Boron Chemistry for Medical Applications
Abstract
1. Introduction
2. Boron Clusters for Medical Applications
2.1. Structure Features of Boron Clusters
2.2. Properties of Boron Clusters for Medical Applications
2.3. Boron Cluster Implication for Drug Design
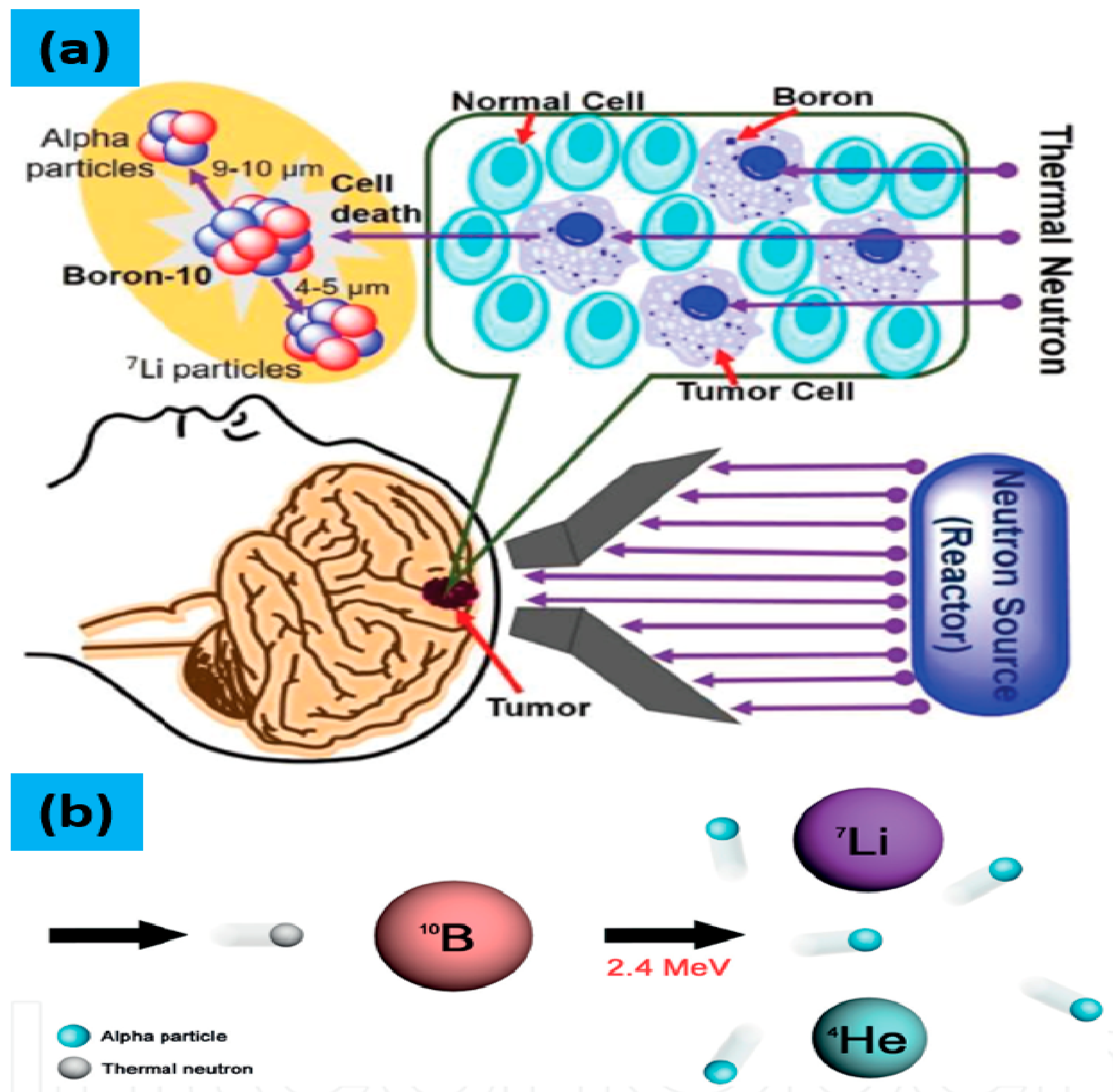
3. Boron Neutron Capture Therapy (BNCT)
3.1. Mechanism of BNCT
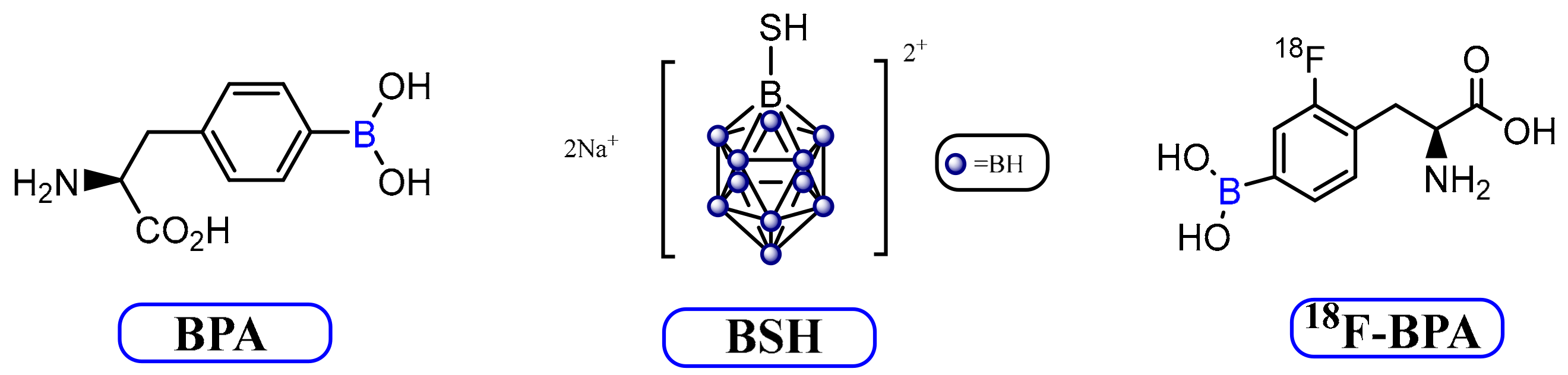
3.2. Current BNCT Agents
3.3. Development of Novel BNCT Agents
3.3.1. Boron Nanoparticles with BNCT
3.3.2. Boron Nitride Nanotubes/Nanoparticles with BNCT
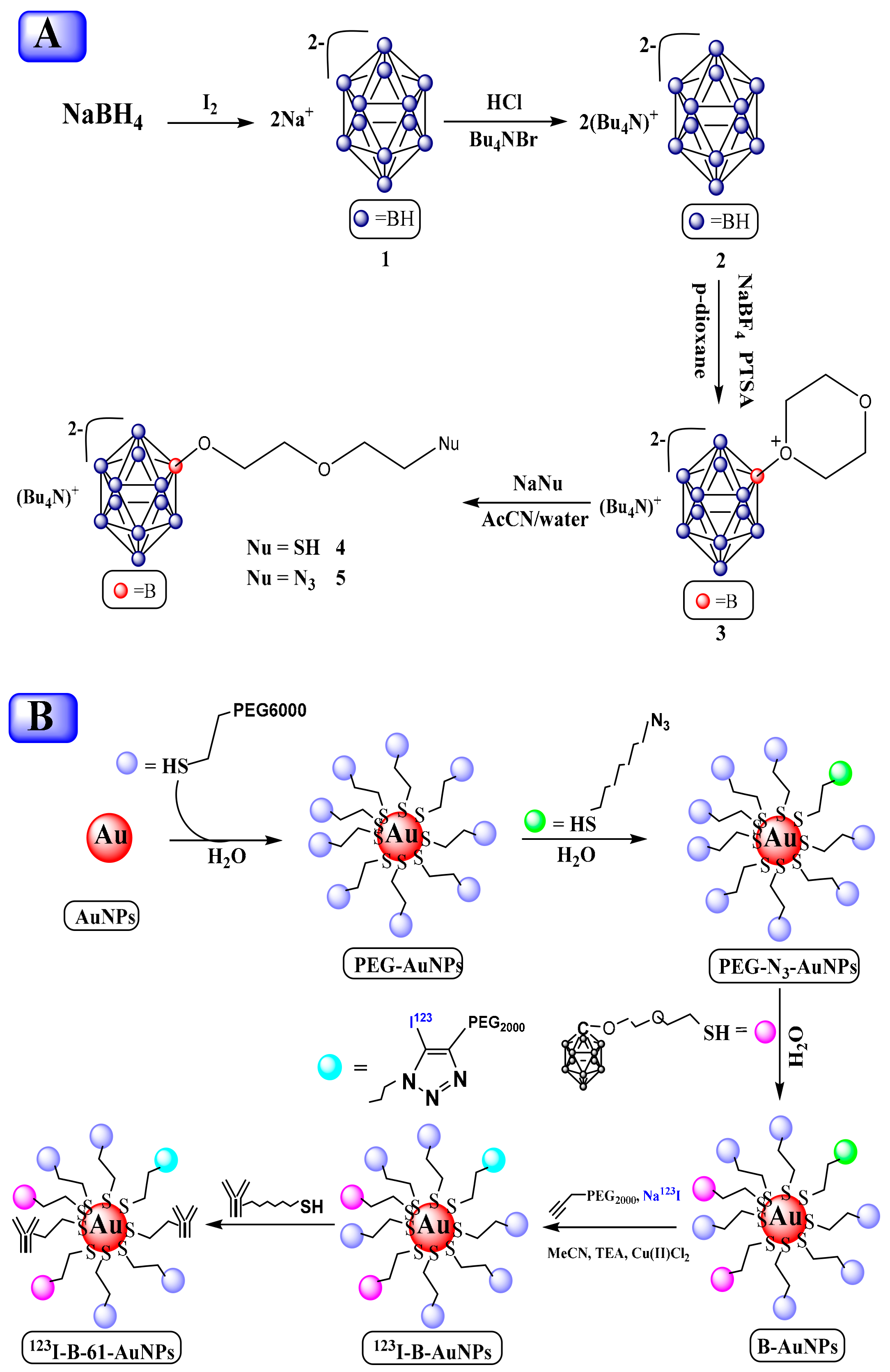
3.3.3. Gold Nanocluster/Nanoparticles with Borane for BNCT
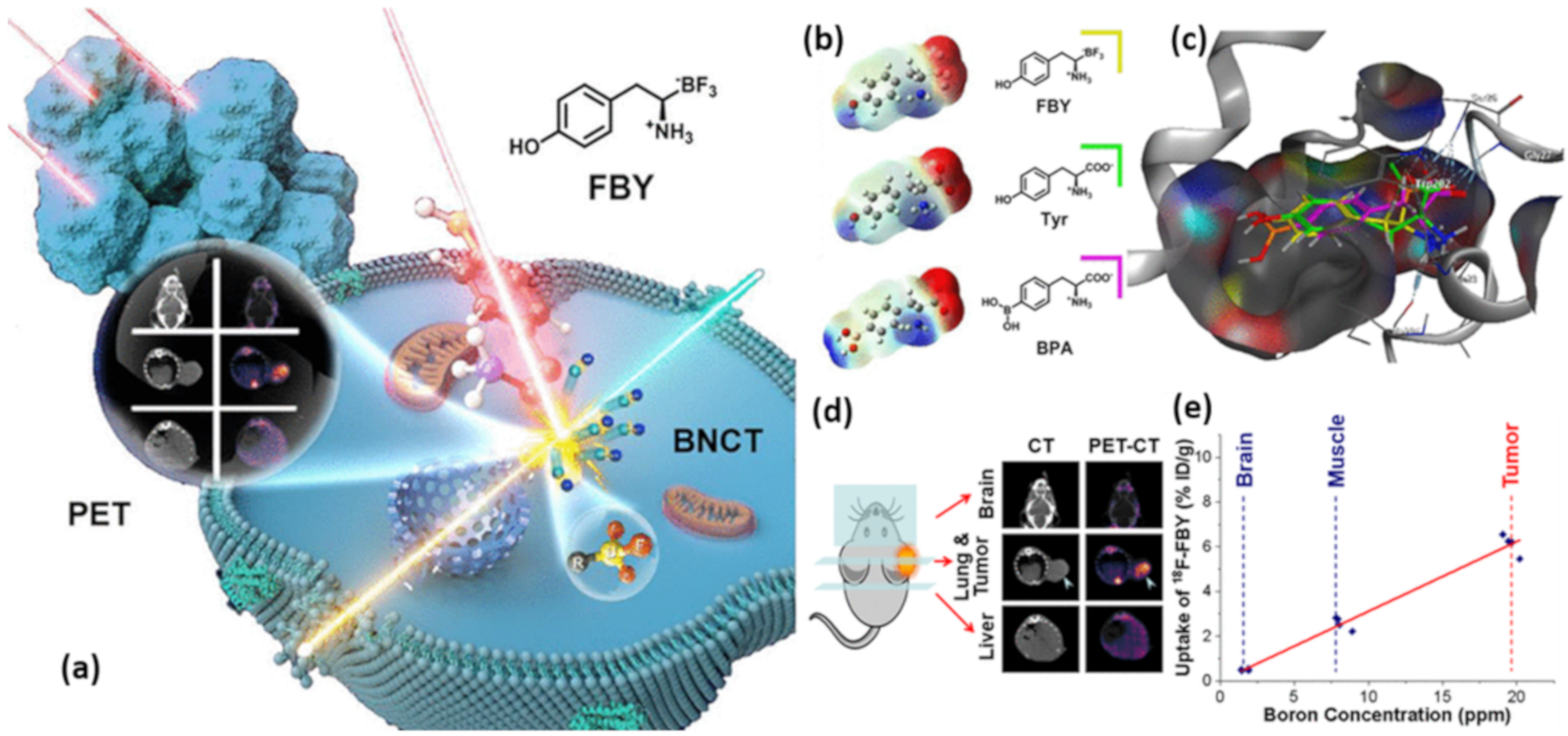
3.3.4. Boron-Based Amino Acids for BNCT
3.3.5. Boron-based Polymers for BNCT
4. Accelerator-Based BNCT (AB-BNCT)
5. Treatment of Different Cancer Tumor with BNCT
5.1. Recurrent Head and Neck Regional Tumor Treatment with BNCT
- 1)
- Most patients with local recurrent HNSCC responded to BNCT.
- 2)
- A high minimum dose delivered to the tumor was a key predictive factor for treatment response, and the number of BNCT treatments was a minimally important factor for progression-free survival and overall survival.
- 3)
- Tumor size < 25 cm3 was found to be a favorable prognostic factor for survival and achieving complete response.
- 4)
- The minimum dose to the gross tumor volume was associated with the survival rates [128,129]. This was the first study to inspect the treatment outcomes in locally recurrent HNSCC patients in association with tumor dose from BNCT. However, this is a survey study. Thus, some key statistics on critical factors, such as adverse effects related to BNCT, human papilloma virus infection, and treatment-related deaths, were not measured or recorded, and could not control the consequences. This study delivers imperative evidence-based grounds for originating random clinical trials for the comparison of BNCT efficacy with other radiotherapies. To improve patient survival, this type of study is required to determine alternative therapies.
5.2. Cutaneous and Genital Cancer Treatment with BNCT
6. Secondary Cancer risk with BNCT
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Axtell, J.C.; Saleh, L.M.; Qian, E.A.; Wixtrom, A.I.; Spokoyny, A.M. Synthesis and applications of perfunctionalized boron clusters. Inorg. Chem. 2018, 57, 2333–2350. [Google Scholar] [CrossRef]
- Pitochelli, A.R.; Hawthorne, F.M. The isolation of the icosahedral B12H12-2 Ion. J. Am. Chem. Soc. 1960, 82, 3228–3229. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An AZ Guide to the Elements; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Das, B.C.; Thapa, P.; Karki, R.; Schinke, C.; Das, S.; Kambhampati, S.; Banerjee, S.K.; Van Veldhuizen, P.; Verma, A.; Weiss, L.M. Boron chemicals in diagnosis and therapeutics. Future Med. Chem. 2013, 5, 653–676. [Google Scholar] [CrossRef]
- Leśnikowski, Z.J. Recent developments with boron as a platform for novel drug design. Expert Opin. Drug Discov. 2016, 11, 569–578. [Google Scholar] [CrossRef]
- Lesnikowski, Z.J. Challenges and opportunities for the application of boron clusters in drug design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Spokoyny, A.M. New ligand platforms featuring boron-rich clusters as organomimetic substituents. Pure Appl. Chem. 2013, 85, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, W.; Crawford, B., Jr.; Lipscomb, W.N. The valence structure of the boron hydrides. J. Chem. Phys. 1954, 22, 989–1001. [Google Scholar] [CrossRef]
- Longuet-Higgins, H.C.; Roberts, M.d.V. The electronic structure of an icosahedron of boron atoms. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1955, 230, 110–119. [Google Scholar]
- Hawthorne, M.F.; Pitochelli, A.R. The reactions of bis-acetonitrile decaborane with amines. J. Am. Chem. Soc. 1959, 81, 5519. [Google Scholar] [CrossRef]
- Sivaev, I.; Bregadze, V.; Kuznetsov, N. Derivatives of the closo-dodecaborate anion and their application in medicine. Russ. Chem. Bull. 2002, 51, 1362–1374. [Google Scholar] [CrossRef]
- Goswami, L.N.; Ma, L.; Chakravarty, S.; Cai, Q.; Jalisatgi, S.S.; Hawthorne, M.F. Discrete nanomolecular polyhedral borane scaffold supporting multiple gadolinium (III) complexes as a high performance MRI contrast agent. Inorg. Chem. 2013, 52, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F. New horizons for therapy based on the boron neutron capture reaction. Mol. Med. Today 1998, 4, 174–181. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Valliant, J.F. The bioinorganic and medicinal chemistry of carboranes: From new drug discovery to molecular imaging and therapy. Dalton Trans. 2007, 38, 4240–4251. [Google Scholar] [CrossRef] [PubMed]
- Bregadze, V.; Sivaev, I.; Glazun, S. Polyhedral boron compounds as potential diagnostic and therapeutic antitumor agents. Anti-Cancer Agents Med. Chem. 2006, 6, 75–109. [Google Scholar] [CrossRef]
- Lesnikowski, Z.J. New opportunities in boron chemistry for medical applications. In Boron Sciences. New Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2011; pp. 3–19. [Google Scholar]
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. The medicinal chemistry of carboranes. Coord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Żołnierczyk, J.D.; Lesnikowski, Z.J. Boron Cluster Modifications with Antiviral, Anticancer, and Modulation of Purinergic Receptors’ Activities Based on Nucleoside Structures. In Boron-Based Compounds: Potential and Emerging Applications in Medicine; Wiley: Hoboken, NJ, USA, 2018; p. 20. [Google Scholar]
- Hawthorne, M.F.; Maderna, A. Applications of radiolabeled boron clusters to the diagnosis and treatment of cancer. Chem. Rev. 1999, 99, 3421–3434. [Google Scholar] [CrossRef]
- Qian, E.A.; Wixtrom, A.I.; Axtell, J.C.; Saebi, A.; Jung, D.; Rehak, P.; Han, Y.; Moully, E.H.; Mosallaei, D.; Chow, S. Atomically precise organomimetic cluster nanomolecules assembled via perfluoroaryl-thiol S N Ar chemistry. Nat. Chem. 2017, 9, 333. [Google Scholar] [CrossRef]
- Hawthorne, M.F. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew. Chem. Int. Ed. Engl. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Hey-Hawkins, E.; Teixidor, C.V. Boron-Based Compounds: Potential and Emerging Applications in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yinghuai, Z.; Lin, X.; Xie, H.; Li, J.; Hosmane, N.S.; Zhang, Y. The Current Status and Perspectives of Delivery Strategy for Boronbased Drugs. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hosmane, N.S. Ionic liquids: Recent advances and applications in boron chemistry. Eur. J. Inorg. Chem. 2017, 2017, 4369–4377. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, S.; Hosmane, N.S. Boron-enriched advanced energy materials. Inorg. Chim. Acta 2018, 471, 577–586. [Google Scholar] [CrossRef]
- Zhu, Y.; Hosmane, N.S. Nanostructured boron compounds for cancer therapy. Pure Appl. Chem. 2018, 90, 653–663. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. 2018, 38, 36. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef]
- Körbe, S.; Schreiber, P.J.; Michl, J. Chemistry of the Carba-closo-dodecaborate (−) Anion, CB11H12. Chem. Rev. 2006, 106, 5208–5249. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.R.; Ferrer-Ugalde, A.; de Biani, F.F.; Teixidor, F. Electrochemistry and photoluminescence of icosahedral carboranes, boranes, metallacarboranes, and their derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Núñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Icosahedral boron clusters: A perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef]
- Olid, D.; Nunez, R.; Vinas, C.; Teixidor, F. Methods to produce B–C, B–P, B–N and B–S bonds in boron clusters. Chem. Soc. Rev. 2013, 42, 3318–3336. [Google Scholar] [CrossRef] [PubMed]
- Hosmane, N.S. Boron Science: New Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Grimes, R.N. Carboranes; Academic Press: London, UK, 2016. [Google Scholar]
- Ferrari, P.; Vanbuel, J.; Hansen, K.; Lievens, P.; Janssens, E.; Fielicke, A. Effect of radiative cooling on the size-dependent stability of small boron clusters. Phys. Rev. A 2018, 98, 012501. [Google Scholar] [CrossRef]
- Wade, K. Skeletal Electron Counting in Cluster and Ring Compounds. Nat. Phys. Sci. 1972, 240, 71. [Google Scholar] [CrossRef]
- Olah, G.A. 100 Years of Carbocations and Their Significance in Chemistry1. J. Org. Chem. 2001, 66, 5943–5957. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Wade, K.; Williams, R.E.; Lipscomb, W.N. Electron Deficient Boron and Carbon Clusters; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Welch, A.J. The significance and impact of Wade’s rules. Chem. Commun. 2013, 49, 3615–3616. [Google Scholar] [CrossRef] [PubMed]
- Heying, T.; Ager, J., Jr.; Clark, S.; Mangold, D.; Goldstein, H.; Hillman, M.; Polak, R.; Szymanski, J. A new series of organoboranes. I. Carboranes from the reaction of decaborane with acetylenic compounds. Inorg. Chem. 1963, 2, 1089–1092. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. π Aromaticity and Three—Dimensional Aromaticity: Two sides of the Same Coin? Angew. Chem. Int. Ed. 2014, 53, 12191–12195. [Google Scholar] [CrossRef]
- Lo, R.; Fanfrlík, J.; Lepšík, M.; Hobza, P. The properties of substituted 3D-aromatic neutral carboranes: The potential for σ-hole bonding. Phys. Chem. Chem. Phys. 2015, 17, 20814–20821. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shi, Y.; Du, P.; Zhang, Z.; Liu, T.; Zhang, R.; Liu, Z. On-Demand Biodegradable Boron Nitride Nanoparticles for Treating Triple Negative Breast Cancer with Boron Neutron Capture Therapy. ACS Nano 2019, 13, 13843–13852. [Google Scholar] [CrossRef]
- Goszczyński, T.M.; Kowalski, K.; Leśnikowski, Z.J.; Boratyński, J. Solid state, thermal synthesis of site-specific protein–boron cluster conjugates and their physicochemical and biochemical properties. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 411–418. [Google Scholar] [CrossRef]
- Assaf, K.I.; Wilińska, J.; Gabel, D. Ionic Boron Clusters as Superchaotropic Anions: Implications for Drug Design. In Boron-Based Compounds: Potential and Emerging Applications in Medicine; Wiley: Hoboken, NJ, USA, 2018; p. 109. [Google Scholar]
- Ban, H.S.; Shimizu, K.; Minegishi, H.; Nakamura, H. Identification of HSP60 as a Primary Target of o-Carboranylphenoxyacetanilide, an HIF-1α Inhibitor. J. Am. Chem. Soc. 2010, 132, 11870–11871. [Google Scholar] [CrossRef] [PubMed]
- Schaffran, T.; Li, J.; Karlsson, G.; Edwards, K.; Winterhalter, M.; Gabel, D. Interaction of N, N, N-trialkylammonioundecahydro-closo-dodecaborates with dipalmitoyl phosphatidylcholine liposomes. Chem. Phys. Lipids 2010, 163, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Schaffran, T.; Justus, E.; Elfert, M.; Chen, T.; Gabel, D. Toxicity of N, N, N-trialkylammoniododecaborates as new anions of ionic liquids in cellular, liposomal and enzymatic test systems. Green Chem. 2009, 11, 1458–1464. [Google Scholar] [CrossRef]
- Genady, A.R.; Ioppolo, J.A.; Azaam, M.M.; Mohamed, E. New functionalized mercaptoundecahydrododecaborate derivatives for potential application in boron neutron capture therapy: Synthesis, characterization and dynamic visualization in cells. Eur. J. Med. Chem. 2015, 93, 574–583. [Google Scholar] [CrossRef]
- Tiwari, R.; Mahasenan, K.; Pavlovicz, R.; Li, C.; Tjarks, W. Carborane clusters in computational drug design: A comparative docking evaluation using AutoDock, FlexX, Glide, and Surflex. J. Chem. Inf. Model. 2009, 49, 1581–1589. [Google Scholar] [CrossRef]
- Calvaresi, M.; Zerbetto, F. In silico carborane docking to proteins and potential drug targets. J. Chem. Inf. Model. 2011, 51, 1882–1896. [Google Scholar] [CrossRef]
- Scholz, M.; Blobaum, A.L.; Marnett, L.J.; Hey-Hawkins, E. Synthesis and evaluation of carbaborane derivatives of indomethacin as cyclooxygenase inhibitors. Bioorg. Med. Chem. 2011, 19, 3242–3248. [Google Scholar] [CrossRef]
- Lee Jr, M.W.; Sevryugina, Y.V.; Khan, A.; Ye, S.Q. Carboranes increase the potency of small molecule inhibitors of nicotinamide phosphoribosyltranferase. J. Med. Chem. 2012, 55, 7290–7294. [Google Scholar]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-dodecaborate anion [B12H12]2−: A review. Collect. Czechoslov. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.V. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. [Google Scholar] [CrossRef]
- Kaim, W.; Hosmane, N.S.; Záliš, S.; Maguire, J.A.; Lipscomb, W.N. Boron Atoms as Spin Carriers in Two-and Three-Dimensional Systems. Angew. Chem. Int. Ed. 2009, 48, 5082–5091. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, J.; Kamitani, N.; Tanaka, R.; Yoden, E.; Tokiya, R.; Suzuki, M.; Barth, R.F.; Ono, K. Boron neutron capture therapy for vulvar melanoma and genital extramammary Paget’s disease with curative responses. Cancer Commun. 2018, 38, 38. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kim, B.K.; Mackeyev, Y.; Rohani, P.; Mahajan, S.D.; Swihart, M.T.; Krishnan, S.; Prasad, P.N. Boron-Nanoparticle-Loaded Folic-Acid-Functionalized Liposomes to Achieve Optimum Boron Concentration for Boron Neutron Capture Therapy of Cancer. J. Biomed. Nanotechnol. 2019, 15, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Sköld, K.; Stenstam, H.B.; Diaz, A.; Giusti, V.; Pellettieri, L.; Hopewell, J. Boron Neutron Capture Therapy for glioblastoma multiforme: Advantage of prolonged infusion of BPA-f. Acta Neurol. Scand. 2010, 122, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Gorlia, T.; Pellettieri, L.; Giusti, V.; Stenstam, H.B.; Sköld, K. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: An assessment of clinical potential. Appl. Radiat. Isot. 2011, 69, 1737–1740. [Google Scholar] [CrossRef]
- Grimes, R. Carboranes in medicine. In Carboranes, 3rd ed.; Grimes, R.N., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 945–984. [Google Scholar]
- Luderer, M.J. Development of Novel Tumor-Targeted Compounds for Boron Neutron Capture Therapy. Ph.D. Thesis, Washington University in St. Louis, St. Louis, MO, USA, 2019. Available online: https://openscholarship.wustl.edu/art_sci_etds/1782/ (accessed on 20 January 2020).
- Tani, H.; Kurihara, H.; Hiroi, K.; Honda, N.; Yoshimoto, M.; Kono, Y.; Murakami, R.; Kumita, S.; Arai, Y.; Itami, J. Correlation of 18F-BPA and 18F-FDG uptake in head and neck cancers. Radiother. Oncol. 2014, 113, 193–197. [Google Scholar] [CrossRef]
- Ishiwata, K. 4-Borono-2-18 F-fluoro-l-phenylalanine PET for boron neutron capture therapy-oriented diagnosis: Overview of a quarter century of research. Ann. Nucl. Med. 2019, 33, 223–236. [Google Scholar] [CrossRef]
- Evangelista, L.; Jori, G.; Martini, D.; Sotti, G. Boron neutron capture therapy and 18F-labelled borophenylalanine positron emission tomography: A critical and clinical overview of theliterature. Appl. Radiat. Isot. 2013, 74, 91–101. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Hosmane, N. Nanostructured Boron Compounds for Boron Neutron Capture Therapy (BNCT) in Cancer Treatment. In Boron-Based Compounds: Potential and Emerging Applications in Medicine, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 371–388. [Google Scholar]
- Shikata, F.; Tokumitsu, H.; Ichikawa, H.; Fukumori, Y. In vitro cellular accumulation of gadolinium incorporated into chitosan nanoparticles designed for neutron-capture therapy of cancer. Eur. J. Pharm. Biopharm. 2002, 53, 57–63. [Google Scholar] [CrossRef]
- Soenen, S.J.; Montenegro, J.-M.; Abdelmonem, A.M.; Manshian, B.B.; Doak, S.H.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. The effect of nanoparticle degradation on poly (methacrylic acid)-coated quantum dot toxicity: The importance of particle functionality assessment in toxicology. Acta Biomater. 2014, 10, 732–741. [Google Scholar] [CrossRef]
- Gu, L.; Fang, R.H.; Sailor, M.J.; Park, J.-H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano 2012, 6, 4947–4954. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Justin, R.; Chen, B. Characterisation and drug release performance of biodegradable chitosan–graphene oxide nanocomposites. Carbohydr. Polym. 2014, 103, 70–80. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, Y.; Liu, Z. Intelligent Albumin–MnO2 Nanoparticles as pH-/H2O2-Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; André, A.A.; Men, Y.; Srinivas, M.; Wilson, D.A. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 2017, 11, 1957–1963. [Google Scholar] [CrossRef]
- Clapper, J.D.; Guymon, C.A. Nanostructured biodegradable polymer composites generated using lyotropic liquid crystalline media. Macromolecules 2007, 40, 7951–7959. [Google Scholar] [CrossRef]
- Song, J.; Pu, L.; Zhou, J.; Duan, B.; Duan, H. Biodegradable theranostic plasmonic vesicles of amphiphilic gold nanorods. ACS Nano 2013, 7, 9947–9960. [Google Scholar] [CrossRef]
- Steinbach, T.; Wurm, F.R. Poly (phosphoester) s: A new platform for degradable polymers. Angew. Chem. Int. Ed. 2015, 54, 6098–6108. [Google Scholar] [CrossRef]
- Di Meo, C.; Panza, L.; Capitani, D.; Mannina, L.; Banzato, A.; Rondina, M.; Renier, D.; Rosato, A.; Crescenzi, V. Hyaluronan as carrier of carboranes for tumor targeting in boron neutron capture therapy. Biomacromolecules 2007, 8, 552–559. [Google Scholar] [CrossRef]
- Sumitani, S.; Nagasaki, Y. Boron neutron capture therapy assisted by boron-conjugated nanoparticles. Polym. J. 2012, 44, 522. [Google Scholar] [CrossRef]
- Takeuchi, I.; Nomura, K.; Makino, K. Hydrophobic boron compound-loaded poly (l-lactide-co-glycolide) nanoparticles for boron neutron capture therapy. Colloids Surf. B Biointerfaces 2017, 159, 360–365. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal–Organic Framework—Based Stimuli—Responsive Systems for Drug Delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-responsive therapeutic metallodrugs. Chem. Rev. 2018, 119, 1138–1192. [Google Scholar] [CrossRef]
- Blum, A.P.; Kammeyer, J.K.; Rush, A.M.; Callmann, C.E.; Hahn, M.E.; Gianneschi, N.C. Stimuli-responsive nanomaterials for biomedical applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. [Google Scholar] [CrossRef]
- Yuan, T.Z.; Xie, S.Q.; Qian, C.N. Boron neutron capture therapy of cancer: Critical issues and future prospects. Thorac. Cancer 2019, 10, 2195. [Google Scholar] [CrossRef]
- Si, P.; Zhang, M.; You, C.; Geng, D.; Du, J.; Zhao, X.; Ma, X.; Zhang, Z. Amorphous boron nanoparticles and BN encapsulating boron nano-peanuts prepared by arc-decomposing diborane and nitriding. J. Mater. Sci. 2003, 38, 689–692. [Google Scholar] [CrossRef]
- Bellott, B.J.; Noh, W.; Nuzzo, R.G.; Girolami, G.S. Nanoenergetic materials: Boron nanoparticles from the pyrolysis of decaborane and their functionalisation. Chem. Commun. 2009, 3214–3215. [Google Scholar] [CrossRef]
- He, X.; Joo, S.; Xiao, H.; Liang, H. Boron-based nanoparticles for chemical-mechanical polishing of copper films. ECS J. Solid State Sci. Technol. 2013, 2, P20–P25. [Google Scholar] [CrossRef]
- Icten, O.; Hosmane, N.S.; Kose, D.A.; Zumreoglu-Karan, B. Production of Magnetic Nano-bioconjugates via Ball Milling of Commercial Boron Powder with Biomolecules. Z. Anorg. Allg. Chem. 2016, 642, 828–832. [Google Scholar] [CrossRef]
- Icten, O.; Hosmane, N.S.; Kose, D.A.; Zumreoglu-Karan, B. Magnetic nanocomposites of boron and vitamin C. New J. Chem. 2017, 41, 3646–3652. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, P.; Singh, K.; Gaharwar, U.S.; Meena, R.; Kumar, M.; Nakagawa, F.; Wu, S.; Suzuki, M.; Nakamura, H. Boron nitride (10BN) a prospective material for treatment of cancer by boron neutron capture therapy (BNCT). Mater. Lett. 2020, 259, 126832. [Google Scholar] [CrossRef]
- Nakamura, H.; Koganei, H.; Miyoshi, T.; Sakurai, Y.; Ono, K.; Suzuki, M. Antitumor effect of boron nitride nanotubes in combination with thermal neutron irradiation on BNCT. Bioorg. Med. Chem. Lett. 2015, 25, 172–174. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Xie, R.; Sekiguchi, T.; Golberg, D. Perfectly dissolved boron nitride nanotubes due to polymer wrapping. J. Am. Chem. Soc. 2005, 127, 15996–15997. [Google Scholar] [CrossRef]
- Wang, W.; Bando, Y.; Zhi, C.; Fu, W.; Wang, E.; Golberg, D. Aqueous noncovalent functionalization and controlled near-surface carbon doping of multiwalled boron nitride nanotubes. J. Am. Chem. Soc. 2008, 130, 8144–8145. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Elliman, R.G.; Petravic, M. Isotopically enriched 10BN nanotubes. Adv. Mater. 2006, 18, 2157–2160. [Google Scholar] [CrossRef]
- Menichetti, L.; De Marchi, D.; Calucci, L.; Ciofani, G.; Menciassi, A.; Forte, C. Boron nitride nanotubes for boron neutron capture therapy as contrast agents in magnetic resonance imaging at 3 T. Appl. Radiat. Isot. 2011, 69, 1725–1727. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Hosmane, N.S. Applications and perspectives of boron-enriched nanocomposites in cancer therapy. Future Med. Chem. 2013, 5, 705–714. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Folate functionalized boron nitride nanotubes and their selective uptake by glioblastoma multiforme cells: Implications for their use as boron carriers in clinical boron neutron capture therapy. Nanoscale Res. Lett. 2009, 4, 113. [Google Scholar] [CrossRef]
- Li, W.; Xie, X.; Wu, T.; Yang, H.; Peng, Y.; Luo, L.; Chen, Y. Targeted delivery of Auristatin PE to Hep G2 cells using folate-conjugated boron nitride nanotubes. Mater. Sci. Eng: C 2019, 109, 110509. [Google Scholar] [CrossRef]
- Ribeiro, H.; Luciano, M.A.; Paula Von Randow, C.; Vilela, D.N.; Andrade, L.M. Functionalized Boron Nitride Applications in Biotechnology. In Recent Advances in Boron-Containing Materials; Intech Open: London, UK, 2019. [Google Scholar]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, J.-J.; Chang, W.-Y.; Hsieh, C.-Y.; Wu, C.-C.; Chen, H.-S.; Hsu, H.-J.; Yang, A.-S.; Hsu, M.-H.; Kuo, W.-Y. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Ye, J.; Li, Z.; Jiang, H.; Yan, H.; Stogniy, M.Y.; Sivaev, I.B.; Bregadze, V.I.; Wang, X. Carborane derivative conjugated with gold nanoclusters for targeted cancer cell imaging. Biomacromolecules 2017, 18, 1466–1472. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Sivaev, I.B.; Godovikov, I.A.; Starikova, Z.A.; Bregadze, V.I.; Qi, S. Synthesis of new ω-amino-and ω-azidoalkyl carboranes. New J. Chem. 2013, 37, 3865–3868. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, C.; Yu, M.; Qin, Y.; Wang, J.; Kim, M.; Zheng, J. Luminescent gold nanoparticles with mixed valence states generated from dissociation of polymeric Au (I) thiolates. J. Phys. Chem. C 2010, 114, 7727–7732. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Gona, K.B.; Gómez-Vallejo, V.; Meijer, J.; Zilberfain, C.; Estrela-Lopis, I.; Baz, Z.; Cossío, U.; Llop, J. Gold Nanoparticles as Boron Carriers for Boron Neutron Capture Therapy: Synthesis, Radiolabelling and In Vivo Evaluation. Molecules 2019, 24, 3609. [Google Scholar] [CrossRef]
- Timonen, J.M. Amino Acids in Boron Neutron Capture Therapy—Prospects for Precise Treatment of Malignant Brain Tumors. Gen. Chem. 2019, 5, 190024. [Google Scholar]
- Li, J.; Shi, Y.; Zhang, Z.; Liu, H.; Lang, L.; Liu, T.; Chen, X.; Liu, Z. A Metabolically stable boron-derived tyrosine serves as a theranostic agent for positron emission tomography guided boron neutron capture therapy. Bioconj. Chem. 2019, 30, 2870–2878. [Google Scholar] [CrossRef]
- Chauhan, N.; Hosmane, N.; Mozafari, M. Boron-based polymers: Opportunities and challenges. Mater. Today Chem. 2019, 14, 100184. [Google Scholar] [CrossRef]
- Ochiai, T.; Tago, S.; Hayashi, M.; Hirota, K.; Kondo, T.; Satomura, K.; Fujishima, A. Boron-doped diamond powder (BDDP)-based polymer composites for dental treatment using flexible pinpoint electrolysis unit. Electrochem. Commun. 2016, 68, 49–53. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Q.; Liu, M.; Lin, M.; Wang, T.; Zhang, Z.; Zhong, X.; Guo, N.; Lu, Y.; Xu, J. Remarkable Boron Delivery Of iRGD-Modified Polymeric Nanoparticles For Boron Neutron Capture Therapy. Int. J. Nanomed. 2019, 14, 8161. [Google Scholar] [CrossRef] [PubMed]
- Kiyanagi, Y.; Sakurai, Y.; Kumada, H.; Tanaka, H. Status of Accelerator-Based BNCT Projects Worldwide; AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; p. 050012. [Google Scholar]
- Hirose, K.; Konno, A.; Yoshimoto, S.; Ono, K.; Otsuki, N.; Hatazawa, J.; Hiratsuka, J.; Takai, Y. A Prospective Phase 2 Trial of Accelerator-Based Boron Neutron Capture Therapy for Recurrent and Locally Advanced Head and Neck Cancer: Initial Report of Treatment Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E374. [Google Scholar] [CrossRef]
- Barth, R.F.; Vicente, M.H.; Harling, O.K.; Kiger, W.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Saarilahti, K.; Mäkitie, A.; Välimäki, P.; Tenhunen, M.; Joensuu, H. Boron neutron capture therapy (BNCT) followed by intensity modulated chemoradiotherapy as primary treatment of large head and neck cancer with intracranial involvement. Radiother. Oncol. 2011, 99, 98–99. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: Final analysis of a phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef]
- Haapaniemi, A.; Kankaanranta, L.; Saat, R.; Koivunoro, H.; Saarilahti, K.; Mäkitie, A.; Atula, T.; Joensuu, H. Boron neutron capture therapy in the treatment of recurrent laryngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 404–410. [Google Scholar] [CrossRef]
- Wang, L.-W.; Liu, Y.-W.H.; Chou, F.-I.; Jiang, S.-H. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing-Hua Open Pool Reactor. Cancer Commun. 2018, 38, 37. [Google Scholar] [CrossRef]
- Yong, Z.; Song, Z.; Zhou, Y.; Liu, T.; Zhang, Z.; Zhao, Y.; Chen, Y.; Jin, C.; Chen, X.; Lu, J. Boron neutron capture therapy for malignant melanoma: First clinical case report in China. Chin. J.Cancer Res. 2016, 28, 634. [Google Scholar] [CrossRef]
- Sorace, A.G.; Korb, M.; Warram, J.M.; Umphrey, H.; Zinn, K.R.; Rosenthal, E.; Hoyt, K. Ultrasound-stimulated drug delivery for treatment of residual disease after incomplete resection of head and neck cancer. Ultrasound Med. Boil. 2014, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Chan, P.-C.; Chou, L.-S.; Chang, C.-W.; Yang, F.-Y.; Liu, R.-S.; Chiou, S.-H.; Chen, Y.-W.; Yen, S.-H.; Wang, H.-E. Pulsed-focused ultrasound enhances boron drug accumulation in a human head and neck cancer xenograft-bearing mouse model. Mol. Imaging Biol. 2014, 16, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, G.; Poon, I.; Karam, I.; Higgins, K.; Pichardo, S.; Hynynen, K.; Enepekides, D. Magnetic resonance-guided high-intensity focused ultrasound combined with radiotherapy for palliation of head and neck cancer—A pilot study. J. Ther. Ultrasound 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Chuang, K.-S.; Liu, Y.-W.H.; Lin, T.-Y.; Teng, Y.-C.; Wang, L.-W. A comparison of dose distributions in gross tumor volume between boron neutron capture therapy alone and combined boron neutron capture therapy plus intensity modulation radiation therapy for head and neck cancer. PLoS ONE 2019, 14, e0210626. [Google Scholar] [CrossRef] [PubMed]
- Koivunoro, H.; Kankaanranta, L.; Seppälä, T.; Haapaniemi, A.; Mäkitie, A.; Joensuu, H. Boron neutron capture therapy for locally recurrent head and neck squamous cell carcinoma: An analysis of dose response and survival. Radiother. Oncol. 2019, 137, 153–158. [Google Scholar] [CrossRef]
- Sun, Y. Boron neutron capture therapy: Moving towards targeted therapy for locally recurrent head and neck squamous cell carcinoma. Mil. Med. Res. 2019, 6, 32. [Google Scholar] [CrossRef]
- Zhang, Z.; Chong, Y.; Chen, X.; Jin, C.; Yang, L.; Liu, T. PGNAA system preliminary design and measurement of In-Hospital Neutron Irradiator for boron concentration measurement. Appl. Radiat. Isot. 2015, 106, 161–165. [Google Scholar] [CrossRef]
- Achkar, T.; Tarhini, A.A. The use of immunotherapy in the treatment of melanoma. J. Hematol. Oncol. 2017, 10, 88. [Google Scholar] [CrossRef]
- Yu, Z.; Si, L. Immunotherapy of patients with metastatic melanoma. Chin. Clin. Oncol. 2017, 6, 20. [Google Scholar] [CrossRef]
- Alvarado-Luna, G.; Morales-Espinosa, D. Treatment for small cell lung cancer, where are we now?—A review. Transl. Lung Cancer Res. 2016, 5, 26. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Geng, C.; Tang, X.; Bortolussi, S.; Han, Y.; Shu, D.; Gong, C.; Zhang, X.; Tian, F. Dosimetric impact of respiratory motion during boron neutron capture therapy for lung cancer. Radiat. Phys. Chem. 2020, 168, 108527. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakurai, Y.; Masunaga, S.; Kinashi, Y.; Nagata, K.; Maruhashi, A.; Ono, K. Feasibility of boron neutron capture therapy (BNCT) for malignant pleural mesothelioma from a viewpoint of dose distribution analysis. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1584–1589. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, O.; Sakurai, Y.; Tanaka, H.; Kondo, N.; Kinashi, Y.; Masunaga, S.-i.; Maruhashi, A.; Ono, K. In Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT). Int. Cancer Conf. J. 2012, 1, 235–238. [Google Scholar] [CrossRef]
- Farías, R.O.; Bortolussi, S.; Menéndez, P.R.; González, S.J. Exploring boron neutron capture therapy for non-small cell lung cancer. Phys. Med. 2014, 30, 888–897. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, C.; Tang, X. Assessment of Secondary Cancer Risk for Patients Treated with BNCT. Available online: https://ybnct10.org/wp-content/uploads/2019/09/26.pdf (accessed on 20 January 2020).
- Geng, C.; Tang, X.; Hou, X.; Shu, D.; Chen, D. Development of Chinese hybrid radiation adult phantoms and their application to external dosimetry. Sci. China Technol. Sci. 2014, 57, 713–719. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]






| Materials | In Cell/In Tumor Model | Observation | Ref. |
|---|---|---|---|
| 10BN | normal (HEK-293) and cancer (HeLa, MCF-7) cells | The promising antitumor effect was observed in HeLa cells. The thermal neutron fluence of ~6.3 × 1012/cm2 resulted in almost 50% cell killing of BN treated HeLa cells | [93] |
| BNNTs | B16-melanoma cell | The accumulation of BNNTs in B16-melanoma cell was three times higher than BSH and their antitumor effect with BNCT was also observed higher than BSH. | [94] |
| BNNTs-FA@PE | Hep G2 cells | Exhibited stronger cytotoxicity than free PE and BNNTs@PE complexes. Also, excellent antiproliferative activities in time- and dose-dependent manners. Additionally, it induced apoptosis of Hep G2 cells by reducing the mitochondrial membrane potential, activating Caspase-9 and Caspase-3. | [102] |
| PTL coated BNNPs | Triple negative breast cancer in mice | The coated BNNPs showed high boron accumulation in the tumor while maintaining its good ratio between tumor and nontumor cells. Suppression of tumor growth was observed with almost negligible side effects. Even these BNNPs shows high accumulation of B atoms inside the cells but they have failure in the therapeutic window for BNCT. | [46] |
| B-AuNP, 61-B-AuNP, 123I-B-AuNP, and 123I-61-B-AuNP | HER2 | The The micro CT/SPECT imaging show the T/M ratio, assessed by ICP-MS, of mice injected by B-AuNP, 61-B-AuNP, 123I-B-AuNP, and 123I-61-B-AuNP was 4.91 ± 2.75, 41.05 ± 11.15, 1.91 ± 0.17, and 12.02 ± 0.94 respectively, at 12 h post-injection. The results show the successfully developed detectable HER2-targeting boron-containing AuNPs with high RCP and an acceptable yield. | [105] |
| (NH2NH3)+[7-NH2(CH2)3S- 7,8-C2B9H11]- in Fluorescent GNCs dispersed in PBS | Cancer tumor in mice model | The accurate tumor imaging by EPR effect and long-term accumulation in tumor cell by nanometer size effect have been observed. This strategy is good for attaining the accurate position of tumor cells with CB and thus decreases the chances of normal tissue damage. In addition, it facilitates the real-time fluorescent visualization to monitor delivery process of the carborane to the targeted tumor, thus endorsing the effect of BNCT treatment through imaging guided therapy. | [106] |
| Gold NPs with PEG, functionalized with bis(dicarbollide), radiolabeled with 124I | in vivo with a mouse model | The results showed a poor accumulation in tumor and major accumulation in liver, lungs and spleen suggesting that the tuning the size and geometry of the gold core is essential | [109] |
| [18F]FBY PET | B16-F10 tumor | showed up to 6 % ID/g in B16-F10 tumor and notably low normal tissue uptake (tumor/muscle = 3.16 ± 0.48; tumor/blood = 3.13 ± 0.50; tumor/brain = 14.25 ± 1.54). Moreover, the administration of [18F]FBY tracer along with a therapeutic dose of FBY showed high accumulation in B16-F10 tumor and low normal tissue uptake. | [111] |
| iRGD-Modified Polymeric Nanoparticles | A549 tumor-bearing mice | Boron concentration ratios for tumor:normal tissue (tumor:muscle = 19.49, tumor:blood = 14.11) in A549 tumor-bearing mice was observed after 24 h of injection. The highest tumor accumulation of DOX was confirmed from both quantitative measurement and fluorescence imaging at 24 hrs after injecting iRGD-modified polymers | [114] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.; S Hosmane, N.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. https://doi.org/10.3390/molecules25040828
Ali F, S Hosmane N, Zhu Y. Boron Chemistry for Medical Applications. Molecules. 2020; 25(4):828. https://doi.org/10.3390/molecules25040828
Chicago/Turabian StyleAli, Fayaz, Narayan S Hosmane, and Yinghuai Zhu. 2020. "Boron Chemistry for Medical Applications" Molecules 25, no. 4: 828. https://doi.org/10.3390/molecules25040828
APA StyleAli, F., S Hosmane, N., & Zhu, Y. (2020). Boron Chemistry for Medical Applications. Molecules, 25(4), 828. https://doi.org/10.3390/molecules25040828







