Structural Diversity of Nickel and Manganese Chloride Complexes with Pyridin-2-One
Abstract
:1. Introduction
2. Results and Discussion
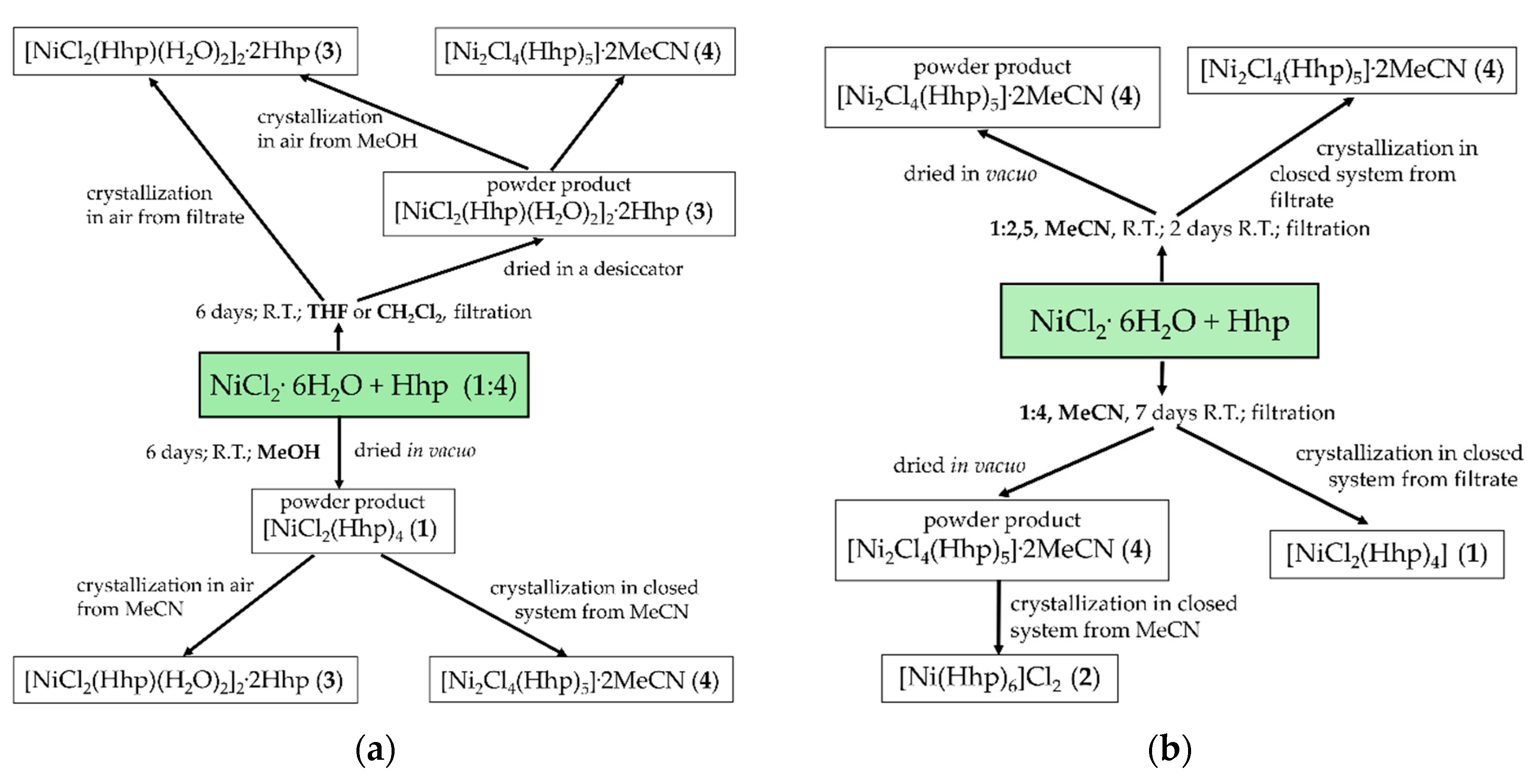
2.1. Synthetic Aspects
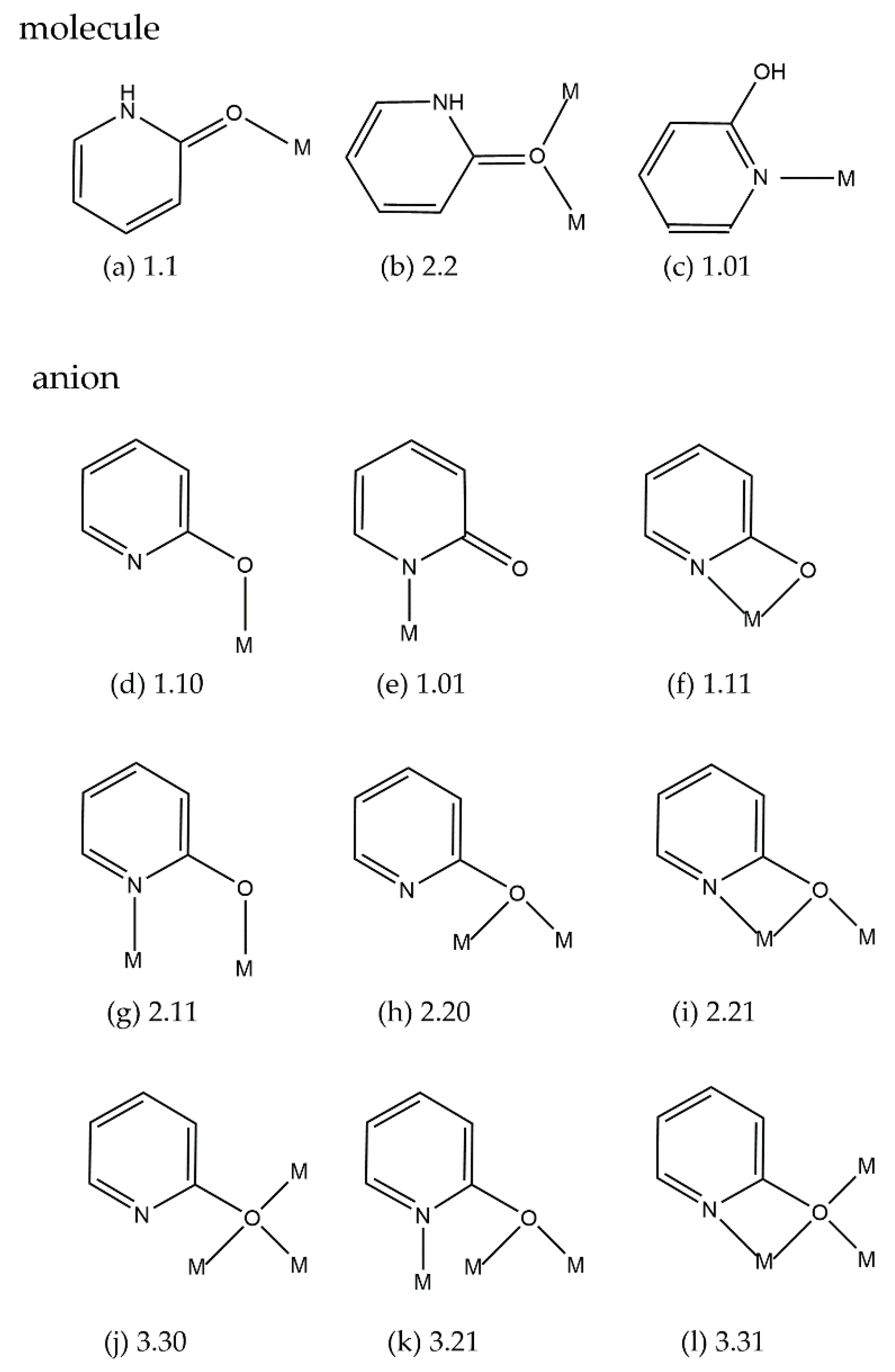
2.2. IR Spectra
2.3. Description of the Structures
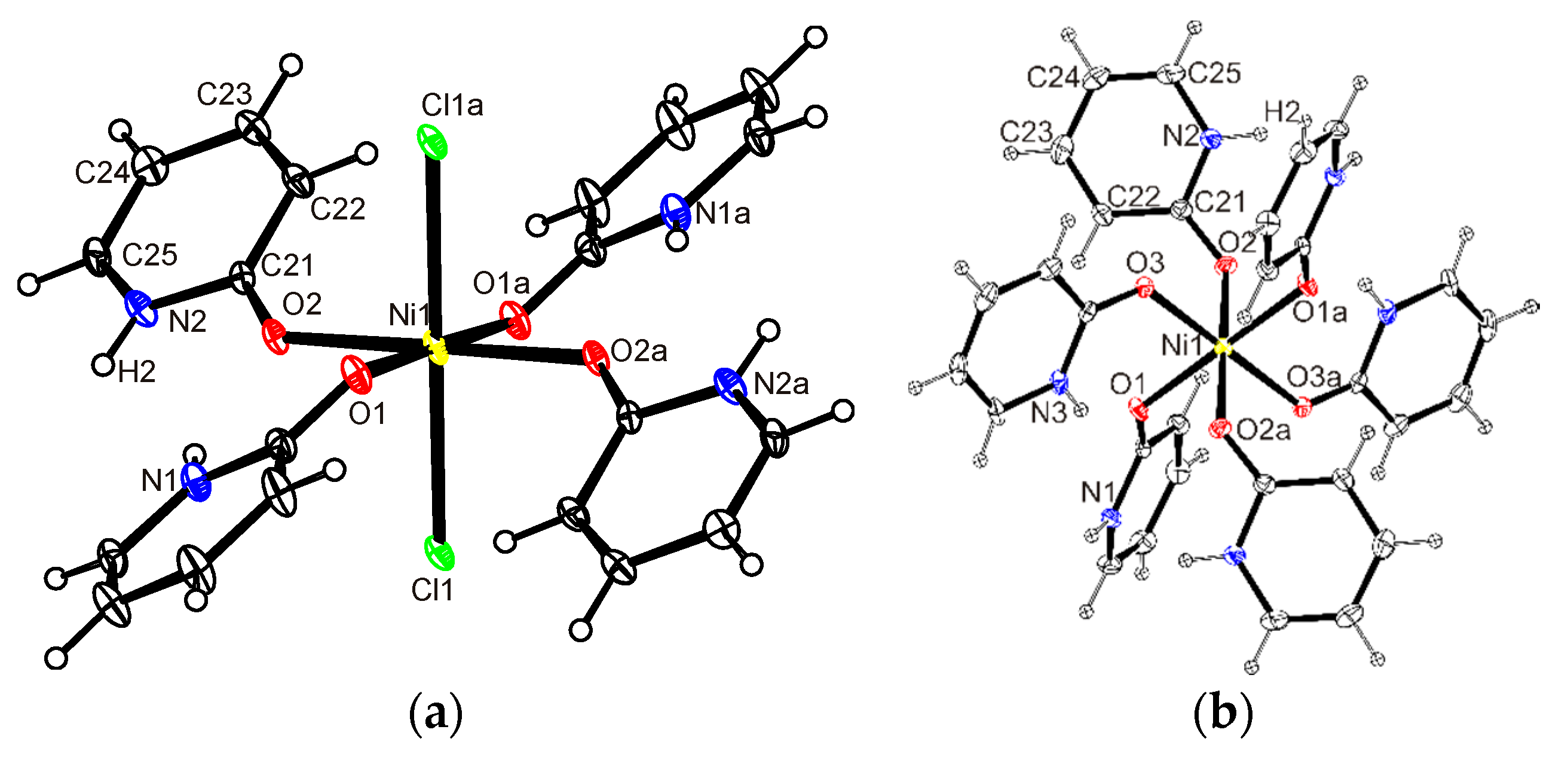
2.3.1. Crystal Structure of 1, [NiCl2(Hhp)4], 2, [Ni(Hhp)6]Cl2 and 5, [MnCl2(Hhp)4]
2.3.2. Crystal Structure of 3, [NiCl2(Hhp)(H2O)2]2 2Hhp
2.3.3. Crystal Structure of 4, [Ni2Cl4(Hhp)5]·2MeCN
2.4. Thermal Analysis of 1 and 3
3. Materials and Methods
3.1. General
3.2. Preparation Procedures
3.2.1. Synthesis of [NiCl2(Hhp)4], 1
3.2.2. Synthesis of [Ni(Hhp)6]Cl2, 2
3.2.3. Synthesis of [NiCl2(Hhp)(H2O)2]2·2(Hhp), 3
3.2.4. Synthesis of [Ni2Cl4(Hhp)5]·2MeCN, 4
3.2.5. Synthesis of [MnCl2(Hhp)4], 5
3.3. X-ray Structure Determinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coxall, R.A.; Harris, S.G.; Henderson, D.K.; Parsons, S.; Tasker, P.A.; Winpenny, R.E.P. Inter-ligand reactions: In situ formation of new polydentate ligands. J. Chem. Soc. Dalton Trans. 2000, 2349–2356. [Google Scholar] [CrossRef]
- Cotton, F.A.; Fanwick, P.E.; Niswander, R.H.; Sekutowski, J.C. A Triad of Homologous, Air-Stable Compounds Containing Short, Quadruple Bonds between Metal Atoms of Group 6. Am. Chem. Soc. 1978, 100, 4725–4732. [Google Scholar] [CrossRef]
- Cotton, F.A.; Ilsley, W.H.; Kaim, W. Homologous Chromium, Molybdenum, and Tungsten Derivatives of 6-Chloro-2-hydroxypyridine. Inductive Effects on the Metal-Metal Bond Length. Inorg. Chem. 1980, 19, 1453–1457. [Google Scholar] [CrossRef]
- Cotton, F.A.; Falvello, L.R.; Han, S.; Wang, W. Preparation, Structures, and Spectra of Tetrakis(6-fluoro-2-oxypyridine) dichromium, -dimolybdenum, and -ditungsten: A Series of Polar Quadruple Bonds. Inorg. Chem. 1983, 22, 4106–4112. [Google Scholar] [CrossRef]
- Berry, M.; Garner, C.D.; Hillier, I.H.; MacDowell, A.A.; Clegg, W. Crystal structure and u.v. photoelectron spectra of tetrakis-(6-methyl-2-oxopyridinato)dirhodium. Chem. Soc. Chem. Commun. 1980, 494–495. [Google Scholar] [CrossRef]
- Berry, M.; Garner, C.D.; Hillier, I.H.; MacDowell, A.A.; Clegg, W. Diruthenium(II) tetrakis-6-methyl-2-oxypyridine, [Ru2(mph)4], and comparisons of the metal–metal bonding in [M2(mhp)4] (M = Mo, Ru, or Rh) complexes. Inorg. Chim. Acta 1981, 53, 61–63. [Google Scholar] [CrossRef]
- Kozlevčar, B.; Radišek, M.; Jagličič, Z.; Merzel, F.; Glažar, L.; Golobič, A.; Šegedin, P. Strong antiferromagnetism in the dinuclear 2-pyridone complex with N–C–O bridges: A paddle-wheel analogue of the dinuclear tetracarboxylates. Polyhedron 2007, 26, 5414–5419. [Google Scholar] [CrossRef]
- Cotton, F.A.; Hanson, B.E. A Well-Characterized Compound Containing a Heteronuclear (Molybdenum-Tungsten) Quadruple Bond. Inorg. Chem. 1978, 17, 3237–3240. [Google Scholar] [CrossRef]
- Blake, A.J.; Milne, P.E.Y.; Thornton, P.; Winpenny, R.E.P. Heterometallic Compounds Involving d- and f-Block Elements: Synthesis, Structure, and Magnetic Properties of Two New LnxCu4 Complexes. Angew. Chem. Int. Ed. 1991, 30, 1139–1141. [Google Scholar] [CrossRef]
- Wang, S. Heterometallic Yttrium-Copper Complexes. Synthesis and Crystal Structure of Y2Cu8(µ-PyO)12(µ-Cl)2(µ4-O)2(NO3)4(H2O)2.2H2O (PyO− = Deprotonated 2-Hydroxypyridine). Inorg. Chem. 1991, 30, 2252–2253. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Williams, D.J.; Winpenny, R.E.P. Bimetallic hexanuclear compounds containing unusual copper-lanthanide arrays: the crystal structures of Cu4Ln2L8(LH)4(OH)2(NO3)4(H2O)2 (Ln = Dy or Gd; LH = 2-(1H)-Pyridone, C5H5NO). Polyhedron 1989, 8, 1531–1536. [Google Scholar] [CrossRef]
- Wang, S.; Pang, Z.; Wagner, M.J. Comparative Study of Crystal Structures and Thermal and Magnetic Properties of a Y2Cu8 and a Nd2Cu8 Complex. Inorg. Chem. 1992, 31, 5381–5388. [Google Scholar] [CrossRef]
- Borta, A.; Jeanneau, E.; Chumakov, Y.; Luneau, D.; Ungur, L.; Chibotaru, L.F.; Wernsdorfer, W. Synthesis, structure, magnetism and theoretical study of a series of complexes with a decanuclear core [Ln(III)2Cu(II)8] (Ln = Y, Gd, Tb, Dy). New J. Chem. 2011, 35, 1270–1279. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhang, D.K.; Hao, X.; Zhu, D.B. Trinuclear [CoIII2–LnIII] (Ln=Tb, Dy) Single-Ion Magnets with Mixed 6-Chloro-2-Hydroxypyridine and Schiff Base Ligands. Chem. Asian J. 2014, 9, 1847–1853. [Google Scholar] [CrossRef]
- Rawson, J.M.; Winpenny, R.E.P. The coordination chemistry of 2-pyridone and its Derivatives. Coord. Chem. Rev. 1995, 139, 313–374. [Google Scholar] [CrossRef]
- Blake, A.J.; Grant, C.M.; Parsons, S.; Rawson, J.M.; Solan, G.A.; Winpenny, R.E.P. Syntheses, Structures and Magnetic Studies of Homometallic Manganese and Heterometallic Iron-Sodium One-dimensional Polymers. J. Chem. Soc. Dalton Trans. 1995, 2311–2314. [Google Scholar] [CrossRef]
- Langley, S.K.; Chilton, N.F.; Moubaraki, B.; Murray, K.S. Unusual oxidation state distributions observed for two mixed-valence heptanuclear manganese disc-like clusters. Dalton Trans. 2012, 41, 9789–9796. [Google Scholar] [CrossRef]
- Brechin, E.K.; Christou, G.; Soler, M.; Helliwell, M.; Teat, S.J. Novel octanuclear and enneanuclear manganese clusters with carboxylate and pyrimidine ligands. Dalton Trans. 2003, 513–514. [Google Scholar] [CrossRef]
- Langley, S.K.; Moubaraki, B.; Berry, K.J.; Murray, K.S. Supertetrahedral icosanuclear and ring-like decanuclear mixed valent manganese(II/III) triethanolamine clusters. Dalton Trans. 2010, 39, 4848–4855. [Google Scholar] [CrossRef]
- Langley, S.K.; Helliwell, M.; Teat, S.J.; Winpenny, R.E.P. Synthesis and Characterization of Nickel(II) Phosphonate Complexes Utilizing Pyridonates and Carboxylates as Co-ligands. Inorg. Chem. 2014, 53, 1128–1134. [Google Scholar] [CrossRef]
- Brechin, E.K.; Graham, A.; Harris, S.G.; Parsons, S.; Winpenny, R.E.P. Overcrowding leads to prism reform: New polyhedra for polymetallic cages. J. Chem. Soc. Dalton Trans. 1997, 3405–3406. [Google Scholar] [CrossRef]
- Blake, A.J.; Grant, C.M.; Parsons, S.; Rawson, J.M.; Winpenny, R.E.P. The Synthesis, Structure and Magnetic Properties of a Cyclic Dodecanuclear Nickel Complex. Chem. Commun. 1994, 2363–2364. [Google Scholar] [CrossRef]
- Parsons, S.; Buchanan, C.; Winpenny, R.; Wood, P.A. (Refcode HAJFUU. The Cambridge Structural Database Communication). Private communication, 2004. [Google Scholar]
- Brechin, E.K.; Harris, S.G.; Parsons, S.; Winpenny, R.E.P. Clusters from Vertex- and Face-Sharing Adamantane-Like Units: A New Topology for Multinuclear Complexes. Angew. Chem. Int. Ed. 1997, 36, 1967–1969. [Google Scholar] [CrossRef]
- Brechin, E.K.; Clegg, W.; Murrie, M.; Parsons, S.; Teat, S.J.; Winpenny, R.E.P. Nanoscale Cages of Manganese and Nickel with “Rock Salt” Cores. J. Am. Chem. Soc. 1998, 120, 7365–7366. [Google Scholar] [CrossRef]
- Brechin, E.K.; Parsons, S.; Winpenny, R.E.P. Uncapped and polar capped prisms of cobalt and nickel. J. Chem. Soc. Dalton Trans. 1996, 3745–3746. [Google Scholar] [CrossRef]
- Graham, A.; Meier, S.; Parsons, S.; Winpenny, R.E.P. Changing cage structures through inter-ligand repulsions. Chem. Commun. 2000, 10, 811–812. [Google Scholar] [CrossRef]
- Santana, M.D.; Garcia, G.; Rufete, A.; Sinchez, G.; Ramkez de Arellano, M.C.; Lopez, G. Preparation, NOESY characterisation and structure of [Ni(N3-macrocycle)(hp)]ClO4: The first crystallographic characterised mononuclear nickel complex containing the 2-pyridonate (hp) ligand. Inorg. Chem. Commun. 1998, 1, 267–269. [Google Scholar] [CrossRef]
- Reedijk, J. The Ligand Properties of Hydroxypyridines. Part I. Hexakis(2-Pyridone) Metal Perchlorates and Tetrafluoroborates. Recl. Trav. Chim. 1969, 88, 1139–1155. [Google Scholar] [CrossRef]
- Reedijk, J.; Smit, J.A. The Ligand Properties of Hydroxypyridines. Part II. Metal(II) Nitrates Containing Coordinated 2-Pyridone. Recl. Trav. Chim. 1972, 91, 681–687. [Google Scholar] [CrossRef]
- Houk, C.C.; Emerson, K. Complexes of 2-Pyridone with MnCl2, CoCl2, NiCl2 and CuCl2. J. Inorg. Nucl. Chem. 1968, 30, 1493–1502. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Williams, D.J.; Winpenny, R.E.P. Formation of some 2-pyridone Hg/M [M=Mn(II), Fe(III) and Cu(II)] heterometallic complexes and crystal structure of hexakis(2-pyridone)iron(III) nitrate. Inorg. Chim. Acta 1989, 166, 159–162. [Google Scholar] [CrossRef]
- Dojer, B.; Pevec, A.; Jagličič, Z.; Kristl, M. Cobalt(II) complexes with hydroxypyridines and halogenides. J. Mol. Struct. 2017, 1128, 724–729. [Google Scholar] [CrossRef]
- Taylor, D. The crystal structure of Hexakis(2-pyridone)copper(II) perchlorate. Aust. J. Chem. 1975, 28, 2615–2622. [Google Scholar] [CrossRef]
- Tong, M.L.; Lin, Z.J.; Li, W.; Zheng, S.L.; Chen, X.M. Metal Cation-Supported Supramolecular Crown Ethers Featuring Hydrogen-Bonded Tetrameric Unit of 2-Hydroxy Pyridines. Cryst. Growth Des. 2002, 2, 443–448. [Google Scholar] [CrossRef]
- Shortsleeves, K.C.; Turnbull, M.M.; Seith, C.B.; Tripodakis, E.N.; Xiao, F.; Landee, C.P.; Dawe, L.N.; Garrett, D.; de Delgado, G.D.; Foxman, B.M. Crystallographic and magnetic studies of the 2-pyridone/copper halide system. Polyhedron 2013, 64, 110–121. [Google Scholar] [CrossRef]
- Małecki, J.G.; Mrozinski, J.; Michalik, K. Structural, spectroscopic and magnetic properties of Mn(II), Co(II) and Ni(II)complexes with 2-hydroxy-6-methylpyridine ligand. Polyhedron 2011, 30, 1806–1814. [Google Scholar] [CrossRef]
- Mochida, T.; Ueda, M.; Aoki, C.; Mori, H. Structures and properties of trans-dichloro{tetrakis (5-chloro-2(1H)-pyridone-O)}M(II) [M = Mn, Fe, Co, Ni, Cu]; formation of quasimacrocyclic metal complexes through hydrogen bonding. Inorg. Chim. Acta 2002, 335, 151–155. [Google Scholar] [CrossRef]
- Dobravc Koleša, T.; Meden, A.; Perdih, F. Supramolecular Potential of Vanadium β-Diketonate and Picolinate Compounds and The First One-dimensional Oxidovanadium(IV) Complex with β-Diketonate Ligand. Acta Chim. Slov. 2015, 62, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Berg, N.; Rajeshkumar, T.; Taylor, S.M.; Brechin, E.K.; Rajaraman, G.; Jones, L.F. What Controls the Magnetic Interaction in bis-µ-Alkoxo MnIII Dimers? A Combined Experimental and Theoretical Exploration. Chem. Eur. J. 2012, 18, 5906–5918. [Google Scholar] [CrossRef] [Green Version]
- Supriya, S.; Das, S.K. Reversible Single Crystal to Single Crystal Transformation through Fe-O(H)Me/Fe-OH2 Bond Formation/Bond Breaking in a Gas-Solid Reaction at an Ambient Condition. J. Am. Chem. Soc. 2007, 129, 3464–3465. [Google Scholar] [CrossRef]
- Supriya, S.; Manikumari, S.; Raghavaiah, P.; Das, S.K. A cyclic supramolecular (H2O)4 cluster in an unusual Fe3 complex that aggregates to {Fe3}n with a zig-zag chainlike structure. New J. Chem. 2003, 27, 218–220. [Google Scholar] [CrossRef]
- Blake, A.J.; Gould, R.O.; Winpenny, R.E.P. Tetrakis(μ-acetato)bis(2-pyridone)dicopper. Acta Crystallogr. C 1991, 47, 1077–1079. [Google Scholar] [CrossRef]
- Dar, A.A.; Sen, S.; Gupta, S.K.; Naresh Patwari, G.; Murugavel, R. Octanuclear Zinc Phosphates with Hitherto Unknown Cluster Architectures: Ancillary Ligand and Solvent Assisted Structural Transformations Thereof. Inorg. Chem. 2015, 54, 9458–9469. [Google Scholar] [CrossRef] [PubMed]
- Selmani, V.; Landee, C.P.; Turnbull, M.M.; Wikaira, J.L.; Xiao, F. An extremely well isolated 2D-antiferromagnetic layer. Inorg. Chem. Comm. 2010, 13, 1399–1401. [Google Scholar] [CrossRef]
- Escuer, A.; Vicente, R.; Goher, M.A.S.; Mautner, F.A. Antiferromagnetic Alternating and Homogeneous Manganese-Azido Chains: Structural Characterization and Magnetic Behavior of Two New One-Dimensional [Mn(L)2(µ1,3-N3)2]n Compounds (L = 3-Ethylpyridine and 2-Hydroxypyridine). Inorg. Chem. 1998, 37, 782–787. [Google Scholar] [CrossRef]
- Hollis, L.S.; Lippard, S.J. Mononuclear Complexes of cis-Diammineplatmum(II) and -(IV) with α-Pyridone. Structures of cis-[Pt(NH3)2(C5H4NOH)2]Cl2, mer-[Pt(NH3)2(C5H4NO)CI3], and cis-[Pt(NH3)2(C5H4N0H)Cl](NO3). Inorg. Chem. 1983, 22, 2708–2713. [Google Scholar] [CrossRef]
- Chang, Q.W.; Hu, C.Y.; Chen, J.L.; Ye, Q.S.; Chen, X.Z.; Yu, Y.; Chen, L.Q.; Liu, W.P. Crystal structure of bis(acetylacetonato-κ2O,O′)-(diacetylmethanido-κ)C)-(2-hydroxypyridine)iridium(III), Ir(C5H7O2)3(C5H5NO). Z. Krist. New Cryst. St. 2011, 226, 357–358. [Google Scholar]
- Forrest, S.J.K.; Manojveer, S.; Johnson, M.T. Cooperative or Oxidative Hydrogen Addition to 2-Hydroxypyridonate Iridium Complexes: Dependence on Oxidation State. Eur. J. Inorg. Chem. 2017, 3239–3243. [Google Scholar] [CrossRef]
- Hollis, L.S.; Lippard, S.J. New Reaction Chemistry of cis-Diammineplatinum(II) with α-Pyridone. Crystalline Relatives of the α-Pyridone Blue. J. Am. Chem. Soc. 1981, 103, 1230–1232. [Google Scholar] [CrossRef]
- Brewster, T.P.; Nguyen, T.H.; Li, Z.; Eckenhoff, W.T.; Schley, N.D.; DeYonker, N.J. Synthesis and Characterization of Heterobimetallic Iridium−Aluminum and Rhodium−Aluminum Complexes. Inorg. Chem. 2018, 57, 1148–1157. [Google Scholar] [CrossRef]
- Andreu, P.L.; Cabeza, J.A.; Carriedo, G.A.; Riera, V.; Garcia-Granda, S.; Van der Maelen, J.F.; Mori, G. The chemical and electrochemical oxidation of pyridonate-bridged ruthenium(I) dimers. X-Ray structure of [Ru2(μ-pyO)2(CO)4(pyOH)2] (pyOH = 2-pyridone). J. Organomet. Chem. 1991, 421, 305–314. [Google Scholar] [CrossRef]
- Nguyen, A.I.; Wang, J.; Levine, D.S.; Ziegler, M.S.; Tilley, T.D. Synthetic control and empirical prediction of redox potentials for Co4O4 cubanes over a 1.4 V range: implications for catalyst design and evaluation of high-valent intermediates in water oxidation. Chem. Sci. 2017, 8, 4274–4284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breeze, S.R.; Wang, S. Hydrogen-Bond-Directed Assembly of One-Dimensional and Two-Dimensional Polymeric Copper(II) Complexes with Trifluoroacetate and Hydroxypyridine as Ligands: Syntheses and Structural Investigations. Inorg. Chem. 1993, 32, 5981–5989. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Effendy; Nitiatmodjo, M.; Skelton, B.W.; White, A.H. Syntheses and structures of some adducts of silver(I) oxyanion salts with some 2-N,O-donor-substituted pyridine bases. Inorg. Chim. Acta 2005, 358, 4327–4341. [Google Scholar] [CrossRef]
- Arman, H.D.; Miller, T.; Tiekink, E.R.T. catena-Poly[[[(2-pyridone-κO)silver(I)]-µ-2-pyridone-κ2O:O] hexafluoridophosphate]. Acta Cryst. 2010, E66, m1212. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.E.; Sidorov, A.A.; Aleksandrov, G.G.; Ikorskii, V.N.; Smolyaninov, I.V.; Okhlobystin, A.O.; Berberova, N.T.; Eremenko, I.L. Replacement of carboxylate bridges in polynuclear nickel pivalates with 2-hydroxy-6-methylpyridine anions. Russ. Chem. Bull. Int. Ed. 2007, 56, 943–952. [Google Scholar] [CrossRef]
- Parsons, S.; Brechin, E.; Winpenny, R.; Wood, P.A. (Refcode HAJFII. The Cambridge Structural Database Communication). Private communication, 2004. [Google Scholar]
- Burkovskaya, N.P.; Nikiforova, M.E.; Kiskin, M.A.; Pekhn’o, V.I.; Sidorov, A.A.; Novotortsev, V.M.; Eremenko, I.L. New Nickel(II) Carboxylate–Phosphonate Cluster: Synthesis and Structure. Russ. J. Coord. Chem. 2012, 38, 331–336. [Google Scholar] [CrossRef]
- Petriček, S. Syntheses and crystal structures of metal (Mn, Co, Ni) chloride complexes with 3-hydroxypyridin-2-one and contribution of O–H⋯ Cl hydrogen bonds to their structural diversity. Polyhedron 2019, 167, 11–25. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Petriček, S.; Demšar, A. Syntheses and crystal structures of manganese, nickel and zinc chloride complexes with dimethoxyethane and di(2-methoxyethyl) ether. Polyhedron 2010, 29, 3329–3334. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Blake, A.J.; Gould, R.O.; Grant, C.M.; Milne, P.E.Y.; Winpenny, R.E.P. Use of a hexanuclear copper complex as a ligand transfer agent: crystal structures of hexsakis(6-methyl-2-pyridone)iron(III) nitrate and tetrakis(6-methyl-2-pyridone)bis(nitrato)cobalt(II). Polyhedron 1994, 13, 187–191. [Google Scholar] [CrossRef]
- Awwadi, F.F.; AlWahsh, M.I.; Dawe, L.N.; Turnbull, M.M. Polymorphism in diaquatetrakis(6-chloro-2-hydroxypyridine) copper(II) perchlorate: Crystallographic, solution and theoretical studies and solid phase transformations. J. Mol. Struct. 2018, 1171, 294–304. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Nishio, M. The CH/π hydrogen bond: Implication in chemistry. J. Mol. Struct. 2012, 1018, 2–7. [Google Scholar] [CrossRef]
- Litsis, O.O.; Ovchynnikov, V.A.; Shishkina, S.V.; Sliva, T.Y.; Amirkhanov, V.M. Dinuclear 3D metal complexes based on a carbacylamidophosphate ligand: Redetermination of the ligand crystal structure. Transition Met. Chem. 2013, 38, 473–479. [Google Scholar] [CrossRef]
- Ikotun, O.F.; Ouellette, W.; Lloret, F.; Julve, M.; Doyle, R.P. Synthesis, X-ray Structure, Thermal and Magnetic Behavior of [(bipy)2Ni2(μ-Cl)2Cl2(H2O)2]: The First Neutral Ferromagnetically Coupled Six-Coordinate Dichlorido-Bridged Nickel(II) Dimer. Eur. J. Inorg. Chem. 2007, 2007, 2083–2088. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Wang, X.; Mahmood, Q.; Hao, X.; Hu, X.; Guo, C.Y.; Solan, G.A.; Sun, W.H. Highly branched unsaturated polyethylenes achievable using strained imino-cyclopenta[b]pyridyl-nickel precatalysts. Polym. Chem. 2017, 8, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zeng, Y.; Huang, W.; Hao, X.; Sun, W.H. N-(5,6,7-Trihydroquinolin-8-ylidene)arylaminonickel dichlorides as highly active single-site pro-catalysts in ethylene polymerization. Dalton Trans. 2011, 40, 8436–8443. [Google Scholar]
- Sun, W.H.; Song, S.; Li, B.; Redshaw, C.; Hao, X.; Li, Y.S.; Wang, F. Ethylene polymerization by 2-iminopyridylnickel halide complexes: Synthesis, characterization and catalytic influence of the benzhydryl group. Dalton Trans. 2012, 41, 11999–12010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Li, B.Z.; Yang, P.; Wu, J.Z. Di-µ-chlorido-bis[chlorido(4′-p-tolyl-2,2′:6′,2″-terpyridine-κ3N,N′,N″)-nickel(II)]: A supramolecular system constructed by C–H⋯ Cl interactions. Acta Cryst. 2009, C65, m238–m240. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.E.; Kwak, C.H. Dimeric Ni(II)2 and polymeric Ni(II)4Fe(II) type complexes bridged with Cl− and CN− ligands: X-ray structures and magnetic properties of a dimeric complex of [(tren)Ni(μ-Cl)2Ni(tren)](ClO4)2 and a polymeric complex of {[Fe(CN)6][Ni(tren)]2[Ni(tren)(H2O)]2}Cl2(ClO4)2·4H2O. Inorg. Chem. Commun. 2013, 33, 95–98. [Google Scholar]
- Junk, P.C.; Steed, J.W. A structural study of late transition metal diethylenetriamine complexes. Inorg. Chim. Acta 2007, 360, 1661–1668. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef] [Green Version]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Cotton, F.A.; Lewis, G.E.; Mott, G.N. Dinuclear and Polynuclear Oxovanadium(IV) Compounds. 1. Synthesis and Structural Study of V2O2CI4(µ-Hmhp)3, a Novel Complex Containing Three Neutral Bridging Ligands. Inorg. Chem. 1983, 22, 378–382. [Google Scholar] [CrossRef]
- Modak, R.; Sikdar, Y.; Mandal, S.; Chatterjee, S.; Bienko, A.; Mrozinski, J.; Goswami, S. Syntheses, crystallographic characterization, catecholase activity and magnetic properties of three novel aqua bridged dinuclear nickel(II) complexes. Inorg. Chim. Acta 2014, 416, 122–134. [Google Scholar] [CrossRef]
- Biswas, R.; Diaz, C.; Bauzá, A.; Barceló-Oliver, M.; Frontera, A.; Ghosh, A. Triple-bridged ferromagnetic nickel(II) complexes: A combined experimental and theoretical DFT study on stabilization and magnetic coupling. Dalton Trans. 2014, 43, 6455–6467. [Google Scholar] [CrossRef]
- Biswas, R.; Giri, S.; Saha, S.K.; Ghosh, A. One Ferromagnetic and Two Antiferromagnetic Dinuclear Nickel(II) Complexes Derived from a Tridentate N,N,O-Donor Schiff Base Ligand: A Density Functional Study of Magnetic Coupling. Eur. J. Inorg. Chem. 2012, 2916–2927. [Google Scholar] [CrossRef]
- Bhardwaj, V.K.; Hundal, M.S.; Corbella, M.; Gomez, V.; Hundal, G. Salicylaldimine Schiff bases—Generation of self-assembled and chiral complexes with Ni(II) and Zn(II) ions. An unusual antiferromagnetic interaction in a triply bridged Ni(II) dimer. Polyhedron 2012, 38, 224–234. [Google Scholar] [CrossRef]
- Biswas, R.; Diaz, C.; Ghosh, A. Three nickel(II) complexes derived from a tridentate NNO donor Schiff base ligand: Syntheses, crystal structures and magnetic properties. Polyhedron 2013, 56, 172–179. [Google Scholar] [CrossRef]
- Biswas, R.; Kar, P.; Song, Y.; Ghosh, A. The importance of an additional water bridge in making the exchange coupling of bis(L-phenoxo)dinickF3el(II) complexes ferromagnetic. Dalton Trans. 2011, 40, 5324–5331. [Google Scholar] [CrossRef] [PubMed]
- Enamullah, M.; Quddus, M.A.; Hasan, M.R.; Pescitelli, G.; Berardozzi, R.; Reiß, G.J.; Janiak, C. Syntheses, Spectroscopy, and Structural Analyses of Dinuclear Chiral-at-Metal μ-Aqua-tetrakis[(R or S)-N-1-(Ar)ethylsalicylaldiminato]di-Λ- or -Δ-nickel(II) Complexes. Eur. J. Inorg. Chem. 2015, 2758–2768. [Google Scholar] [CrossRef]
- Suthan, T.; Rajesh, N.P.; Mahadevan, C.K.; Sajan, D.; Bhagavannarayana, G. Growth and characterization of organic material 2-hydroxypyridine single crystal by modified vertical Bridgman technique. Mater. Chem. Phys. 2011, 130, 915–920. [Google Scholar] [CrossRef]
- CrysAlis PRO; Agilent Technologies UK: Yarnton, Oxfordshire, 2013.
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal-structures with SIR–92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, J.J. ORTEP-3 for Windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Molecular formula | C20H20Cl2N4NiO4 | C30H30Cl2N6NiO6 | C20H28Cl4N4Ni2O8 | C29H31Cl4N7Ni2O5 | C20H20Cl2MnN4O4 |
| Colour | yellow | yellow | yellow | yellow | colorless |
| For.mass (g/mol) | 509.99 | 700.19 | 711.64 | 816.83 | 506.24 |
| Cryst. syst. | monoclinic | monoclinic | triclinic | monoclinic | monoclinic |
| Radiation type | Mo Kα | Mo Kα | Cu Kα | Mo Kα | Mo Kα |
| Space gr. | P21/n (no. 14) | P21/n (no. 14) | P (no. 2) | C2/c (no. 15) | P21/n (no. 14) |
| a (Å) | 6.8918(6) | 10.1083(10) | 7.1191(4) | 20.3325(6) | 6.9071(3) |
| b (Å) | 9.7906(7) | 8.7836(6) | 9.7675(5) | 9.9564(3) | 9.6873(4) |
| c (Å) | 15.6618(15) | 18.9985(15) | 10.3811(4) | 17.4575(5) | 16.1391(8) |
| α (°) | 90 | 90 | 68.572(4) | 90 | 90 |
| β (°) | 90.316(5) | 99.128(8) | 86.606(4) | 104.344(3) | 90.020(4) |
| γ (°) | 90 | 90 | 79.957(5) | 90 | 90 |
| V (Å3) | 1056.8(2) | 1665.5(2) | 661.7(1) | 3423.9(3) | 1079.9(1) |
| Z (form.) | 2 | 2 | 1 | 4 | 2 |
| Dcal. (g cm−3) | 1.603 | 1.396 | 1.786 | 1.584 | 1.557 |
| μ (mm−1) | 1.207 | 0.793 | 5.958 | 1.461 | 0.894 |
| Crystal size (mm) | 0.16 × 0.16 × 0.12 | 0.4 × 0.4 × 0.5 | 0.17 × 0.17 × 0.12 | 0.4 × 0.3 × 0.2 | 0.16 × 0.14 × 0.12 |
| θ Range (o) | 3.0–27.5 | 3.1–27.5 | 4.6–74.6 | 3.1–27.5 | 3.2–27.5 |
| Collected refl. | 10229 | 9273 | 6162 | 16091 | 9807 |
| Unique refl. | 2409 | 3824 | 2694 | 3920 | 2476 |
| Rint | 0.041 | 0.069 | 0.021 | 0.023 | 0.03 |
| Observed refl. | 2181 | 2600 | 2648 | 3575 | 2178 |
| No. param. | 143 | 205 | 184 | 218 | 142 |
| Ra (I > 2.0 σ(I)) | 0.037 | 0.049 | 0.034 | 0.0327 | 0.027 |
| wR2b | 0.092 | 0.085 | 0.094 | 0.09 | 0.062 |
| S | 1.07 | 0.994 | 1.04 | 1.14 | 1.044 |
| Max/min res. elec. d. (e Å−3) | −0.54, 0.53 | −0.61, 0.53 | −0.76, 0.48 | −0.85, 0.74 | −0.23, 0.28 |
| 1 | 5 | 1 | 5 | ||
| M1–O1 | 2.036(2) | 2.1401(11) | O1–M1–O2 | 89.59(7) | 87.50(4) |
| M1–O2 | 2.078(2) | 2.1764(11) | O1–M1–O2 i | 90.41(7) | 92.50(4) |
| M1–Cl1 | 2.4539(2) | 2.5762(4) | O1–M1–Cl1 | 86.79(6) | 87.78(3) |
| O1–C11 | 1.254(3) | 1.2562(18) | O2–M1–Cl1 | 91.69(6) | 91.52(3) |
| O2–C21 | 1.266(3) | 1.2626(19) | O1–M1–Cl1 i | 93.21(6) | 92.22(3) |
| O2–M1–Cl1 i | 88.31(6) | 88.48(3) | |||
| M1–O1–C11 | 133.23(19) | 136.35(10) | |||
| M1–O2–C21 | 130.64(18) | 131.91(9) | |||
| Symmetry codes in 1 and 5: (i) 1 − x, −y, 1 − z. | |||||
| 2 | |||||
| Ni1–O1 | 2.0387(19) | O1–Ni1–O2 | 88.27(7) | ||
| Ni1–O2 | 2.0489(19) | O1–Ni1–O3 | 89.19(7) | ||
| Ni1–O3 | 2.0530(19) | O2–Ni1–O3 | 93.90(7) | ||
| O1–C11 | 1.256(3) | O1–Ni1–O1 i | 180.00 | ||
| O2–C21 | 1.273(4) | O1–Ni1–O2 i | 91.73(7) | ||
| O3–C31 | 1.274(3) | O1–Ni1–O3 i | 90.81(7) | ||
| O2–Ni1–O3 i | 86.10(7) | ||||
| Ni1–O1–C11 | 128.64(19) | ||||
| Ni1–O2–C21 | 128.41(17) | ||||
| Ni1–O3–C31 | 127.80(17) | ||||
| Symmetry codes in 2: (i) −x, −y, −z. | |||||
| Ni1–Cl1 | 2.4111(6) | Cl1–Ni1–Cl2 | 90.19(2) |
| Ni1–Cl2 | 2.3975(6) | Cl1–Ni1–O1 | 174.75(4) |
| Ni1–Cl2 i | 2.4010(6) | Cl1–Ni1–O2 | 93.02(4) |
| Ni1–O1 | 2.1503(15) | Cl1–Ni1–O10 | 96.53(5) |
| Ni1–O2 | 2.0639(15) | Cl1–Ni1–Cl2 i | 94.15(2) |
| Ni1–O10 | 2.0293(15) | Cl2–Ni1–O1 | 84.56(4) |
| O10–C10 | 1.265(3) | Cl2–Ni1–O2 | 174.77(5) |
| Cl2–Ni1–O10 | 97.46(4) | ||
| Cl2–Ni1–Cl2 i | 89.29(2) | ||
| O1–Ni1–O2 | 92.22(6) | ||
| Ni1…Ni1 i | 3.4141(5) | O1–Ni1–O10 | 84.13(6) |
| Cl2 i–Ni1–O1 | 85.86(4) | ||
| O2–Ni1–O10 | 86.28(6) | ||
| Cl2 i–Ni1–O2 | 86.37(5) | ||
| Cl2 i–Ni1–O10 | 167.33(5) | ||
| Ni1–Cl2–Ni1 i | 90.71(2) |
| Ni1–Cl1 | 2.3690(7) | Cl1–Ni1–Cl2 | 93.64(2) |
| Ni1–Cl2 | 2.3628(6) | Cl1–Ni1–O1 | 96.86(5) |
| Ni1–O1 | 2.0219(16) | Cl1–Ni1–O2 | 94.34(5) |
| Ni1–O2 | 2.0882(16) | Cl1–Ni1–O3 | 96.52(3) |
| Ni1–O2 i | 2.1078(17) | Cl1–Ni1–O2 i | 167.82(5) |
| Ni1–O3 | 2.0892(14) | Cl2–Ni1–O1 | 94.96(5) |
| O1–C11 | 1.263(3) | Cl2–Ni1–O2 | 96.25(5) |
| O2–C21 | 1.271(3) | Cl2–Ni1–O3 | 166.97(4) |
| O3–C31 | 1.268(4) | Cl2–Ni1–O2 i | 94.21(5) |
| O1–Ni1–O2 | 163.60(7) | ||
| Ni1…Ni1 i | 2.9802(5) | O1–Ni1–O3 | 91.91(5) |
| O1–Ni1–O2i | 91.72(7) | ||
| O2–Ni1–O3 | 74.91(6) | ||
| O2–Ni1–O2 i | 74.50(6) | ||
| Ni1–O1–C11 | 128.76(16) | ||
| Ni1–O2–C21 | 134.85(15) | ||
| Ni1–O3–C31 | 134.49(4) | ||
| Ni1–O2–Ni1 i | 90.53(7) | ||
| Ni1–O3–Ni1i | 91.01(8) |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petriček, S. Structural Diversity of Nickel and Manganese Chloride Complexes with Pyridin-2-One. Molecules 2020, 25, 846. https://doi.org/10.3390/molecules25040846
Petriček S. Structural Diversity of Nickel and Manganese Chloride Complexes with Pyridin-2-One. Molecules. 2020; 25(4):846. https://doi.org/10.3390/molecules25040846
Chicago/Turabian StylePetriček, Saša. 2020. "Structural Diversity of Nickel and Manganese Chloride Complexes with Pyridin-2-One" Molecules 25, no. 4: 846. https://doi.org/10.3390/molecules25040846
APA StylePetriček, S. (2020). Structural Diversity of Nickel and Manganese Chloride Complexes with Pyridin-2-One. Molecules, 25(4), 846. https://doi.org/10.3390/molecules25040846




