) nanoparticles on HaCaT and A549 cell Lines. J. Toxicol. Pharmacol. 2017, 1, 16. [Google Scholar]
- Mukherjee, S.G.; O’Claonadh, N.; Casey, A.; Chambers, G. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol. in Vitro 2012, 26, 238–251. [Google Scholar] [CrossRef] [Green Version]
- Behboodi, S.; Baghbani-Arani, F.; Abdalan, S.; Shandiz, S.A.S. Green engineered biomolecule-capped silver nanoparticles fabricated from Cichorium intybus extract: In vitro assessment on apoptosis properties toward human breast cancer (MCF-7) cells. Biol. Trace Elem. Res. 2019, 187, 392–402. [Google Scholar] [CrossRef]
- Boyadzhiev, L.; Yordanov, B. Pertraction of indole alkaloids from Vinca minor L. Sep. Sci. Technol. 2005, 39, 1321–1329. [Google Scholar] [CrossRef]
- Sottomayor, M.; Barcelo, A.R. The Vinca alkaloids: From biosynthesis and accumulation in plant cells, to uptake, activity and metabolism in animal cells. Nat. Prod. Chem. 2006, 33, 813–857. [Google Scholar]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano. 2019, 13, 7410–7424. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, M.F.; Bielenberg, D.R.; Lenormand, G.; Marinkovic, M.; Waghorne, C.G.; Zetter, B.R.; Fredberg, J.J. Cytoskeletal stiffness, friction, and fluidity of cancer cell lines with different metastatic potential. Clin. Exp. Metastasis. 2013, 30, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Bonin, K.; Scarpinato, K.; Guthold, M. The effect of neighboring cells on the stiffness of cancerous and non-cancerous human mammary epithelial cells. New J. Phys. 2014, 16, 5002. [Google Scholar] [CrossRef] [Green Version]
- Graf, C.; Nordmeyer, D.; Sengstock, C.; Ahlberg, S.; Diendorf, J.R.; Raabe, J.R.; Epple, M.; Koller, M.; Lademann, J.R.; Vogt, A.; et al. Shape-dependent dissolution and cellular uptake of silver nanoparticles. Langmuir 2018, 34, 1506–1519. [Google Scholar] [CrossRef]
- Pogoda, K.; Jaczewska, J.; Wiltowska-Zuber, J.; Klymenko, O.; Zuber, K.; Fornal, M.; Lekka, M. Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur. Biophys. J. 2012, 41, 79–87. [Google Scholar] [CrossRef]
- Lekka, M.; Pogoda, K.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Wiltowska-Zuber, J.; Jaczewska, J.; Lekki, J.; Stachura, Z. Cancer cell recognition–mechanical phenotype. Micron 2012, 43, 1259–1266. [Google Scholar] [CrossRef]
- Clark, A.G.; Vignjevic, D.M. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 2015, 36, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobiepanek, A.; Milner-Krawczyk, M.; Lekka, M.; Kobiela, T. AFM and QCM-D as tools for the distinction of melanoma cells with a different metastatic potential. Biosens. Bioelectron. 2017, 93, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.; Ferreira de Oliveira, J.M.P.; Brown, D.; Jonhston, H.; Malheiro, E.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. The influence of Citrate or PEG coating on silver nanoparticle toxicity to a human keratinocyte cell line. Toxicol. Lett. 2016, 249, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderc, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles–A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Tyagi, N.; Bhardwaj, A.; Rusu, L.; Palanki, R.; Vig, K.; Singh, S.R.; Singh, A.P.; Palanki, S.; Miller, M.E.; et al. Silver nanoparticles protect human keratinocytes against UVB radiation-induced DNA damage and apoptosis: Potential for prevention of skin carcinogenesis. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1265–1275. [Google Scholar] [CrossRef] [Green Version]
- Carrola, J.; Bastos, V.; Jarak, I.; Oliveira-Silva, R.; Malheiro, E.; Daniel-da-Silva, A.L.; Oliveira, H.; Santos, C.; Gil, A.M.; Duarte, I.F. Metabolomics of silver nanoparticles toxicity in HaCaT cells: Structure-activity relationships and role of ionic silver and oxidative stress. Nanotoxicology 2016, 10, 1105–1117. [Google Scholar] [CrossRef]
- Andersson, P.O.; Lejon, C.; Ekstrand-Hammarström, B.; Akfur, C.; Ahlinder, L.; Bucht, A.; Österlund, L. Polymorph- and size-dependent uptake and toxicity of TiO2 nanoparticles in living lung epithelial cells. Toxicology 2011, 7, 514–523. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Habas, K.; Shang, L. Silver nanoparticle-mediated cellular responses in human keratinocyte cell line HaCaT in vitro. Nanoscale Reports 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdörster, G. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Suman, T.Y.; Radhika Rajasree, S.R.; Kanchana, A.; Beena Elizabeth, S. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B. 2013, 106, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Levytskyy, R.M.; Filyak, Y.Z.; Stoika, R.S. Correlation between generation of nitric oxide and cell viability in human peripheral blood mononuclear cells and leukemic jurkat t-cell line. Exp. Oncol. 2004, 26, 217–220. [Google Scholar] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int.l J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Shan, K.; Song, J.; Liu, J.; Rajendran, S.; Pugazhendhi, A.; Jacob, J.A.; Chen, B. Toxic effects of magnetic nanoparticles on normal cells and organs. Life Sci. 2019, 220, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Majidi, F.S.; Mohammadi, E.; Mehravi, B.; Nouri, S.; Ashtari, K.; Neshasteh-riz, A. Investigating the effect of near infrared photo thermal therapy folic acid conjugated gold nano shell on melanoma cancer cell line A375. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Andreicut, A.-D.; Pârvu, A.E.; Mot, A.C.; Pârvu, M.; Fischer Fodor, E.; Cătoi, A.F.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical analysis of anti-inflammatory and antioxidant effects of Mahonia aquifolium flower and fruit extracts. Oxid. Med Cell. Long. 2018, 2018, 2879793. [Google Scholar] [CrossRef] [Green Version]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciorîță, A.; Suciu, M.; Macavei, S.; Kacso, I.; Lung, I.; Soran, M.-L.; Pârvu, M. Green Synthesis of Ag-MnO2 Nanoparticles using Chelidonium majus and Vinca minor Extracts and Their In Vitro Cytotoxicity. Molecules 2020, 25, 819. https://doi.org/10.3390/molecules25040819
Ciorîță A, Suciu M, Macavei S, Kacso I, Lung I, Soran M-L, Pârvu M. Green Synthesis of Ag-MnO2 Nanoparticles using Chelidonium majus and Vinca minor Extracts and Their In Vitro Cytotoxicity. Molecules. 2020; 25(4):819. https://doi.org/10.3390/molecules25040819
Chicago/Turabian StyleCiorîță, Alexandra, Maria Suciu, Sergiu Macavei, Irina Kacso, Ildiko Lung, Maria-Loredana Soran, and Marcel Pârvu. 2020. "Green Synthesis of Ag-MnO2 Nanoparticles using Chelidonium majus and Vinca minor Extracts and Their In Vitro Cytotoxicity" Molecules 25, no. 4: 819. https://doi.org/10.3390/molecules25040819




