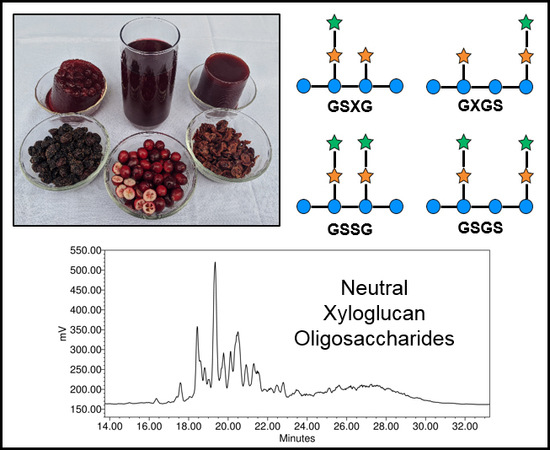
Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity
Abstract
1. Cranberry as a Functional Food
1.1. Diversity of Cranberry Products
1.2. Processing Methods Influence Product Composition
1.3. Common Chemical Components of Cranberry and Related Species
2. Oligosaccharides: Structures, Separations, Occurrence
2.1. Cranberry Xyloglucans
2.1.1. Structural Features
2.1.2. Chromatographic Resolution and Aggregation Behavior
2.2. Characteristics of Xyloglucan Fractions Used by Various Studies
2.2.1. Initial Oligosaccharide Separations
2.2.2. Targeted Oligosaccharide Separations
2.3. Cranberry Pectins: Occurrence and Structures
2.4. The Hidden Occurrence of Oligosaccharides in Cranberry Materials
2.4.1. Oligosaccharide Content Estimates
2.4.2. Composition Ambiguity for Cranberry Products
2.4.3. Oligosaccharide Content Reporting
- (A)
- Minimum requirements (1–3) for oligosaccharide characterization and content descriptions with additional (4) characterization if possible:
- (1)
- Identification of possible monosaccharide composition using one of the following methods:
- (a)
- Monosaccharide composition analysis suitable for neutral or pectic polysaccharides and oligosaccharides to give relative molar percentages of monosaccharide constituents.
- (b)
- 1H-NMR spectrum (D2O) showing distinct anomeric signals corresponding to oligosaccharides previously characterized from cranberry materials.
- (2)
- Full description of the source material and separation methods
- (a)
- (b)
- Inclusion of sufficient separation details (including yields and accurate sorbent descriptions) necessary for another researcher to repeat the methods used.
- (3)
- Reported presence or absence of additional possible constituents
- (a)
- A PDAD max plot UV spectrum will show the presence or absence of trace amounts of organic acids and phenolic compounds, such as flavonoids and PACs. A UV spectrum would also indicate the presence of uronic acids such as those present in pectic oligosaccharides.
- (b)
- Visual description of the dried material. Trace amounts of anthocyanins can be visually detected as a slight pinkish or purplish color when the material is dried.
- (4)
- Additional characterization if possible:
- (a)
- Characterization by MALDI-TOF MS or other MS-based methods customized for oligosaccharide detection using neutral oligosaccharide standards of at least DP 3–10 (include the specific identity of the standards used for comparison).
- (b)
- Glycosyl linkage analysis using chemical derivatization with appropriate methods and standards.
- (c)
- 2D NMR spectra showing anomeric region correlations
- (B)
- Minimum requirements for eliminating oligosaccharides as possible components of a given cranberry material. Any one of the following three recommendations would be acceptable.
- 1.
- 1H-NMR spectrum (D2O) showing the absence of anomeric signals corresponding to oligosaccharides.
- 2.
- UV and LCMS-based detection with negative results may be acceptable if oligosaccharide-appropriate derivatization and separation methods are applied.
- 3.
- The application of a standard monosaccharide analysis method suitable for oligosaccharides and other complex carbohydrates yields 0% monosaccharides.
3. Human ADME of Cranberry Complex Carbohydrates
3.1. ADME of Classic Dietary Fibers
3.2. ADME of Soluble Oligosaccharides
3.3. Detection and Analysis of Soluble Oligosaccharide Components In Vivo
4. Biological Properties of Cranberry Complex Carbohydrates
4.1. Effects on Bacterial Adhesion
4.2. Effects on Biofilm Formation and Bacterial Aggregation
4.3. Effects on Microbial Growth and Cell Viability
4.4. Effects on Metabolic Health Factors
5. Cranberry Carbohydrates and Human Microbiota
5.1. Prebiotic Effects of Cranberry Oligosaccharides
5.1.1. Bacterial Fermentation of Cranberry Oligosaccharides in Monoculture
5.1.2. Bacterial Fermentation of Cranberry Oligosaccharides in Mixed Species Culture
5.2. Effects of Cranberry on Gut Microbiota Profiles In Vivo
5.3. Effects of Cranberry on Urinary Tract Microbiota Profiles
6. Cranberry for Human Health and Disease Prevention
6.1. Recognized Composition Influences Bioactivity Interpretations
6.2. A New Paradigm for UTI Prevention with Cranberry Materials
7. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C.; Vinson, J.A. Chapter 6. Cranberry. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.B.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Sobota, A.E. Inhibition of bacterial adherence by cranberry juice-potential use for the treatment of urinary tract infections. J. Urol. 1984, 131, 1013–1016. [Google Scholar] [CrossRef]
- Zafriri, D.; Ofek, I.; Adar, R.; Pocino, M.; Sharon, N. Inhibitory activity of cranberry juice on adherence of type 1 and type P-fimbriated Escherichia coli to eukaryotic cells. Antimicrob Agents Chemother. 1989, 33, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Avorn, J.; Monane, M.; Gurwitz, J.H.; Glynn, R.J.; Choodnovskiy, I.; Lipsitz, L.A. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. J. Am. Med. Assoc. 1994, 271, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Basu, A.; Krueger, C.G.; Lila, M.A.; Neto, C.C.; Novotny, J.A.; Reed, J.D.; Rodriguez-Mateos, A.; Toner, C.D. Impact of cranberries on gut microbiota and cardiometabolic health: Proceedings of the cranberry health research conference 2015. Adv. Nutr. 2016, 7, 759S–770S. [Google Scholar] [CrossRef] [PubMed]
- Bodet, C.; Grenier, D.; Chandad, F.; Ofek, I.; Steinberg, D.; Weiss, E.I. Potential Oral Health Benefits of Cranberry. Crit. Rev. Food Sci. Nutr. 2008, 48, 672–680. [Google Scholar] [CrossRef]
- Alexander, B.; John, S. Oral health benefits of cranberry: A review. J. Dent. Med. Sci. 2018, 18, 41–44. [Google Scholar]
- Basu, A.; Masek, E.; Ebersole, J.L. Dietary polyphenols and periodontitis. A mini-review of literature. Molecules 2018, 23, 1786. [Google Scholar] [CrossRef]
- Philip, N.; Walsh, L.J. Cranberry Polyphenols: Natural Weapons against Dental Caries. Dent. J. 2019, 7, 20. [Google Scholar] [CrossRef]
- Neto, C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular disease. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Neto, C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, M.; Hadi, A.; Najafgholizadeh, A.; Joukar, F.; Mansour-Ghanaei, F. The effects of cranberry on cardiovascular metabolic risk factors: A systematic review and meta-analysis. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A. Beneficial effects of cranberry in the prevention of obesity and related complications: Metabolic syndrome and diabetes-A review. J. Funct. Foods 2016, 20, 171–181. [Google Scholar] [CrossRef]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef]
- Basu, A. Role of berry bioactive compounds on lipids and lipoproteins in diabetes and metabolic syndrome. Nutrients 2019, 11, 1983. [Google Scholar] [CrossRef]
- Guay, D.R.P. Cranberry and urinary tract infections. Drugs 2009, 69, 775–807. [Google Scholar] [CrossRef]
- Jepson, R.G.; Williams, G.; Craig, J.C. Cranberries for preventing urinary tract infections (review). Cochrane Database Syst. Rev. 2012, 10, CD001321:1-82. [Google Scholar]
- Wang, C.H.; Fang, C.C.; Chen, N.C.; Liu, S.S.; Yu, P.H.; Wu, T.Y.; Chen, W.T.; Lee, C.C.; Chen, S.C. Cranberry-containing products for prevention of urinary tract infections in susceptible populations-a systematic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2012, 172, 988–996. [Google Scholar] [CrossRef]
- Hyson, D.A. A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Adv. Nutr. 2015, 6, 37–51. [Google Scholar] [CrossRef]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: A systematic review and meta-analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Wawrysiuk, S.; Naber, K.; Rechberger, T.; Miotla, P. Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance-non-antibiotic approaches: A systemic review. Arch. Gynecol. Obs. 2019, 300, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.M.; Ferreira, D.; Howell, A.B.; Reed, J.D.; Krueger, C.G.; Marais, J.P.J. Anti-Adhesive Urinary Metabolites Produced as a Result of Cranberry Juice Consumption. In Proceedings of the Oral session presentation at the Joint Meeting of the American Society of Pharmacognosy and the Phytochemical Society of North America, St. Petersburg Beach, FL, USA, 13 July 2010. [Google Scholar]
- Coleman, C.M. Cranberry metabolites with urinary anti-adhesion activity. Ph.D. Thesis, University of Mississippi, University, MS, USA, 2014. [Google Scholar]
- Coleman, C.M.; Auker, K.M.; Killday, K.B.; Azadi, P.; Black, I.; Ferreira, D. Arabinoxyloglucan oligosaccharides may contribute to the antiadhesive properties of porcine urine after cranberry consumption. J. Nat. Prod. 2019, 82, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Auker, K.M. Structural Characterization and Bioactivity of Cranberry Oligosaccharides. Master’s Thesis, University of Mississippi, University, MS, USA, 2013. [Google Scholar]
- Auker, K.M.; Coleman, C.M.; Wang, M.; Avula, B.; Bonnet, S.L.; Kimble, L.L.; Mathison, B.D.; Chew, B.P.; Ferreira, D. Structural characterization of cranberry arabinoxyloglucan oligosaccharides. J. Nat. Prod. 2019, 82, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.M.; Akgul, Y.; Kimble, L.L.; Mathison, B.D.; Chew, B.P.; Ferreira, D. Cranberry juice concentrate: Arabinoxyloglucan content and anti-adhesion properties. Submitted February 2020.
- Coleman, C.M.; Auker, K.M.; Ferreira, D. Oligosaccharide content of commercially available cranberry juice and dietary supplement products, as determined by RP-HPLC-PDAD-ELSD. Submitted February 2020.
- Alston, J.M.; Medellin-Azuara, J.; Saitone, T.L. Economic impact of the North American cranberry industry. Published 2014. Available online: https://www.uscranberries.com/wp-content/uploads/2018/09/Economic_Impact_of_the_NA_Cranberry_Industry_August2014-1.pdf (accessed on 13 December 2018).
- Jesse, E.V.; Rogers, R.T. The cranberry industry and Ocean. Spray Cooperative: Lessons in cooperative governance. Food System Research Group, FSRG Monograph Series 19 January 2006. Available online: https://www.semanticscholar.org/paper/The-Cranberry-Industry-and-Ocean-Spray-Cooperative%3A-Jesse-Rogers/8e5944d8d5e50179db0d35cf18b307bf3ebdc409 (accessed on 31 December 2019).
- Reiss, R. Ocean Spray’s Secrets of co-op Success. Published Sept 15, 2010. Available online: https://www.forbes.com/2010/09/15/papadellis-ocean-spray-leadership-managing-interview.html#3ff069a13bd4 (accessed on 13 February 2019).
- Diaz-Garcia, L.; Rodriguez-Bonilla, L.; Phillips, M.; Lopez-Hernandez, A.; Grygleski, E.; Atucha, A.; Zalapa, J. Comprehensive analysis of the internal structure and firmness in American cranberry (Vaccinium macrocarpon Ait.) fruit. PLoS ONE 2019, 14, e0222451:1–17. [Google Scholar] [CrossRef]
- USDA Commercial Item Description A-A-20327, Cranberry Juice, Blends, One Hundred Percent. Available online: https://www.ams.usda.gov/sites/default/files/media/CID%20Cranberry%20Juice%20Blends%2C%20One%20Hundred%20Percent.pdf (accessed on 15 March 2019).
- USDA Commercial Item Description A-A-20121A, Cranberry Juice Cocktail (Single Strength and Concentrate). Available online: https://www.ams.usda.gov/sites/default/files/media/CID%20Cranberry%20Juice%20Cocktail%20%28Single%20Strength%20and%20Concentrate%29.pdf (accessed on 15 March 2019).
- UN Food and Agriculture Organization-Codex Standard 247-2005 General Standard for Fruit Juices and Nectars. Available online: www.fao.org/input/download/standards/10154/CXS_247e.pdf (accessed on 15 March 2019).
- Roopchand, D.E.; Krueger, C.G.; Moskal, K.; Fridlender, B.; Lila, M.A.; Raskin, I. Food-compatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chem. 2013, 141, 3664–3669. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Proximate and polyphenolic characterization of cranberry pomace. J. Agric. Food Chem. 2010, 58, 4030–4036. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Gornas, P.; Seglina, D.; Pilarska, A.; Jesionowski, T. Physical and bioactive properties of muffins enriched with raspberry and cranberry pomace powder: A promising application of fruit by-products rich in biocompounds. Plant. Foods Hum. Nutr. 2016, 71, 165–173. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Cranberry processing waste for solid state fungal inoculant production. Process. Biochem. 1998, 33, 323–329. [Google Scholar] [CrossRef]
- Mantius, H.L.; Peterson, P.R. Fruit Extraction and Infusion. US Patent 5,419,251, 30 May 1995. [Google Scholar]
- Pease, M.A. Influence of Preparation and Processing on Cranberry Gel Properties. Master’s Thesis, University of Massachusetts, Amhurst, MA, USA, 2007. [Google Scholar]
- Anderson, E.E.; Anti, A.W. Process for Preparing Cranberry Sauce. US Patent 3,023,108, 27 February 1962. [Google Scholar]
- Skelskie, S.I. Process for Making Cranberry Sauce. US Patent 3,326,694, 20 June 1967. [Google Scholar]
- Smith, T.; Kawa, K.; Eckl, V.; Stredney, R. Herbal supplement sales in U.S. increase 7.7% in 2016. Herbalgram 2017, 115, 56–65. [Google Scholar]
- Lager, B.G. Activated Cranberry Powder. US Patent Application US 2008/0020094 A1, 24 January 2008. [Google Scholar]
- Lee, J. Anthocyanin analysis of Vaccinium fruit dietary supplements. Food Sci. Nutr. 2016, 4, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Cardellina II, J.H.; Gafner, S. Cranberry products laboratory guidance document. Austin, TX: ABCAHP-NCNPR Botanical Adulterants Prevention Program. 2018. [Google Scholar]
- Parets, L.; Alechaga, E.; Nunez, O.; Saurina, J.; Hernandez-Cassou, S.; Puignou, L. Ultrahigh pressure liquid chromatography atmospheric pressure photoionization-tandem mass spectrometry for the determination of polyphenolic profiles in the characterization and classification of cranberry-based pharmaceutical preparations and natural extracts. Anal. Methods 2016, 8, 4363–4378. [Google Scholar]
- Chughtai, B.; Howell, A. Variability of commercial cranberry dietary supplements for the prevention or uropathogenic bacterial adhesion. Am. J. Obs. Gynecol. 2016, 7, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Pardo-Mates, N.; Hidalgo-Serrano, M.; Saurina, J.; Puignou, L.; Nunez, O. UHPLC-HRMS (Orbitrap) fingerprinting in classification and authentication of cranberry-based natural products and pharmaceuticals using multivariate calibration methods. Anal. Methods 2019, 11, 3341–3349. [Google Scholar] [CrossRef]
- Turbitt, J.R.; Colson, K.L.; Killday, K.B.; Milstead, A.; Neto, C.C. Application of 1H-NMR-based metabolomics to the analysis of cranberry (Vaccinium macrocarpon) supplements. Phytochem. Anal. 2020, 31, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, G.; Zhang, B.; Fu, X. Current applications and new opportunities for the thermal and non-thermal processing technologies to generate berry product or extracts with high nutraceutical contents. Food Res. Int. 2017, 100, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.G. Methods of Stabilizing Fruit-Concentrate Powders. International Patent WO 02/43510 A2, 6 June 2002. [Google Scholar]
- Ghaedian, R.; Shinde, R.M.; Karava, N. Process for Spray Drying Botanical Food. US Patent Application US 2013/0122146 A1, 16 May 2013. [Google Scholar]
- Nowacka, M.; Laghi, L.; Rybak, K.; Rosa, M.D.; Witrowa-Rajchert, D.; Tylewicz, U. Water state and sugars in cranberry fruits subjected to combined treatments: cutting, blanching and sonication. Food Chem. 2019, 299, 125122:1-8. [Google Scholar] [CrossRef] [PubMed]
- Mantius, H.L. Juice Enriched in Beneficial Compounds. International Patent WO 01/03520 A1, 18 January 2001. [Google Scholar]
- Coneybear, J.F.; Chandler, C.H.; Andrisin, J.J. Crusher Separator Apparatus and Method. US Patent 3,463,311, 26 August 1969. [Google Scholar]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Mantius, H.L.; Rose, L. Process for Producing Sugars and Acids-Rich Juice and Phytochemical-Rich Juice. US Patent Application US 2006/0177560 A1, 10 August 2006. [Google Scholar]
- Hoskin, R.T.; Xiong, J.; Lila, M.A. Comparison of berry juice concentrates and pomaces and alternative plant proteins to produce spray dried protein–polyphenol food ingredients. Food Funct. 2019, 10, 6286–6299. [Google Scholar] [CrossRef] [PubMed]
- O’May, C.; Amzallag, O.; Bechir, K.; Tufenkji, N. Cranberry derivatives enhance biofilm formation and transiently impair swarming motility of the uropathogen Proteus mirabilis HI4320. Can. J. Microbiol. 2016, 62, 464–474. [Google Scholar] [CrossRef]
- Strobel, R.G.K.; Tarr, R.E. Process for Making Concentrated Low Calorie Fruit Juice. US Patent 4,971,813, 20 November 1990. [Google Scholar]
- Willems, J.L.; Low, N.H. Oligosaccharide formation during commercial pear juice processing. Food Chem. 2016, 204, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Mantius, H.L. Xyloglucan Extraction Process. US Patent 9,738,732, 9 July 2015. [Google Scholar]
- Murayama, H.; Katsumata, T.; Endou, H.; Fukushima, T.; Sakurai, N. Effect of storage period on the molecular-mass distribution profile of pectic and hemicellulosic polysaccharides in pears. Postharvest Biol. Technol. 2006, 40, 141–148. [Google Scholar] [CrossRef]
- Anderson, E.E.; Nelson, C.P.; Hampton, W.F. Food Process. US Patent 3,003,881, 10 October 1961. [Google Scholar]
- Harrison, J.E.; Oomah, B.D.; Diarra, M.S.; Ibarra-Alvarado, C. Bioactivites of pilot-scale extracted cranberry juice and pomace. J. Food Process. Preserv. 2013, 37, 356–365. [Google Scholar] [CrossRef]
- Zielinska, M.; Zielinska, D. Effects of freezing, convective and microwave-vacuum drying on the content of bioactive compounds and color of cranberries. Lwt--Food Sci. Technol. 2019, 104, 202–209. [Google Scholar] [CrossRef]
- Zielinska, M.; Markowski, M.; Zielinska, D. The effect of freezing on the hot air and microwave vacuum drying kinetics and texture of whole cranberries. Dry. Technol. 2019, 37, 1714–1730. [Google Scholar] [CrossRef]
- Hotchkiss, A.T.; Nunez, A.; Khoo, C.; Strahan, G.D. Cranberry Xyloglucan Oligosaccharide Composition. U.S. Patent 9,314,494 B2, 19 April 2016. [Google Scholar]
- Zhang, J.; Zhang, C.; Chen, X.; Quek, S.Y. Effect of spray drying on phenolic compounds of cranberry juice and their stability during storage. J. Food Eng. 2020, 269, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Matthan, N.R.; Liu, J.; De La Torre, R.; Chen, C.Y.O. Cranberries attenuate animal-based diet-induced changes in microbiota composition and functionality: A randomized crossover controlled feeding trial. J. Nutr. Biochem. 2018, 62, 76–86. [Google Scholar] [CrossRef]
- Cai, X.; Han, Y.; Gu, M.; Song, M.; Wu, X.; Li, Z.; Li, F.; Goulette, T.; Xiao, H. Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct. 2019, 10, 6331–6341. [Google Scholar] [CrossRef]
- Moreno, F.J.; Sanz, M.L. (Eds.) Food Oligosaccharides: Production, Analysis and Bioactivity; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Holck, J.; Hotchkiss, A.T.; Meyer, A.S.; Mikkelsen, J.D.; Rastall, R.A. Production and Bioactivity of Pectic Oligosaccharides from Fruit and Vegetable Biomass. In Food Oligosaccharides: Production, Analysis and Bioactivity, 1st ed.; Moreno, F.J., Sanz, M.L., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 76–87. [Google Scholar]
- Anjou, K.; Von Sydow, E. The aroma of cranberries IV. Juice of Vaccinium macrocarpon Ait. Ark. Kemi. 1968, 30, 9–14. [Google Scholar]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Effect of dried powder preparation process on polyphenolic content and antioxidant capacity of cranberry (Vaccinium macrocarpon L.). Ind. Crop. Prod. 2015, 77, 658–665. [Google Scholar]
- USDA SCI Division Inspection Series Technical Procedures Manual, February 2013. Available online: http://agnis/sites/FV/PPB/default.aspx (accessed on 29 January 2020).
- Nowacka, M.; Wiktor, A.; Dadan, M.; Rybak, K.; Anuszewska, A.; Materek, L.; Witrowa-Rajchert, D. The application of combined pre-treatment with utilization of sonication and reduced pressure to accelerate the osmotic dehydration process and modify the selected properties of cranberries. Foods 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Megias-Perez, R.; Gamboa-Santos, J.; Soria, A.C.; Villamiel, M.; Montilla, A. Survey of quality indicators in commercial dehydrated fruits. Food Chem. 2014, 150, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Wojdylo, A.; Honke, J.; Ciska, E.; Andlauer, W. Drying-induced physio-chemical changes in cranberry products. Food Chem. 2018, 240, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Nowacka, M.; Anuszewska, A.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D. Drying kinetics and quality of dehydrated cranberries pretreated by traditional and innovative techniques. J. Food Sci. 2019, 84, 1820–1828. [Google Scholar] [CrossRef]
- Kokubu, E.; Kinoshita, E.; Ishihara, K. Inhibitory Effects of Lingonberry Extract on Oral Streptococcal Biofilm Formation and Bioactivity. Bull. Tokyo Dent. Coll. 2019, 60, 1–9. [Google Scholar] [CrossRef]
- Ofek, I.; Goldhar, J.; Sharon, N. Anti-Escherichia coli adhesion activity of cranberry and blueberry juices. Adv. Exp. Med. Biol. 1996, 408, 179–183. [Google Scholar]
- Ofek, I.; Goldhar, J.; Zafriri, D.; Lis, H.; Adar, R.; Sharon, N. Anti-Escherichia coli adhesion activity of cranberry and blueberry juices. N. Eng. J. Med. 1991, 324, 1599. [Google Scholar]
- Rocha, D.M.U.P.; Caldas, A.P.S.; Da Silva, B.P.; Hermsdorff, H.H.M.; Alfenas, R.C.G. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1816–1828. [Google Scholar] [CrossRef]
- Pedersen, C.B.; Kyle, J.; McE Jenkinson, A.; Gardner, P.T.; McPhail, D.B.; Duthie, G.C. Effects of blueberry and cranberry juice consumption on the plasma antioxidant capacity of healthy female volunteers. Eur. J. Clin. Nutr. 2000, 54, 405–408. [Google Scholar] [CrossRef]
- Brown, P.N.; Murch, S.J.; Shipley, P. Phytochemical diversity of cranberry (Vaccinium macrocarpon Aiton) cultivars by anthocyanin determination and metabolomic profiling with chemometric analysis. J. Agric. Food Chem. 2012, 60, 261–271. [Google Scholar] [CrossRef]
- Caesar, L.K.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Opportunities and Limitations for Untargeted Mass Spectrometry Metabolomics to Identify Biologically Active Constituents in Complex Natural Product Mixtures. J. Nat. Prod. 2019, 82, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Jones, O.A.H.; Beale, D.J.; Boughton, B.A.; Benheim, D.; Kouremenos, K.A.; Wolfender, J.L.; Wishart, D.S. Current and future perspectives on the structural identification of small molecules in biological systems. Metabolites 2016, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.H.; Stone, M.J.; Hauck, P.R.; Rahman, S.K. Why are secondary metabolites (natural products) biosynthesized? J. Nat. Prod. 1989, 52, 1189–1208. [Google Scholar] [CrossRef] [PubMed]
- Maplestone, R.A.; Stone, M.J.; Williams, D.H. The evolutionary role of secondary metabolites-a review. Gene 1992, 115, 151–157. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Komai, K. Plant secondary metabolites as plant defense systems. Recent Res. Devel. Phytochem. 2000, 4, 99–114. [Google Scholar]
- Sims, I.M.; Monro, J.A. Fiber: Composition, structures, and functional properties. Adv. Food Nutr. Res. 2013, 68, 81–99. [Google Scholar]
- Porter, N.T.; Martens, E.C. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol. 2017, 71, 349–369. [Google Scholar] [CrossRef]
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 527–537. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Abraham, S.N.; Sharon, N.; Ofek, I. Adhesion and Colonization. Mol. Med. Microbiol. 2002, 1, 629–644. [Google Scholar]
- Schlautman, B.; Diaz-Garcia, L.; Covarrubias-Pazaran, G.; Schlautman, N.; Vorsa, N.; Polashock, J.; Ogden, E.L.; Brown, A.; Lin, Y.C.; Bassil, N.; et al. Comparative genetic mapping reveals synteny and collinearity between the American cranberry and diploid blueberry genomes. Mol. Breed. 2018, 38, 1–19. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Marlett, J.A.; Vollendorf, N.W. Dietary fiber content and composition of different forms of fruits. Food Chem. 1994, 51, 39–44. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its interactions with other components of the growing cell wall. Plant. Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frías, J. Production and Bioactivity of Oligosaccharides in Plant Foods. In Food Oligosaccharides: Production, Analysis and Bioactivity, 1st ed.; Moreno, F.J., Sanz, M.L., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 35–54. [Google Scholar]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Venema, K. In vitro assessment of the bioactivity of food oligosaccharides. In Food Oligosaccharides: Production, Analysis and Bioactivity, 1st ed.; Moreno, F.J., Sanz, M.L., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 219–237. [Google Scholar]
- Clemente, A. In Vivo Assessment of the Bioactivity of Food Oligosaccharides. In Food Oligosaccharides: Production, Analysis and Bioactivity, 1st ed.; Moreno, F.J., Sanz, M.L., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 238–254. [Google Scholar]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Hotchkiss, A.T.; Nunez, A.; Strahan, G.D.; Chau, H.K.; White, A.K.; Marais, J.P.J.; Hom, K.; Vakkalanka, M.S.; Di, R.; Yam, K.L.; et al. Cranberry xyloglucan structure and inhibition of Escherichia coli adhesion to epithelial cells. J. Agric. Food Chem. 2015, 63, 5622–5633. [Google Scholar] [CrossRef]
- Neto, C.C.; Penndorf, K.A.; Feldman, M.; Meron-Sudaim, S.; Zakay-Rones, Z.; Steinberg, D.; Fridman, M.; Kashman, Y.; Ginsburg, I.; Ofek, I.; et al. Characterization of non-dialyzable constituents from cranberry juice that inhibit adhesion, co-aggregation and biofilm formation by oral bacteria. Food Funct. 2017, 8, 1955–1965. [Google Scholar] [CrossRef]
- Sun, J.; Marais, J.P.J.; Khoo, C.; LaPlante, K.; Vejborg, R.M.; Givskov, M.; Tolker-Nielsen, T.; Seeram, N.P.; Rowley, D.C. Cranberry (Vaccinium macrocarpon) oligosaccharides decrease biofilm formation by uropathogenic Escherichia coli. J. Funct. Foods 2015, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, W.; Ma, H.; Marais, J.P.J.; Khoo, C.; Dain, J.A.; Rowley, D.C.; Seeram, N.P. Effect of cranberry (Vaccinium macrocarpon) oligosaccharides on the formation of advanced glycation end-products. J. Berry Res. 2016, 6, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Tuomivaara, S.T.; Yaoi, K.; O’Neill, M.A.; York, W.S. Generation and structural validation of a library of diverse xyloglucan-derived oligosaccharides, including an update on xyloglucan nomenclature. Carbohydr. Res. 2015, 402, 56–66. [Google Scholar] [CrossRef] [PubMed]
- York, W.S.; Kolli, V.S.K.; Orlando, R.; Albersheim, P.; Darvill, A.G. The structures of arabinoxyloglucans produced by solanaceous plants. Carbohydr. Res. 1996, 285, 99–128. [Google Scholar] [CrossRef]
- Vincken, J.P.; York, W.S.; Beldman, G.; Voragen, A.G.J. Two general branching patterns of xyloglucan, XXXG and XXGG. Plant. Physiol. 1997, 114, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lütteke, T.; O’Boyle, N.; Packer, N.H.; Stanley, P.; Toukach, P.; et al. Updates to the Symbol Nomenclature for Glycans guidelines. Glycobiology 2019, 29, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Levy, S.; York, W.S.; Stuike-Prill, R.; Meyer, B.; Staehelin, L.A. Simulations of the static and dynamic molecular conformations of xyloglucan. The role of the fucosylated side chain in surface-specific sidechain folding. Plant. J. 1991, 1, 195–215. [Google Scholar] [CrossRef]
- Picard, C.; Gruza, J.; Derouet, C.; Renard, C.M.G.C.; Mazeau, K.; Koca, J.; Imberty, A.; Du Penhoat, C.H. A conformation study of the xyloglucan oligomer, XXXG by NMR spectroscopy and molecular modeling. Biopolymers 2000, 54, 11–26. [Google Scholar] [CrossRef]
- Umemura, M.; Yuguchi, Y. Conformational folding of xyloglucan side chains in aqueous solution from molecular dynamics simulation. Carbohydr. Res. 2005, 340, 2520–2532. [Google Scholar] [CrossRef]
- Umemura, M.; Yuguchi, Y. Solvation of xyloglucan in water/alcohol systems by molecular dynamics simulation. Cellulose 2009, 16, 361–371. [Google Scholar] [CrossRef]
- Amoako, D.; Awika, J.M. Polyphenol interaction with food carbohydrates and consequences on availability of dietary glucose. Curr. Opin. Food Sci. 2016, 8, 14–18. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Smith, G.E. In vitro binding of bile acids by blueberries (Vaccinium spp.), plums (Prunus spp.), prunes (Prunus spp.), strawberries (Fragaria X ananassa), cherries (Malpighia punicifolia), cranberries (Vaccinium macrocarpon) and apples (Malus sylvestris). Food Chem. 2007, 100, 1182–1187. [Google Scholar] [CrossRef]
- Maury, C.; Sarni-Manchado, P.; Poinsaut, P.; Cheynier, V.; Moutounet, M. Influence of polysaccharides and glycerol on proanthocyanidin precipitation by protein fining agents. Food Hydrocoll. 2016, 60, 598–605. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, D.; Sanguansri, L.; Augustin, M.A. Comparison of the adsorption behaviour of catechin onto cellulose and pectin. Food Chem. 2019, 271, 733–738. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Schulz, D.L.; Kennedy, J.A. Wine polysaccharides influence tannin-protein interactions. Food Hydrocoll. 2017, 63, 571–579. [Google Scholar] [CrossRef]
- Özcan, E.; Sun, J.; Rowley, D.C.; Sela, D.A. A Human Gut Commensal Ferments Cranberry Carbohydrates To Produce Formate. Appl. Env. Microbiol. 2017, 83, e01097-17:1-16. [Google Scholar]
- O’Connor, K.; Morrissette, M.; Strandwitz, P.; Ghiglieri, M.; Caboni, M.; Liu, H.; Khoo, C.; D’Onofrio, A.; Lewis, K. Cranberry extracts promote growth of Bacteroidaceae and decrease abundance of Enterobacteriaceae in a human gut simulator model. PLoS ONE 2019, 14, e0224836:1-14. [Google Scholar]
- Sun, J.; Deering, R.W.; Peng, Z.; Najia, L.; Khoo, C.; Cohen, P.S.; Seeram, N.P.; Rowley, D.C. Pectic Oligosaccharides from Cranberry Prevent Quiescence and Persistence in the Uropathogenic Escherichia coli CFT073. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Polymer Laboratories. Operator’s Manual for PL-ELS 2100; Version 1.01, Revision August 2003, Document 6/26069B; Polymer Laboratories: Amherst, MA, USA, 2003. [Google Scholar]
- Mathews, B.T.; Higginson, P.D.; Lyons, R.; Mitchell, J.C.; Sach, N.W.; Snowden, M.J.; Taylor, M.R.; Wright, A.G. Improving quantitative measurements for the evaporative light scattering detector. Chromatographia 2004, 60, 625–633. [Google Scholar] [CrossRef]
- Goulão, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Hayashi, T.; Kaida, R. Functions of xyloglucan in plant cells. Mol. Plant. 2011, 4, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.L.; Kneeland, R.F. Cranberry Pectin Properties. Ind. Eng. Chem. 1936, 28, 372–375. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Solid state production of polygalacturonase by Lentinus edodes using fruit processing wastes. Process. Biochem. 2000, 35, 825–830. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. Lwt--Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Simonsen, J.; Zhao, Y. Fruit pomace as a source of alternative fibers and cellulose nanofiber as reinforcement agent to create molded pulp packaging boards. Compos. Part. A 2017, 99, 48–57. [Google Scholar] [CrossRef]
- Popov, S.V.; Markov, P.A.; Nikitina, I.R.; Petrishev, S.; Smirnov, V.; Ovodov, Y.S. Preventive effect of a pectic polysaccharide of the common cranberry Vaccinium oxycoccos L. on acetic acid-induced colitis in mice. World J. Gastroenterol. 2006, 12, 6646–6651. [Google Scholar] [CrossRef]
- Polle, A.Y.; Ovodova, R.G.; Chizhov, A.O.; Shashkov, A.S.; Ovodov, Y.S. Structure of tanacetan, a pectic polysaccharide from tansy Tanacetum vulgare L. Biochem. (Mosc) 2002, 67, 1371–1376. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Lim, J.; McMeans, A.R.; Shulman, R.J.; Hamaker, B.R. Evaluation of FODMAP Carbohydrates Content in Selected Foods in the United States. J. Pediatr. 2018, 199, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Meyers, S.L.; Singh, A.P.; Limburg, P.J.; Vorsa, N. Favorable Glycemic Response of Type 2 Diabetics to Low-Calorie Cranberry Juice. J. Food Sci. 2008, 73, H241–H245. [Google Scholar] [CrossRef]
- Diarra, M.S.; Block, G.; Rempe, H.; Oomah, B.D.; Harrison, J.; McCallum, J.; Boulanger, S.; Brouillette, E.; Gattuso, M.; Malouin, F. In vitro and in vivo antibacterial activities of cranberry press cake extracts alone or in combination with β-lactams against Staphylococcus aureus. Bmc Complementary Altern. Med. 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S.; Mateos, R.; Marais, J.P.; Bravo-Clemente, L.; Khoo, C.; Goya, L. Chemical characterization and chemo-protective activity of cranberry phenolic powders in a model cell culture. Response of the antioxidant defenses and regulation of signaling pathways. Food Res. Int. 2015, 71, 68–82. [Google Scholar] [CrossRef]
- Hannon, D.B.; Thompson, J.T.; Khoo, C.; Juturu, V.; Heuvel, J.P.V. Effects of cranberry extracts on gene expression in THP-1 cells. Food Sci. Nutr. 2017, 5, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Bekiares, N.; Krueger, C.G.; Meudt, J.J.; Shanmuganayagam, D.; Reed, J.D. Effect of Sweetened Dried Cranberry Consumption on Urinary Proteome and Fecal Microbiome in Healthy Human Subjects Omics J. Integr. Biol. 2018, 22, 1–9. [Google Scholar]
- Islam, M.R.; Oomah, D.B.; Diarra, M.S. Potential immunomodulatory effects of non-dialyzable materials of cranberry extract in poultry production. Poult. Sci. 2017, 96, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Weiss, E.; Shemesh, M.; Ofek, I.; Bachrach, G.; Rozen, R.; Steinberg, D. Cranberry constituents affect fructosyltransferase expression in Streptococcus mutans. Altern. Health Med. 2009, 15, 32–38. [Google Scholar]
- Tipton, D.A.; Christian, J.; Blumer, A. Effects of cran components on human immune components (NDM). Arch. Oral Biol. 2016, 68, 88–96. [Google Scholar] [CrossRef]
- Peixoto, T.C.; Moura, E.G.; De Oliveira, E.; Soares, P.N.; Guarda, D.S.; Bernardino, D.N.; Ai, X.X.; Da, S.T.; Rodrigues, V.; De Souza, G.R.; et al. Cranberry (Vaccinium macrocarpon) extract treatment improves triglyceridemia, liver cholesterol, liver steatosis, oxidative damage and corticosteronemia in rats rendered obese by high fat diet. Eur. J. Nutr. 2018, 57, 1829–1844. [Google Scholar] [CrossRef]
- Howell, A.; Souza, D.; Roller, M.; Fromentin, E. Comparison of the Anti-Adhesion Activity of Three Different Cranberry Extracts on Uropathogenic P-fimbriated Escherichia coli: A Randomized, Double-blind, Placebo Controlled, Ex Vivo, Acute Study. Nat. Prod. Comm. 2015, 10, 1215–1218. [Google Scholar] [CrossRef]
- Babar, A.; Leblanc, V.; Dudonne, S.; Desjardins, Y.; Howell, A.; Dodin, S. Standardised high dose versus low dose cranberry Proanthocyanidin extracts for the prevention of recurrent urinary tract infection in healthy women [PACCANN]: A double blind randomised controlled trial protocol. Bmc Urol. 2018, 18, 1–7. [Google Scholar]
- Peña, M.J.; Tuomivaara, S.T.; Urbanowicz, B.R.; O’Neill, M.A.; York, W.S. Methods for Structural Characterization of the Products of Cellulose- and Xyloglucan-Hydrolyzing Enzymes. Methods Enzym. 2012, 510, 121–139. [Google Scholar]
- Courts, F.L.; Wells, L.K.; Allen, S.K.; Jones, H.S. Behavior of dietary oligosaccharides in the human gastrointestinal tract. In Oligosaccharides; Schweizer, L.S., Krebs, S.J., Eds.; Nova Science: New York, NY, USA, 2014; pp. 85–113. [Google Scholar]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Olano, A.; Rastall, R.A.; Moreno, F.J. In vitro Digestibility of Dietary Carbohydrates: Toward a Standardized Methodology Beyond Amylolytic and Microbial Enzymes. Front Nutr. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Sela, D.A.; Price, N.P.J.; Mills, D.A. Metabolism of Bifidobacteria. In Bifidobacteria, Genomics and Molecular Aspects; Mayo, B., Van Sinderen, D., Eds.; Caister Academic: Poole, UK, 2010; pp. 45–70. [Google Scholar]
- Ose, R.; Hirano, K.; Maeno, S.; Nakagawa, J.; Salminen, S.; Tochio, T.; Endo, A. The ability of human intestinal anaerobes to metabolize different oligosaccharides: Novel means for microbiota modulation? Anaerobe 2018, 51, 110–119. [Google Scholar] [CrossRef]
- Wegmann, U.; Louis, P.; Goesmann, A.; Henrissat, B.; Duncan, S.H.; Flint, H.J. Complete genome of a new Firmicutes species belonging to the dominant human colonic microbiota (‘Ruminococcus bicirculans’) reveals two chromosomes and a selective capacity to utilize plant glucans. Env. Microbiol. 2014, 16, 2879–2890. [Google Scholar] [CrossRef]
- Hemsworth, G.R.; Thompson, A.J.; Stepper, J.; Sobala, L.F.; Coyle, T.; Larsbrink, J.; Spadiut, O.; Goddard-Borger, E.D.; Stubbs, K.A.; Brumer, H.; et al. Structural dissection of a complex Bacteroides ovatus gene locus conferring xyloglucan metabolism in the human gut. Open Biol. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Vergara-Castañeda, H.A.; Oomah, B.D.; Loarca-Piña, G.; Pool, H.; Campos-Vega, R. Oligosaccharides: A Key for Gut Health. In Oligosaccharides; Schweizer, L.S., Krebs, S.J., Eds.; Nova Science: New York, NY, USA, 2014; pp. 1–48. [Google Scholar]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Tokunaga, M.; Wakamatsu, E.; Yosizawa, Z. Excretion of glucose-containing oligosaccharides in urines of orthopedic patients. Tohoku J. Exp. Med. 1979, 128, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Parkkinen, J.; Finne, J. Isolation and characterization of novel phosphate-containing sialyloligosaccharides from normal human urine. Eur. J. Biochem. 1984, 140, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Matsuura, F.; Nunez, H.; Ledonne Jr., N.; Baltzer, B.; Sweeley, C.C. Glycerol-, Inositol-, and Reducing End Hexose-containing Oligosaccharides in Human Urine. Glycoconj. J. 1984, 1, 17–35. [Google Scholar] [CrossRef]
- Clements, P.R. Determination of Sialylated and Neutral Oligosaccharides in Urine by Mass Spectrometry. Curr. Protoc. Hum. Genet. 2012, 72, u17.10:1-14. [Google Scholar] [CrossRef]
- Xia, B.; Asif, G.; Arthur, L.; Pervaiz, M.A.; Li, X.; Liu, R.; Cummings, R.D.; He, M. Oligosaccharide Analysis in Urine by MALDI-TOF Mass Spectrometry for the Diagnosis of Lysosomal Storage Diseases. Clin. Chem. 2013, 59, 1357–1368. [Google Scholar] [CrossRef]
- Robijn, M.L.M.; Koeleman, C.A.M.; Hokke, C.H.; Deelder, A.M. Schistosoma mansoni eggs excrete specific free oligosaccharides that are detectable in the urine of the human host. Mol. Biochem. Parasitol. 2007, 151, 162–172. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S. Physiology of oligosaccharides in lactating women and breastfed infants. Short and long term effects of breast feeding on child health. In Short and Long Term Effects of Breast Feeding on Child Health; Koletzko, B., Michaelsen, K.F., Hernell, O., Eds.; Kluwer Academic: New York, NY, USA, 2000; pp. 241–250. [Google Scholar]
- Ruhaak, L.R.; Stroble, C.; Underwood, M.A.; Lebrilla, C.B. Detection of milk oligosaccharides in plasma of infants. Anal. Bioanal. Chem. 2014, 406, 5775–5784. [Google Scholar] [CrossRef]
- Dotz, V.; Rudloff, S.; Blank, D.; Lochnit, G.; Geyer, R.; Kunz, C. 13C-labeled oligosaccharides in breastfed infants’ urine: Individual-, structure- and time-dependent differences in the excretion. Glycobiology 2014, 24, 185–194. [Google Scholar] [CrossRef]
- Dotz, V.; Rudloff, S.; Meyer, C.; Lochnit, G.; Kunz, C. Metabolic fate of neutral human milk oligosaccharides in exclusively breast-fed infants. Mol. Nutr. Food Res. 2015, 59, 355–364. [Google Scholar] [CrossRef]
- Difilippo, E.; Bettonvil, M.; Willems, R.H.A.M.; Braber, S.; Fink-Gremmels, J.; Jeurink, P.V.; Schoterman, M.H.C.; Gruppen, H.; Schols, H.A. Oligosaccharides in Urine, Blood, and Feces of Piglets Fed Milk Replacer Containing Galacto-oligosaccharides. J. Agric. Food Chem. 2015, 63, 10862–10872. [Google Scholar] [CrossRef]
- Dotz, V.; Adam, R.; Lochnit, G.; Schroten, H.; Kunz, C. Neutral oligosaccharides in feces of breastfed and formula-fed infants at different ages. Glycobiology 2016, 26, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.O.; Martin, L.; Østergaard, M.V.; Rudloff, S.; Roggenbuck, M.; Nguyen, D.N.; Sangild, P.T.; Bering, S.B. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J. Nutr. Biochem. 2017, 40, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.L.; Garber, S.A.; Marcon, A.A.; Prieto, P.A. In Vitro and In Vivo Effects of Soluble, Monovalent Globotriose on Bacterial Attachment and Colonization. Antimicrob. Agents Chemother. 2005, 49, 3842–3846. [Google Scholar] [CrossRef] [PubMed]
- Barile, D.; Rastall, R.A. Human milk and related oligosaccharides as prebiotics. Curr. Opin. Biotechnol. 2013, 24, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Warren, C.D.; Buescher, C.R.; Pickering, L.K.; Newburg, D.S. Survival of human milk oligosaccharides in the intestine of infants. Adv. Exp. Med. Biol. 2001, 501, 315–323. [Google Scholar] [PubMed]
- Zha, Y.; Punt, P.J. Exometabolomics Approaches in Studying the Application of Lignocellulosic Biomass as Fermentation Feedstock. Metabolites 2013, 3, 119–143. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076:1-28. [Google Scholar] [CrossRef]
- Yu, B.; Zanetti, K.A.; Temprosa, M.; Albanes, D.; Appel, N.; Barrera, C.B.; Ben-Shlomo, Y.; Boerwinkle, E.; Casas, J.P.; Clish, C.; et al. The Consortium of Metabolomics Studies (COMETS): Metabolomics in 47 Prospective Cohort Studies. Am. J. Epidemiol. 2019, 188, 991–1012. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Jane, M.; McKay, J.; Sebely, P. Effects of daily consumption of psyllium, oat bran and polyGlycopleX on obesity-related disease risk factors: A critical review. Nutrition 2019, 57, 84–91. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Manderson, K.; Wells, A.; Hotchkiss, A.T., Jr.; Gibson, G.R.; Formentin, K.; Beer, M.; Rastall, R.A. Oligosaccharide-Mediated Inhibition of the Adhesion of Pathogenic Escherichia coli Strains to Human Gut Epithelial Cells In Vitro. J. Food Prot. 2008, 71, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, J. Food derived anti-adhesive components against bacterial adhesion: Current progresses and future perspectives. Trends Food Sci. Technol. 2017, 69, 148–156. [Google Scholar] [CrossRef]
- Altamimi, M.; Abdelhay, O.; Rastall, R.A. Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe 2016, 39, 136–142. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; He, W.; Muhammad, Z.; Wang, L.; Liu, F.; Pan, S. Regulatory Roles of Pectin Oligosaccharides on Immunoglobulin Production in Healthy Mice Mediated by Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, 1–8. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Jahromi, M.F.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Wu, R.Y.; Määttänen, P.; Napper, S.; Scruten, E.; Li, B.; Koike, Y.; Johnson-Henry, K.C.; Pierro, A.; Rossi, L.; Botts, S.R.; et al. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Microbiome 2017, 5, 1–15. [Google Scholar] [CrossRef]
- Mulvey, M.A. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 2002, 4, 257–271. [Google Scholar] [CrossRef]
- Johnson, J.R. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 1991, 4, 80–128. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Pinzón-Arango, P.A.; Howell, A.B.; Camesano, T.A. Oral consumption of cranberry juice cocktail inhibits molecular-scale adhesion of clinical uropathogenic Escherichia coli. J. Med. Food 2011, 14, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Martinov, J.; Krstić, M.; Spasić, S.; Miletić, S.; Stefanović-Kojić, J.; Nikolić-Kokić, A.; Blagojević, D.; Spasojević, I.; Spasić, M.B. Apple pectin-derived oligosaccharides produce carbon dioxide radical anion in Fenton reaction and prevent growth of Escherichia coli and Staphylococcus aureus. Food Res. Int. 2017, 100, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Mou, H.; Luo, B.; Jiang, X. Inhibition of Adhesion of Intestinal Pathogens (Escherichia coli, Vibrio cholerae, Campylobacter jejuni, and Salmonella Typhimurium) by Common Oligosaccharides. Foodborne Pathog. Dis. 2015, 12, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.F.; Rahman, S.; Dykes, G.A. Pectin and Xyloglucan Influence the Attachment of Salmonella enterica and Listeria monocytogenes to Bacterial Cellulose-Derived Plant Cell Wall Models. Appl. Env. Microbiol. 2016, 82, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Black, M.A.; Caron, L.; Camesano, T.A. Role of Cranberry Juice on Molecular-Scale Surface Characteristics and Adhesion Behavior of Escherichia coli. Biotechnol. Bioeng. 2006, 93, 297–305. [Google Scholar] [CrossRef]
- Gupta, P.; Song, B.; Neto, C.; Camesano, T.A. Atomic force microscopy-guided fractionation reveals the influence of cranberry phytochemicals on adhesion of Escherichia Coli. Food Funct. 2016, 7, 2655–2666. [Google Scholar] [CrossRef]
- Conover, M.S.; Ruer, S.; Taganna, J.; Kalas, V.; De Greve, H.; Pinkner, J.S.; Dodson, K.W.; Remaut, H.; Hultgren, S.J. Inflammation-Induced Adhesin-Receptor Interaction Provides a Fitness Advantage to Uropathogenic E. coli during Chronic Infection. Cell Host Microbe 2016, 20, 482–492. [Google Scholar] [CrossRef]
- Evans, D.G.; Evans, D.J.; Tjoa, W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: Correlation with colonization factor. Infect. Immunol 1977, 18, 330–337. 207. Strӧmberg, N.; Nyholm, P.G.; Pascher, I.; Normark, S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc. Natl. Acad. Sci. USA 1991, 88, 9340–9344. [Google Scholar]
- Schmidt, D.R.; Sobota, A.E. An examination of the anti-adherence activity of cranberry juice on urinary and nonurinary bacterial isolates. Microbios 1988, 55, 173–181. [Google Scholar]
- Howell, A.B.; Vorsa, N.; Der Marderosian, A.; Foo, L.Y. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial cell surfaces by proanthocyanidin extracts from cranberries. N. Eng. J. Med. 1998, 339, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-Type Proanthocyanidin Trimers from Cranberry that Inhibit Adherence of Uropathogenic P-Fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Botto, H.; Combescure, C.; Blanc-Potard, A.B.; Gausa, L.; Matsumoto, T.; Tenke, P.; Sotto, A.; Lavigne, J.P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: A multicentric randomized double blind study. Bmc Infect. Dis. 2010, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mathison, B.D.; Kimble, L.L.; Kaspar, K.L.; Khoo, C.; Chew, B.P. Development and Validation of a Sensitive, High-Throughput Bioassay for the Adhesion of Radiolabeled E. coli to Uroepithelial Cells in Vitro. J. Nat. Prod. 2013, 76, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Kimble, L.L.; Mathison, B.D.; Kaspar, K.L.; Khoo, C.; Chew, B.P. Development of a Fluorometric Microplate Antiadhesion Assay Using Uropathogenic Escherichia coli and Human Uroepithelial Cells. J. Nat. Prod. 2014, 77, 1102–1110. [Google Scholar] [CrossRef]
- Kjelleberg, S.; Givskov, M. (Eds.) The Biofilm Mode of Life: Mechanisms and Adaptations; Horizon Bioscience: Norfolk, UK, 2007. [Google Scholar]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How biofilms evade host defenses. Microbiol. Spectr. 2015, 3, MB-0012-2014:1-10. [Google Scholar] [CrossRef]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef]
- Diaz, P. Microbial Diversity and Interactions in Subgingival Biofilm Communities. Front. Oral Biol. 2012, 15, 17–40. [Google Scholar]
- Buhmann, M.T.; Abt, D.; Nolte, O.; Neu, T.R.; Strempel, S.; Albrich, W.C.; Betschart, P.; Zumstein, V.; Neels, A.; Maniura-Weber, K.; et al. Encrustations on ureteral stents from patients without urinary tract infection reveal distinct urotypes and a low bacterial load. Microbiome 2019, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hashemizadeh, Z.; Kalantar-Neyestanaki, D.; Mansouri, S. Association between virulence profile, biofilm formation and phylogenetic groups of Escherichia coli causing urinary tract infection and the commensal gut microbiota: A comparative analysis. Microb. Pathog. 2017, 110, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A. Microbiome: Focus on causation and mechanism. Cell 2018, 174, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, J.; Koch, M.; Umek, W. The human urinary microbiome and how it relates to urogynecology. Int. Urogynecol. J. 2016, 27, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Wolfe, A.J. The female urinary microbiota, urinary health and common urinary disorders. Ann. Transl. Med. 2017, 5, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Khasriya, R.; Sathiananthamoorthy, S.; Ismail, S.; Kelsey, M.; Wilson, M.; Rohn, J.L.; Malone-Lee, J. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J. Clin. Microbiol. 2013, 51, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Brotman, R.M. Translating the vaginal microbiome: Gaps and challenges. Genome Med. 2016, 8, 1–3. [Google Scholar] [CrossRef]
- Wood, T. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Env. Microbiol. 2009, 11, 1–15. [Google Scholar] [CrossRef]
- Limoli, D.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, MB-0011-2014:1-19. [Google Scholar] [CrossRef]
- Reid, G.; Potter, P.; Lam, D.; Warren, D.; Borrie, M.; Hayes, K. Cranberry juice to reduce bladder biofilms and infection in geriatric and spinal cord injured patients with dysfunctional bladders. Nutraceut. Food 2003, 8, 24–28. [Google Scholar] [CrossRef]
- Pederson, D.B.; Dong, Y.; Blue, L.B.; Smith, S.V.; Cao, M. Water-soluble cranberry extract inhibits Vibrio cholera biofilm formation possibly through modulating the second messenger 3’,5’-cyclic diguanylate level. PLoS ONE 2018, 13, e0207056:1-20. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Duarte, S.; Murata, R.M.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010, 44, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Babu, J.; Blair, C.; Jacob, S.; Itzhak, O. Inhibition of Streptococcus gordonii Metabolic Activity in Biofilm by Cranberry Juice High-Molecular-Weight Component. J. Biomed. Biotechnol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Feldman, M.; Ofek, I.; Weiss, E.L. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J. Antimicrob Chemother. 2004, 54, 86–89. [Google Scholar] [CrossRef]
- Bodet, C.; Chandad, F.; Grenier, D. Inhibition of host extracellular matrix destructive enzyme production and activity by a high-molecular-weight cranberry fraction. J. Periodontal Res. 2007, 42, 159–168. [Google Scholar] [CrossRef]
- Caldas, A.P.S.; Coelho, O.G.L.; Bressan, J. Cranberry antioxidant power on oxidative stress, inflammation and mitochondrial damage. Int. J. Food Prop. 2018, 21, 582–592. [Google Scholar] [CrossRef]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost 2004, 92, 1–9. [Google Scholar] [CrossRef]
- Douëllou, T.; Montel, M.C.; Sergentet, D.T. Anti-adhesive properties of bovine oligosaccharides and bovine milk fat globule membrane-associated glycoconjugates against bacterial food enteropathogens. J. Dairy Sci. 2017, 100, 3348–3359. [Google Scholar] [CrossRef]
- Adam, C.L.; Thomson, L.M.; Williams, P.A.; Ross, A.W. Soluble Fermentable Dietary Fibre (Pectin) Decreases Caloric Intake, Adiposity and Lipidaemia in High-Fat Diet-Induced Obese Rats. PLoS ONE 2015, 10, e0140392:1-4. [Google Scholar] [CrossRef]
- Chang, K.T.; Lampe, J.W.; Schwarz, Y.; Breymeyer, K.L.; Noar, K.A.; Song, X.; Neuhouser, M.L. Low Glycemic Load Experimental Diet More Satiating Than High Glycemic Load Diet. Nutr. Cancer 2012, 64, 666–673. [Google Scholar] [CrossRef]
- Genda, T.; Kondo, T.; Hino, S.; Sugiura, S.; Nishimura, N.; Morita, T. The impact of fructo-oligosaccharides on gut permeability and inflammatory responses in the cecal mucosa quite differs between rats fed semi-purified and non-purified diets. J. Nutr. Sci. Vitam. 2018, 64, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.; Knight, R.; Gordon, J.I. The human microbiome project: Exploring the microbial part of ourselves in a changing world. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Gonze, D.; Coyte, K.Z.; Lahti, L.; Faust, K. Microbial communities as dynamical systems. Curr. Opin. Microbiol. 2018, 44, 41–49. [Google Scholar] [CrossRef]
- Tripathi, A.; Marotz, C.; Gonzalez, A.; Vazquez-Baeza, Y.; Song, S.J.; Bouslimani, A.; McDonald, D.; Zhu, Q.; Sanders, J.G.; Smarr, L.; et al. Are microbiome studies ready for hypothesis driven research? Curr. Opin. Microbiol. 2018, 44, 61–69. [Google Scholar] [CrossRef]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol Hepatol 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalova-Hogenova, H.; Tuckova, L.; Mesteckyy, J.; Kolinskaz, J.; Rossmann, P.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Hrncir, T.; Frolova, L.; et al. Interaction of Mucosal Microbiota with the Innate Immune System. Scand J. Immunol. 2005, 62, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obs. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 533, 427–436. [Google Scholar] [CrossRef]
- Zuo, T.; Kamm, M.A.; Colombel, J.F.; Ng, S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol Hepatol 2018, 15, 441–452. [Google Scholar] [CrossRef]
- Hickey, R.J.; Abdo, Z.; Zhou, X.; Nemeth, K.; Hansmann, M.; Osborn, T.W.; Wang, F.; Forney, L.J. Effects of tampons and menses on the composition and diversity of vaginal microbial communities over time. Int. J. Obs. Gynaecol. 2013, 120, 695–706. [Google Scholar] [CrossRef]
- Mueller, E.R.; Wolfe, A.J.; Brubaker, L. The Female Urinary Microbiota. Curr. Opin. Urol. 2017, 27, 282–286. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; Fitzgerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine is not sterile: Use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4, UTI-0012-2012:1-31. [Google Scholar] [CrossRef] [PubMed]
- Karstens, L.; Asquith, M.; Caruso, V.; Rosenbaum, J.T.; Fair, D.; Braun, J.; Gregory, W.T.; Nardos, R.; McWeeney, S.K. Community profiling of the urinary microbiota: Methodological considerations for low microbial biomass biological samples. Nat. Rev. Urol. 2018, 15, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Tang, J. Microbiome in the urinary system-a review. Aims Microbiol. 2017, 3, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Morand, A.; Cornu, F.; Difpir, K.C.; Tsimaratos, M.; Lagier, J.C.; Raoult, D. Human bacterial repertoire of the urinary tract: A potential paradigm shift. J. Clin. Microbiol. 2019, 57, e00675-18:1-9. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.; Forster, C.S.; Hsieh, M. The role of the genitourinary microbiome in pediatric urology: A review. Curr. Urol. Rep. 2018, 19, 13. [Google Scholar] [CrossRef]
- Govender, Y.; Gabriel, I.; Minassian, V.; Fichorova, R. The current evidence on the association between the urinary microbiome and urinary incontinence in women. Front. Cell Infect. Microbiol. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Drake, M.J.; Morris, N.; Apostolidis, A.; Rahnama’i, M.S.; Marchesi, J.R. The urinary microbiome and its contribution to lower urinary tract symptoms. Neurourol. Urodyn. 2017, 36, 850–853. [Google Scholar] [CrossRef]
- Chen, Z.; Phan, M.D.; Bates, L.J.; Peters, K.M.; Mukerjee, C.; Moore, K.H.; Schembri, M.A. The urinary microbiome in patients with refractory urge incontinence and recurrent urinary tract infection. Int. Urogynecol. J. 2018, 29, 1775–1782. [Google Scholar] [CrossRef]
- Pearce, M.M.; Zilliox, M.J.; Rosenfeld, A.B.; Thomas-White, K.J.; Richter, H.E.; Nager, C.W.; Visco, A.G.; Nygaard, I.E.; Barber, M.D.; Schaffer, J.; et al. The female urinary microbiome in urgency urinary incontinence. Am. J. Obs. Gynecol. 2015, 213, e1–347.e11:1-11. [Google Scholar] [CrossRef]
- Stiverson, J.; Williams, T.; Chen, J.; Adams, S.; Hustead, D.; Price, P.; Guerrieri, J.; Deacon, J.; Yua, Z. Prebiotic Oligosaccharides: Comparative Evaluation Using In Vitro Cultures of Infants’ Fecal Microbiomes. Appl. Env. Microbiol. 2014, 80, 7388–7397. [Google Scholar] [CrossRef]
- Likotrafiti, E.; Tuohy, K.M.; Gibson, G.R.; Rastall, R.A. An in vitro study of the effect of probiotics, prebiotics and synbiotics on the elderly faecal microbiota. Anaerobe 2014, 27, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rogier, R.; Ederveen, T.H.A.; Wopereis, H.; Hartog, A.; Boekhorst, J.; Van Hijum, S.A.F.T.; Knol, J.; Garssen, J.; Walgreen, B.; Helsen, M.M.; et al. Supplementation of diet with non-digestible oligosaccharides alters the intestinal microbiota, but not arthritis development, in IL-1 receptor antagonist deficient mice. PLoS ONE 2019, 14, e0219366:1-17. [Google Scholar]
- Centanni, M.; Ferguson, S.A.; Sims, I.M.; Biswas, A.; Tannock, G.W. Bifidobacterium bifidum ATCC 15696 and Bifidobacterium breve 24b Metabolic Interaction Based on 2’-O-Fucosyl-Lactose Studied in Steady-State Cultures in a Freter-Style Chemostat. Appl. Env. Microbiol. 2019, 85, e02783:1-17. [Google Scholar] [CrossRef] [PubMed]
- Kjølbæk, L.; Benítez-Páez, A.; Gómez del Pulgar, E.M.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H.; et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrients 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Poeker, S.A.; Geirnaert, A.; Berchtold, L.; Greppi, A.; Krych, L.; Steinert, R.E.; De Wouters, T.; Lacroix, C. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS). Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Moraïs, S.; David, Y.B.; Bensoussan, L.; Duncan, S.H.; Koropatkin, N.M.; Martens, E.C.; Flint, H.J.; Bayer, E.A. Enzymatic profiling of cellulosomal enzymes from the human gut bacterium, Ruminococcus champanellensis, reveals a fine-tuned system for cohesin-dockerin recognition. Env. Microbiol. 2016, 18, 542–556. [Google Scholar] [CrossRef]
- Lam, K.L.; Keung, H.Y.; Ko, K.C.; Kwan, H.S.; Cheung, P.C.K. In vitro fermentation of beta-glucans and other selected carbohydrates by infant fecal inoculum: An evaluation of their potential as prebiotics in infant formula. Bioact. Carbohydr. Diet Fibre 2018, 14, 20–24. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Van Den Broek, T.; Schuren, F.H.J.; Steinert, R.R.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Déjean, G.; Tauzin, A.; Bennett, S.W.; Creagh, A.L.; Brumer, H. Adaptation of Syntenic Xyloglucan Utilization Loci of Human Gut Bacteroidetes to Polysaccharide Side Chain Diversity. Appl. Env. Microbiol. 2019, 85, e01491-19:1-17. [Google Scholar]
- Wang, J.; Chen, C.; Yu, Z.; He, Y.; Yong, Q.; Newburg, D.S. Relative fermentation of oligosaccharides from human milk and plants by gut microbes. Eur. Food Res. Technol. 2017, 243, 133–146. [Google Scholar] [CrossRef]
- Bell, T.J.; Draper, S.L.; Centanni, M.; Carnachan, S.M.; Tannock, G.W.; Sims, I.M. Characterization of Polysaccharides from Feijoa Fruits (Acca sellowiana Berg.) and Their Utilization as Growth Substrates by Gut Commensal Bacteroides Species. J. Agric. Food Chem. 2018, 66, 13277–13284. [Google Scholar] [CrossRef] [PubMed]
- Tuncil, Y.E.; Nakatsu, C.H.; Kazem, A.E.; Arioglu-Tuncil, S.; Reuhs, B.; Martens, E.C.; Hamaker, B.R. Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J. Funct. Foods 2017, 32, 347–357. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Basta, P.; Trzeciak, J.; Michalska, A.; Kafkas, M.E.; Woitas-Ślubowska, D. Effects of cranberry (Vaccinum macrocarpon) supplementation on iron status and inflammatory markers in rowers. J. Int. Soc. Sports Nutr. 2017, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.V.; Ovodov, Y.S. Polypotency of the Immunomodulatory Effect of Pectins. Biochem. (Mosc) 2013, 78, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Naylor, S.; Chen, J.Y. Unraveling human complexity and disease with systems biology and personalized medicine. Pers. Med. 2010, 7, 275–289. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Jin, D.; Liu, T.; Dong, W.; Zhang, Y.; Wang, S.; Xie, R.; Wang, B.; Cao, H. Dietary feeding of freeze-dried whole cranberry inhibits intestinal tumor development in Apcmin/+ mice. Oncotarget 2017, 8, 97787–97800. [Google Scholar] [CrossRef]
- Hugenholtz, F.; De Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell Mol. Life. Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- Brahma, S.; Walter, I.M.J.; Clarke, J.; Gonzalez, T.; Menon, R.; Rose, D.J. Impact of dietary pattern of the fecal donor on in vitro fermentation properties of whole grains and brans. J. Funct. Foods 2017, 29, 281–289. [Google Scholar] [CrossRef]
- Bolca, S.; Van De Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gibson, G.R.; Costabile, A.; Sailer, M.; Theis, S.; Rastall, R.A. Prebiotic supplementation of in vitro fecal fermentations inhibits proteolysis by gut bacteria, and host diet shapes gut bacterial metabolism and response to intervention. Appl. Env. Microbiol. 2019, 85, e02749-18:1-14. [Google Scholar] [CrossRef] [PubMed]
- Telle-Hansen, V.H.; Holven, K.B.; Ulven, S.M. Impact of a Healthy Dietary Pattern on Gut Microbiota and Systemic Inflammation in Humans. Nutrients 2018, 10, 1783. [Google Scholar] [CrossRef]
- Xue, B.; Xie, J.; Huang, J.; Chen, L.; Gao, L.; Ou, S.; Wang, Y.; Peng, X. Plant polyphenols alter a pathway of energy metabolism by inhibiting fecal Bacteroidetes and Firmicutes in vitro. Food Funct. 2016, 7, 1501–1507. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Barroso, E.; Van De Wiele, T.; Jiménez-Girón, A.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Bartolomé, B. Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chem. 2015, 183, 273–282. [Google Scholar]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Pierre, J.F.; Heneghan, A.F.; Feliciano, R.P.; Shanmuganayagam, D.; Roenneburg, D.A.; Krueger, C.G.; Reed, J.D.; Kudsk, K.A. Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition. JpenJ. Parenter. Enter. Nutr. 2013, 37, 401–409. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Xue, B.; Liu, Q.; Ou, S.; Wang, Y.; Peng, X. Different Flavonoids Can Shape Unique Gut Microbiota Profile In Vitro. J. Food Sci. 2016, 81, H2273–H2279. [Google Scholar] [CrossRef]
- Jensen, H.D.; Struve, C.; Christensen, S.B.; Krogfelt, K.A. Cranberry Juice and Combinations of Its Organic Acids Are Effective against Experimental Urinary Tract Infection. Front. Microbiol. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Vrancken, G.; Gregory, A.C.; Huys, G.R.B.; Faust, K.; Raes, G. Synthetic ecology of the human gut microbiota. Nat. Rev. Microbiol. 2019, 17, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Nakazawa, H.; Takeda, O.; Kaneko, T.; Koshino, K.; Tanaka, T. Production of a mixture of antimicrobial organic acids from lactose by co-culture of Bifidobacterium longum and Propionibacterium freudenreichii. Biosci. Biotechnol. Biochem. 1998, 62, 1522–1527. [Google Scholar] [CrossRef]
- De Llano, D.G.; Liu, H.; Khoo, C.; Moreno-Arribas, M.V.; Bartolomeé, B. Some New Findings Regarding the Antiadhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Bacteria. J. Agric. Food Chem. 2019, 67, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, S.; Liu, F.; Zhang, P.; Muhammad, Z.; Pan, S. Role of the Gut Microbiota and Their Metabolites in Modulating the Cholesterol-Lowering Effects of Citrus Pectin Oligosaccharides in C57BL/6 Mice. J. Agric. Food Chem. 2019, 67, 11922–11930. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Chang, E.B. Interactions between diet, bile acid metabolism, gut microbiota, and Inflammatory Bowel Diseases. Dig. Dis 2015, 33, 351–356. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Brubaker, L. “Sterile urine” and the presence of bacteria. Eur Urol 2015, 68, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Wolfe, A.J. New world of urinary microbiome in women. Am. J. Obs. Gynecol 2015, 213, 644–649. [Google Scholar] [CrossRef]
- Alteri, C.J.; Mobley, H.L.T. Metabolism and fitness of urinary tract pathogens. Microbiol Spectr. 2015, 3, MBP-0016-2015:1-12. [Google Scholar] [CrossRef]
- Das, Q.; Lepp, D.; Yin, X.; Ross, K.; McCallum, J.L.; Warriner, K.; Marcone, M.F.; Diarra, M.S. Transcriptional profiling of Salmonella enterica serovar Enteritidis exposed to ethanolic extract of organic cranberry pomace. PLoS ONE 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.Y.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Hansson, G. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective Effects of Xyloglucan in Association with the Polysaccharide Gelose in an Experimental Model of Gastroenteritis and Urinary Tract Infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef] [PubMed]
- De Servi, B.; Ranzini, F.; Pique, N. Effect of Utipro® (containing gelatinxyloglucan) against Escherichia coli invasion of intestinal epithelial cells: Results of an in vitro study. Future Microbiol. 2016, 11, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Koradia, P.; Kapadia, S.; Trivedi, Y.; Chanchu, G.; Harper, A. Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: A controlled pilot study. Expert Rev. Anti-Infect. 2019, 17, 733–740. [Google Scholar] [CrossRef]
- Gill, C.M.; Hughes, M.S.A.; LaPlante, K.L. A Review of Nonantibiotic Agents to Prevent Urinary Tract Infections in Older Women. J. Am. Med. Dir. Assoc. 2020, 21, 46–54. [Google Scholar] [CrossRef]
- Peron, G.; Sut, S.; Pellizzaro, A.; Brun, P.; Voinovich, D.; Castagliuolo, I.; Dall’Acqua, S. The antiadhesive activity of cranberry phytocomplex studied by metabolomics: Intestinal PAC-A metabolites but not intact PAC-A are identified as markers in active urines against uropathogenic Escherichia Coli. Fitoterapia 2017, 122, 67–75. [Google Scholar] [CrossRef]
- Scharf, B.; Sendker, J.; Dobrindt, U.; Hensel, A. Influence of cranberry extract on Tamm-Horsfall protein in human urine and its antiadhesive activity against uropathogenic Escherichia coli. Planta Med. 2019, 85, 126–138. [Google Scholar] [CrossRef]
- Tokonami, N.; Takata, T.; Beyeler, J.; Ehrbar, I.; Yoshifuji, A.; Christensen, E.I.; Loffing, J.; Devuyst, O.; Olinger, E.G. Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int. 2018, 94, 701–715. [Google Scholar] [CrossRef]
- Garimella, P.S.; Sarnak, M.J. Uromodulin in kidney health and disease. Curr. Opin. Nephrol. Hypertens 2017, 26, 136–142. [Google Scholar] [CrossRef]
- Sappal, S.; Goetz, L.L.; Vince, R.; Klausner, A.P. Randomized trial of concentrated proanthocyanidins (PAC) for acute reduction of bacteriuria in male veterans with spinal cord injury utilizing clean intermittent catheterization. Spinal Cord Ser. Cases 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Gunnarsson, A.K.; Gunningberg, L.; Larsson, S.; Jonsson, K.B. Cranberry juice concentrate does not significantly decrease the incidence of acquired bacteriuria in female hip fracture patients receiving urine catheter: A double-blind randomized trial. Clin. Interv. Aging 2017, 12, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Thomas-White, K.J.; Gao, X.; Lin, H.; Fok, C.S.; Ghanayem, K.; Mueller, E.R.; Dong, Q.; Brubaker, L.; Wolfe, A.J. Urinary microbes and postoperative urinary tract infection risk in urogynecologic surgical patients. Int. Urogynecol. J. 2018, 29, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Tachon, S. Environmental factors influencing the efficacy of probiotic bacteria. Curr. Opin. Biotechnol. 2013, 24, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, S.; Malik, R.D.; Zimmern, P.E. Financial burden of recurrent urinary tract infections in women: A time-driven activity-based cost analysis. Urology 2019, 128, 47–54. [Google Scholar] [CrossRef] [PubMed]
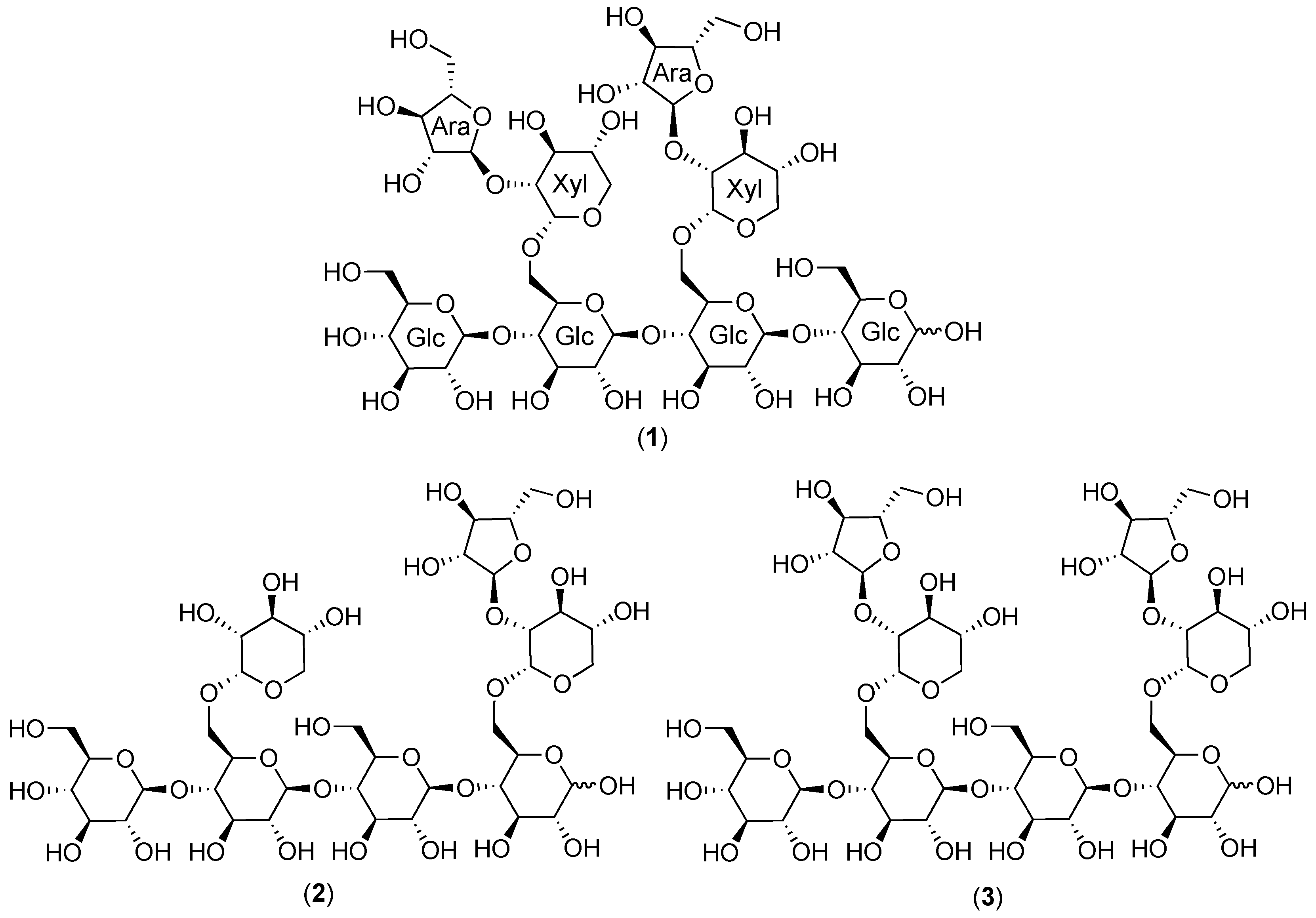
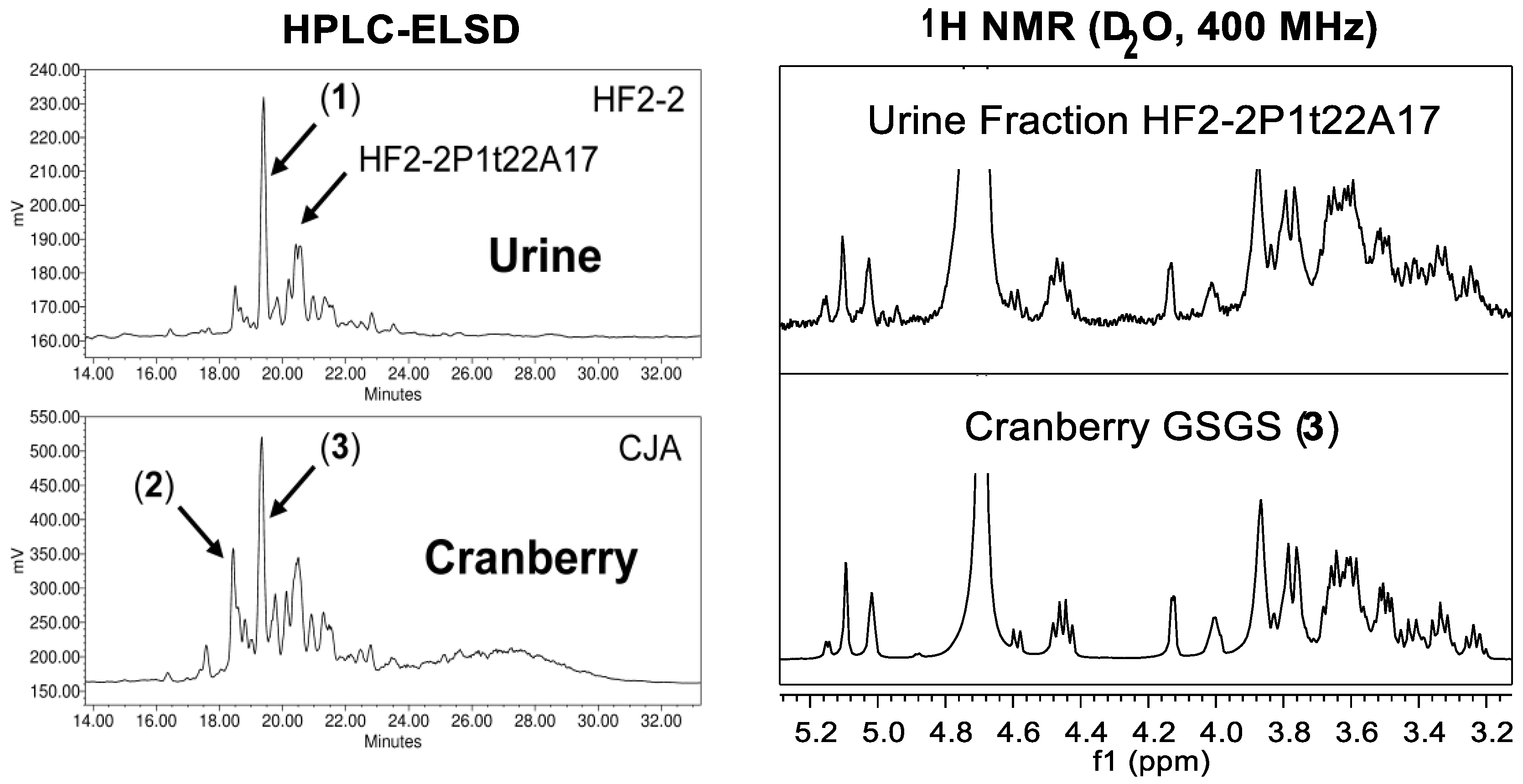



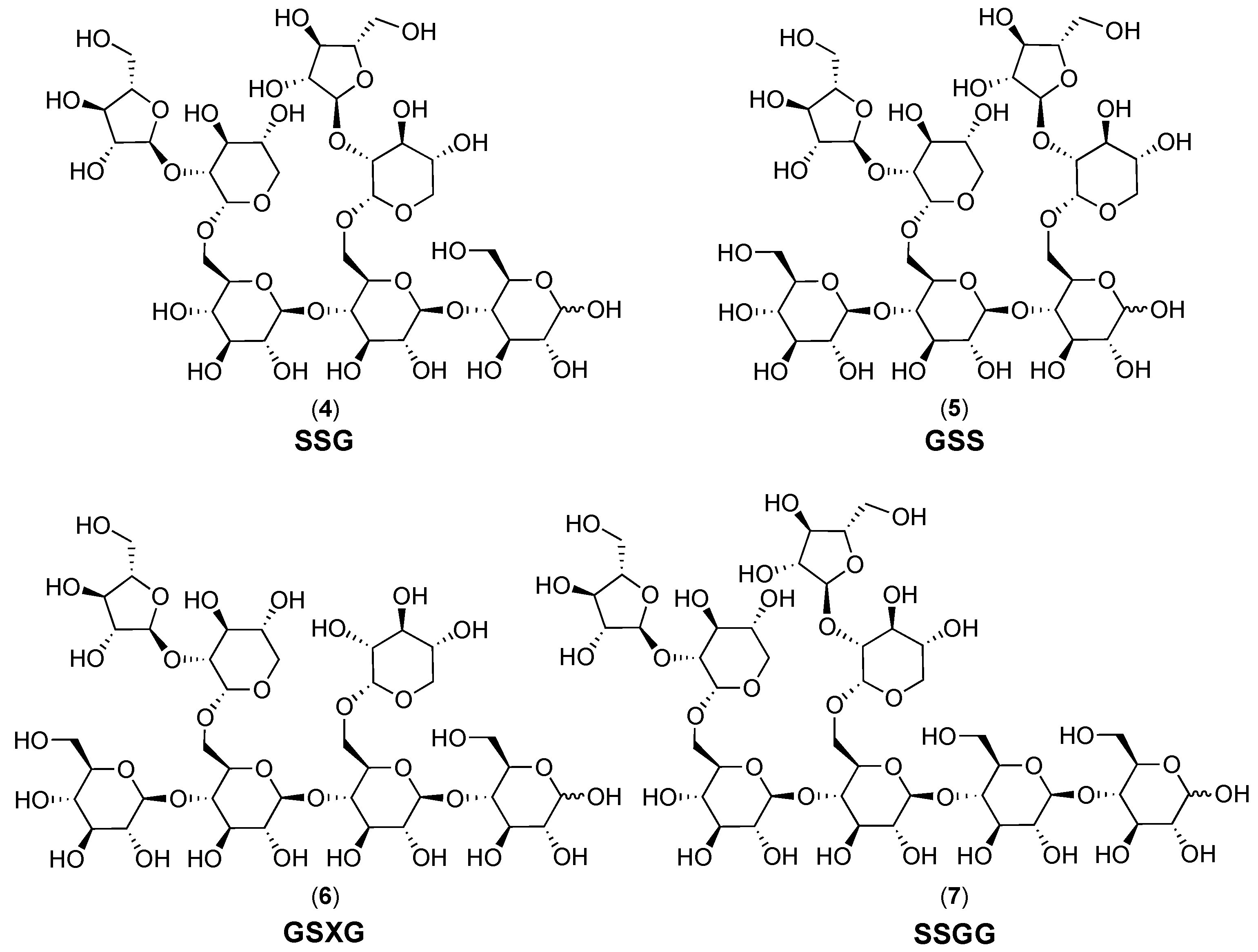





| Description | Abbreviation |
|---|---|
| Cranberry Hull Extract Powder (“Juice” Powder) | CHEP |
| Cranberry Juice (fresh pressed or from CJS, no additives) | CJ |
| Cranberry Juice Blend (with added fruit juices) – from CJS | CJB |
| Cranberry Juice Cocktail (with added sugar) – from CJS | CJK |
| Cranberry Juice Syrup/Concentrated Juice Syrup | CJS |
| Cranberry Pomace (fresh pressed or frozen) | CP |
| Cranberry Sauce (Whole: WCS/Jellied: JCS) | CS |
| Dried Cranberry Pomace – from CP) | DCP |
| Fresh Whole Berries | FWB |
| Sweetened Dried Cranberries – from FWB | SDC |
| Whole Berry Powder – from FWB | WBP |
| Starting Material | Study | Oligosaccharide Fractions | Chemical and Spectroscopic Analyses | Bioassay Testing |
|---|---|---|---|---|
| CHEP | [24,25,26,27,28] | CJA1, CJA1-02, CJA1-02B, CJA1-03B, CJA2, CJA2-02, CJA2-03, CJA2-04, CJA2-05 Pure compounds 2 and 3 | NMR (1D, 2D) PDAD, ELSD Glycosyl Composition Glycosyl Linkage Glycosyl Configuration | Bacterial HRBC Anti-Agglutination Bacterial Anti-Adhesion Microbial Growth Inhibition |
| WBP | [27,28] | SNA, SNA-02, SNA-03, SNA-04 Pure compounds 2 and 3 | NMR (1D, 2D) PDAD, ELSD Glycosyl Composition Glycosyl Linkage Glycosyl Configuration | Bacterial Anti-Adhesion Cell Viability/Cytotoxicity |
| CJS | [24,29] | CCA1, CCA1-01, CCA1-02, CCA1-03, CCA2, CCA2-01, CCA2-02, CCA2-03, CCA2-04, CCA2-05 | 1H-NMR PDAD, ELSD | Bacterial Anti-Adhesion |
| Study | Oligosaccharides Fractions | Separation Sorbent: Elution Solvent | Detection/Fraction Partitioning Basis | Chemical and Spectroscopic Analyses |
|---|---|---|---|---|
| [72,112] | A1 (CHEP) A2 A6 | SNAP KP-C18-HS: water, 15% MeOH, MeOH Sephadex LH-20: water CarboPrep 90 (PGC): 30% MeCN/0.1% TFA 2x TSK GMPWXL: 0.05 M NaNO3/0.01% NaN3 | volume/solvent/time MALLS-DPV-RI (TSK gel) | NMR (1D, 2D); UV (230 & 280 nm) Neutral sugar content Uronic acid content Degree of methyl esterificationDegree of acetylation Glycosyl Composition MALDI-TOF/TOF MS/MS (CID) |
| [114] | Cranf1 (CHEP) Cranf1b Cranf1b-F1 Cranf1b-F2 | SNAP KP-C18-HS: water, 15% MeOH, MeOH Sepharose Q XL 16/10: 0.1 M NaCl Bio-gel P2: water TSK gel G3000PW: water | Phenol sulfuric acid assay [138] for total carb content Refractive Index (TSK gel) | HP-SEC-RI for MW Glycosyl Composition Glycosyl Linkage NMR (1D, 2D); MALDI-TOF-MS |
| [115] | Cranf1 (CHEP) Cranf1b Cranf1bA Cranf1b-CL | RediSep GOLD C18: water, 15% MeOH, MeOH Hypersep Hypercarbon (PGC) SPE: 30% MeCN/0.1% TFA C18 RP SPE (not specified): water | volume/solvent/time | Glycosyl Composition 1H-NMR MALDI-TOF-MS |
| [131] | Cranf1 (CHEP) Cranf1b Cranf1b-XG | same methods as [115] to obtain Cranf1b Sephacryl S-100 HR 16/60: water | Phenol sulfuric acid assay [138] for total carb content | 1H-NMR MALDI-TOF MS |
| [133] | Cranf1 (CHEP) Cranf1b Cranf1bA Cranf1bS-cPOSt Cranf1b-cPOSHDP-cPOS uG3m2 uG4m3 (pure 8) | same methods as [115] to obtain Cranf1b Hypersep Hypercarbon (PGC) SPE: water, 10% MeCN/0.1% TFA, 30% MeCN/0.1% TFA Trituration with 95% EtOH TSK gel Amide-80 HR HILIC | volume/solvent/time ESI-MS profile (TSK gel) | LC-ESI-MS/MS HR-ESI-TOF-MS/MS UV (235 nm) NMR (1D, 2D) Glycosyl Composition Uronic Acid Composition |
| [113] | NDM (from CJS) NDMEt NDMAc NDMAc-MCI-3 | Dialysis membrane (12–14 kDa): water Dialysis membrane (12–14 kDa): 50% EtOH Sephadex LH-20: 50% EtOH, 75% Acetone MCI Gel CHP20P: 30, 50, 70, 100% MeOH | physical partitioning volume/solvent/time | Total Phenolic Content (Folin Ciocalteu) HPLC-PDAD (210–600 nm) 1H-NMRMALDI-TOF MS (DHBA) |
| Bioassay Type (organisms/cells) | Assay Description | Active Fractions | Study |
|---|---|---|---|
| Bacterial HRBC Anti-Agglutination: E. coli (clinical strains) | Inhibition of the agglutination of HRBCs by E. coli. Results based on visual agglutination scores. | CJA, CJA1-02, CJA1-02B, CJA2-02, CJA2-03, CJA2-04, CJA2-05 | [24,25,26,27,28] |
| Bacterial Anti-Adhesion: E. coli (CFT073) (radiolabeled) and T24 uroepithelial cells | Inhibition of the adhesion of E. coli to a confluent layer of epithelial cells. Results based on quantification of radioactivity | SNA, SNA-03, SNA-04 | [27,28] |
| Bacterial Anti-Adhesion: E. coli (CFT073)(fluorescence labeled) and T24 uroepithelial cells | Inhibition of the adhesion of E. coli to a confluent layer of epithelial cells. Results based on quantification of fluorescence intensity | A2, A6, CCA, CCA1-01, CCA1-02, CCA1-03, CCA2-01, CCA2-02, CCA2-03, CCA2-04, CCA2-05, | [29,72,112] |
| Bacterial Anti-Adhesion: E. coli (UTI89) (fluorescence labeled) and T24 uroepithelial cells | Inhibition of the adhesion of E. coli to a confluent layer of epithelial cells. Results based on quantification of fluorescence intensity | A2, A6 | [72,112] |
| Bacterial Anti-Adhesion: E. coli (O157:H7) HT29 colonic epithelial cells | Inhibition of the adhesion of E. coli to a confluent layer of epithelial cells. Results based on viable counts (CFU) of adhered bacteria after disruption of the cell layer. | A2, A6 | [72,112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, C.M.; Ferreira, D. Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity. Molecules 2020, 25, 881. https://doi.org/10.3390/molecules25040881
Coleman CM, Ferreira D. Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity. Molecules. 2020; 25(4):881. https://doi.org/10.3390/molecules25040881
Chicago/Turabian StyleColeman, Christina M., and Daneel Ferreira. 2020. "Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity" Molecules 25, no. 4: 881. https://doi.org/10.3390/molecules25040881
APA StyleColeman, C. M., & Ferreira, D. (2020). Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity. Molecules, 25(4), 881. https://doi.org/10.3390/molecules25040881