Inhibitory Effects of Raw-Extract Centella asiatica (RECA) on Acetylcholinesterase, Inflammations, and Oxidative Stress Activities via In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Quantitative High-Performance Liquid Chromatography (HPLC) Analysis
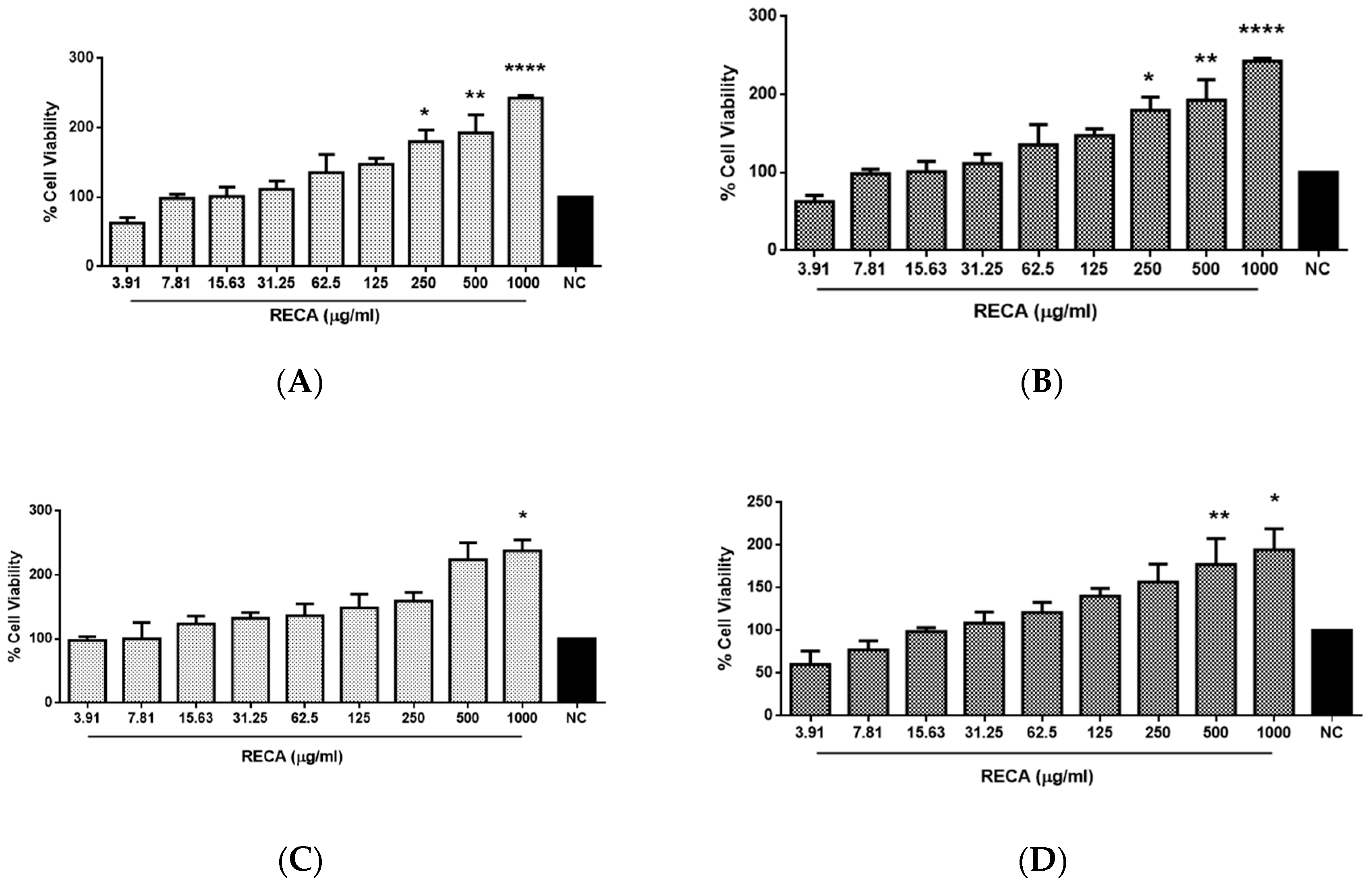
2.2. Effect of RECA on Viability of SH-SY5Y and RAW 264.7 Cells
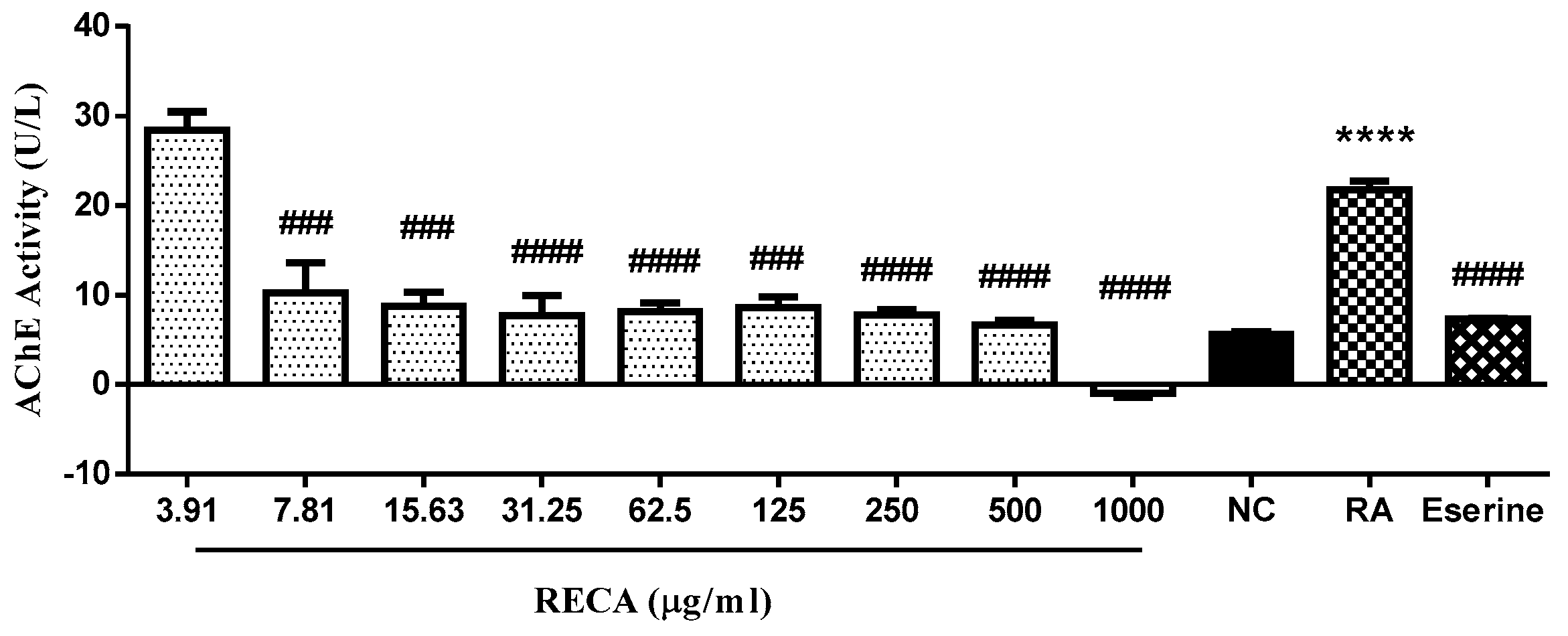
2.3. Inhibitory Effect of RECA on AChE Activity
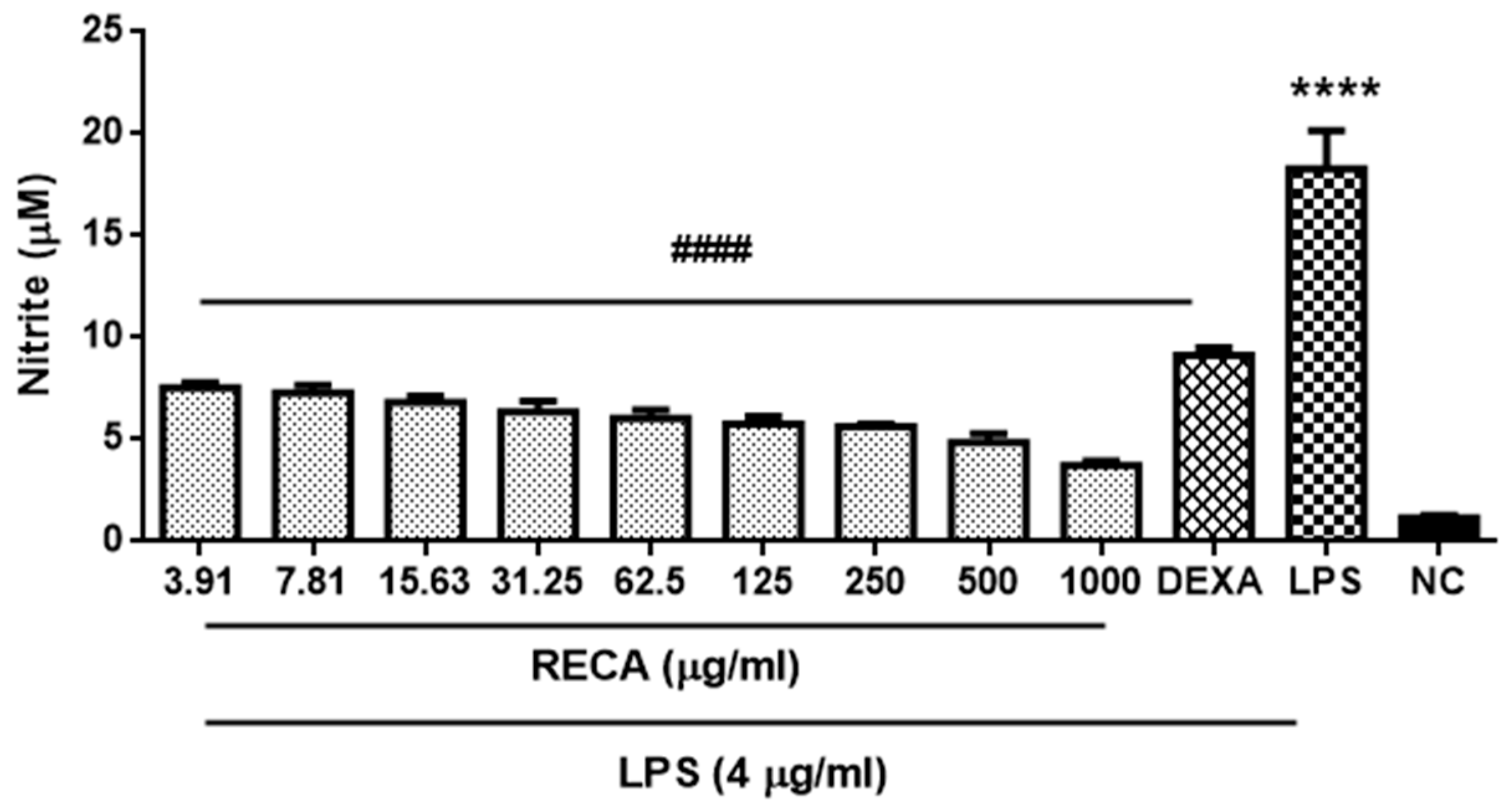
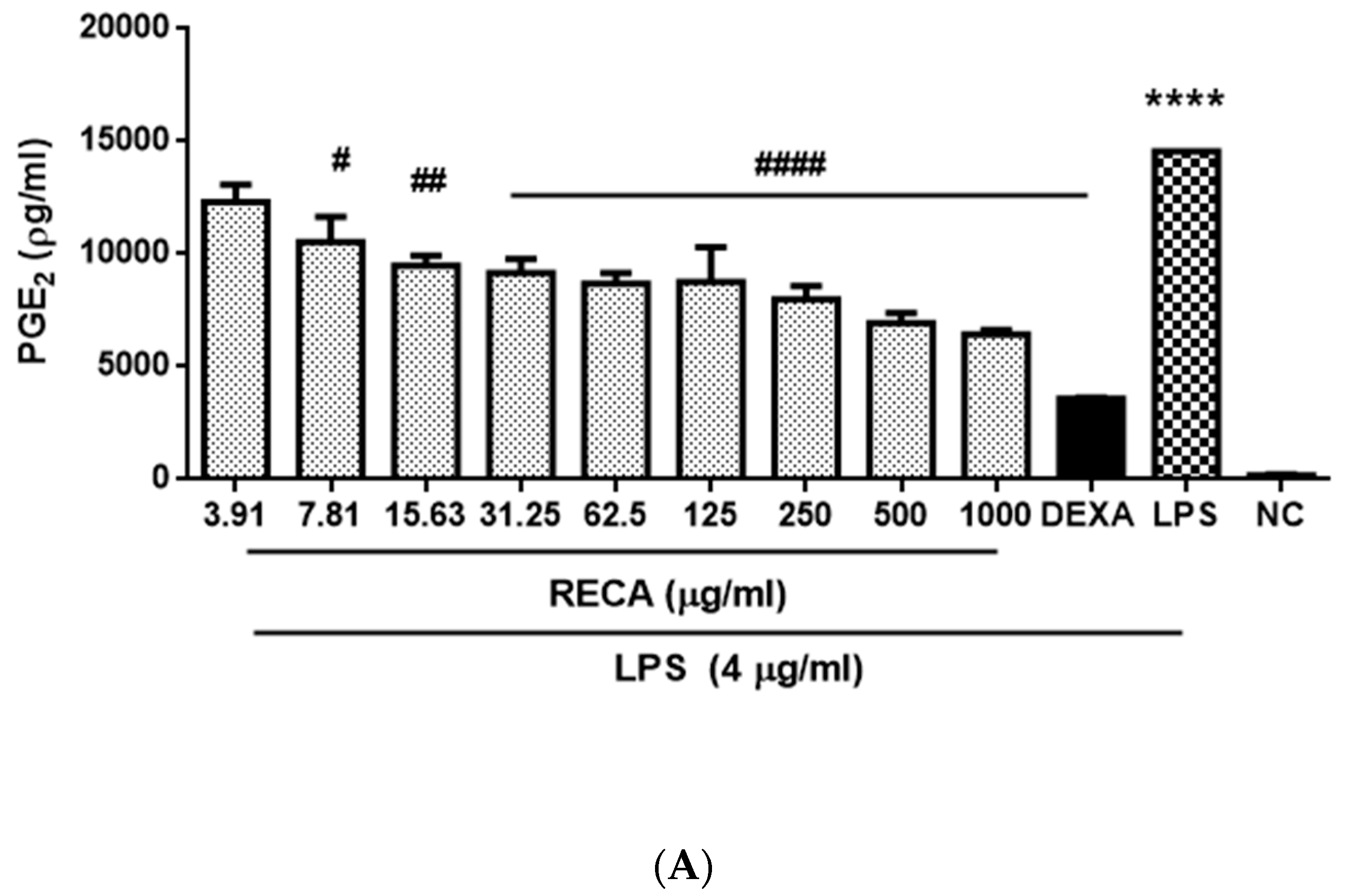
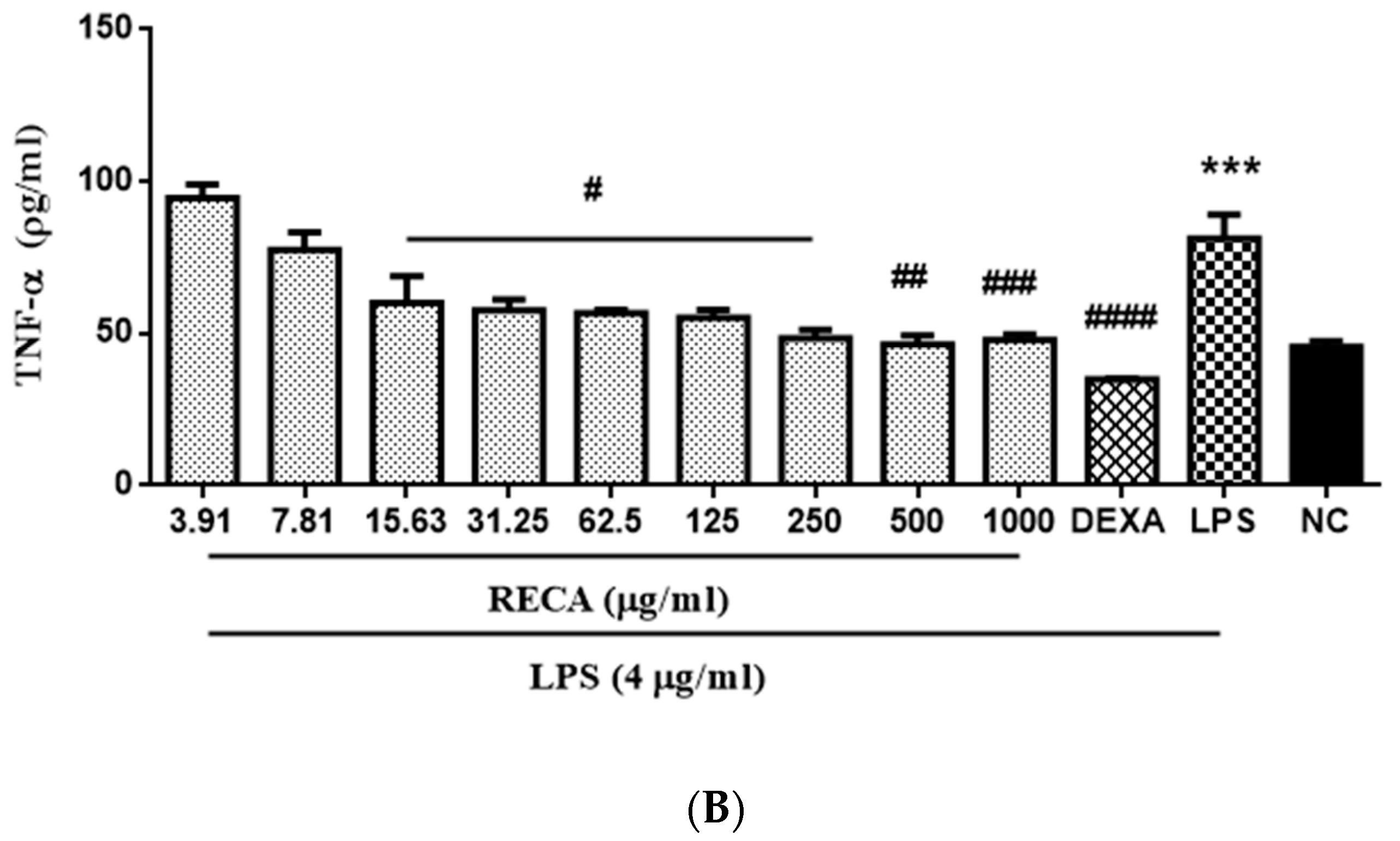
2.4. Inhibitory Effect of RECA on Anti-Inflammatory Activities
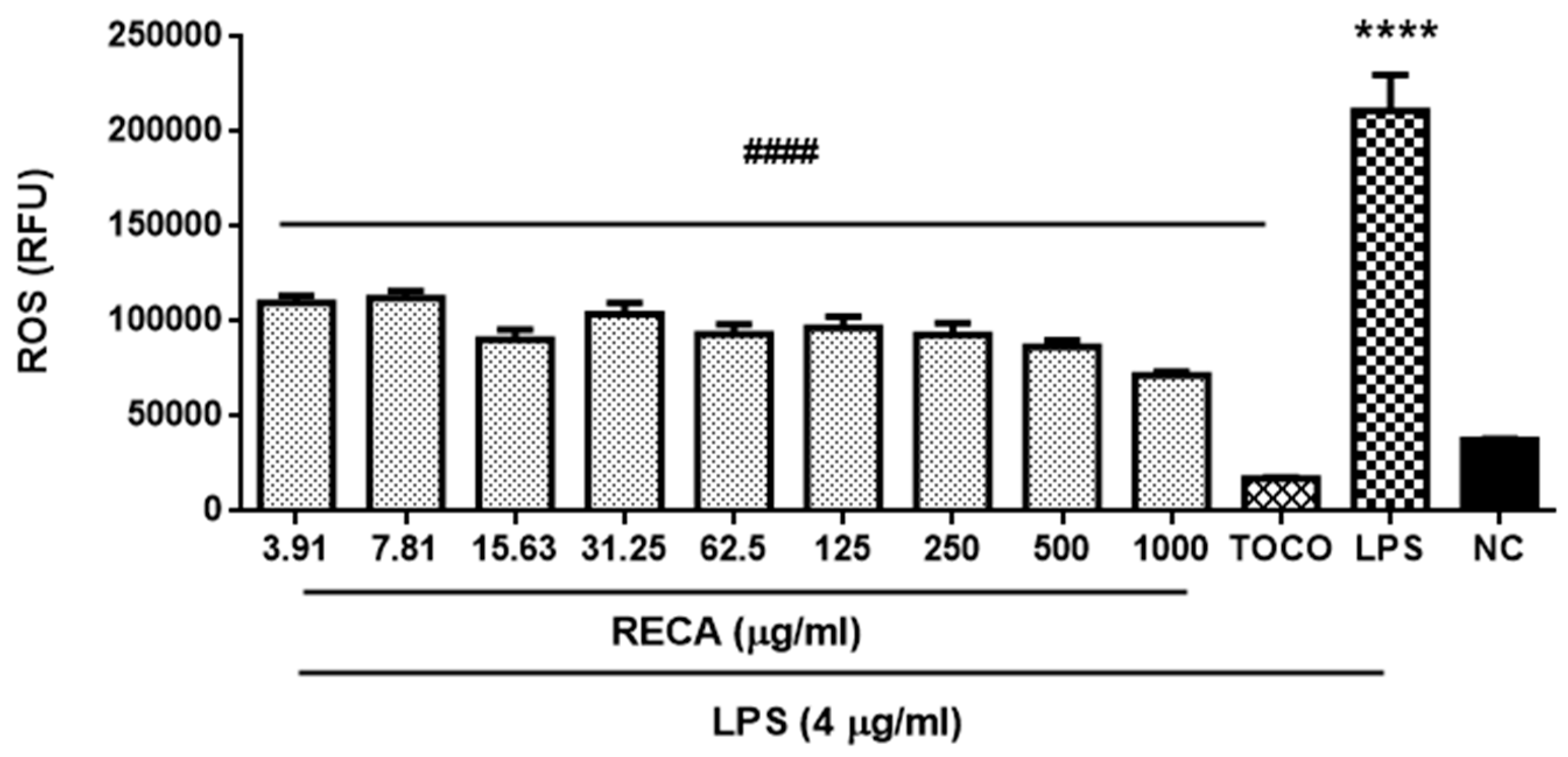
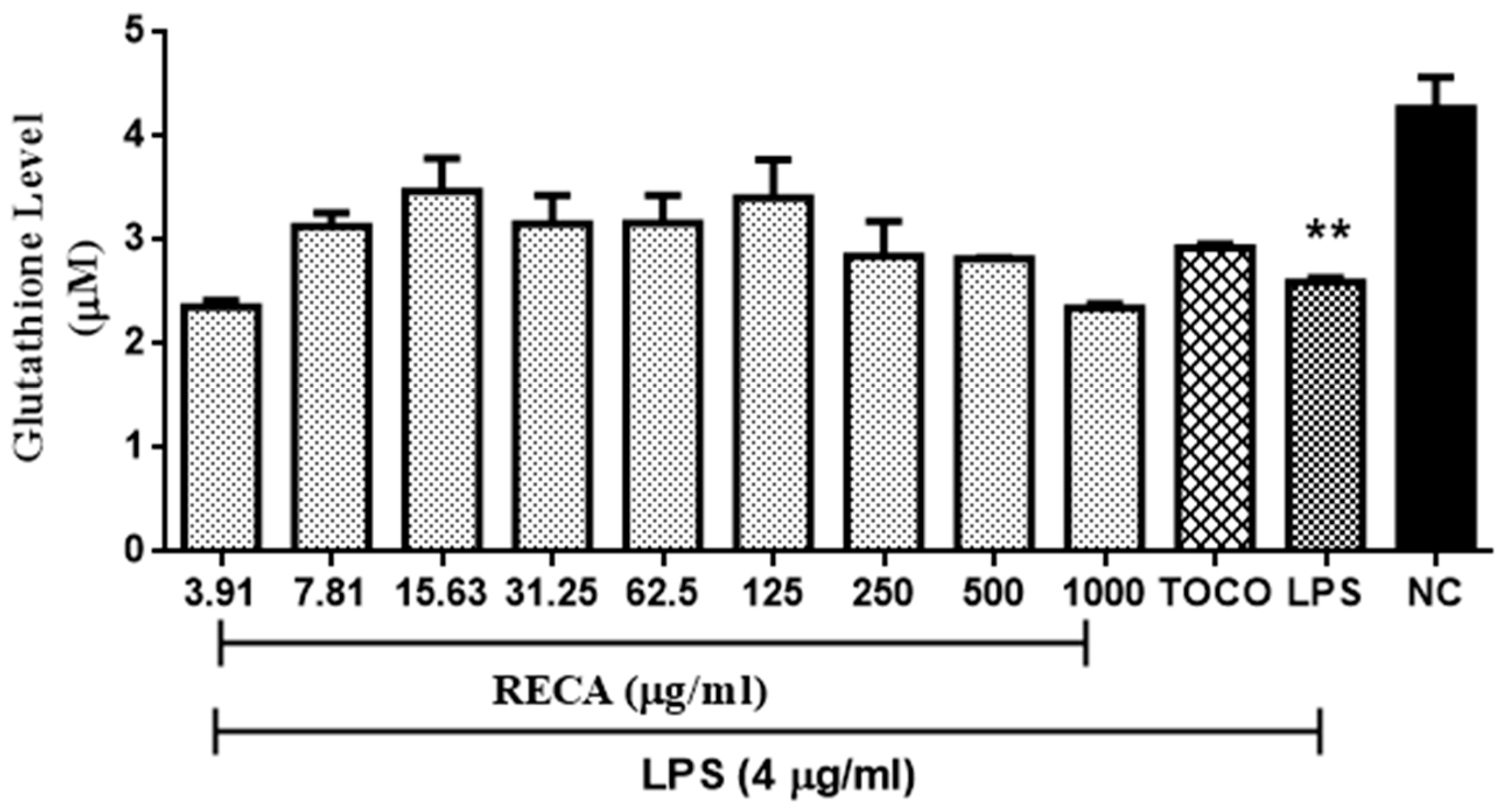
2.5. Inhibitory Effect of RECA on Antioxidant Activities
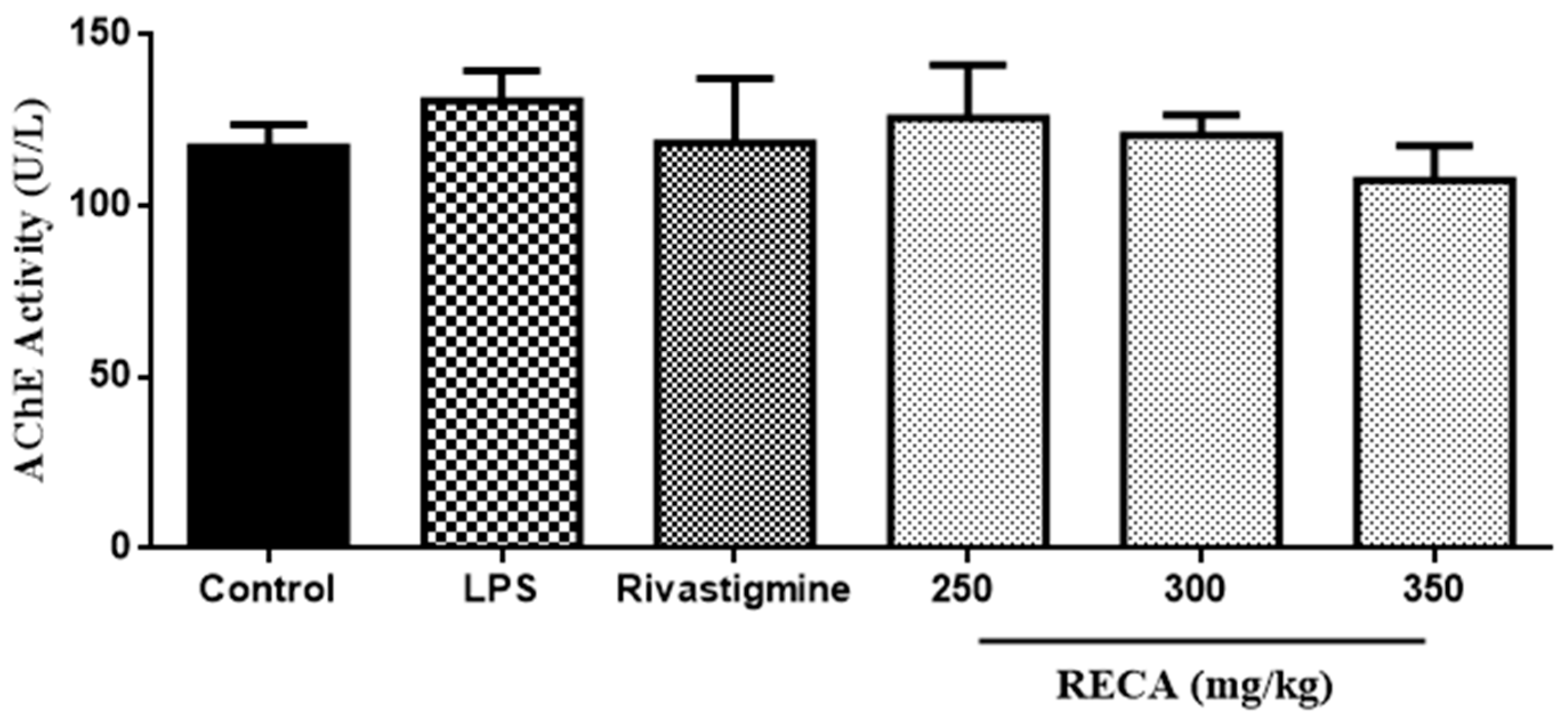
2.6. Inhibitory Effect of RECA on AChE Activity In Vivo
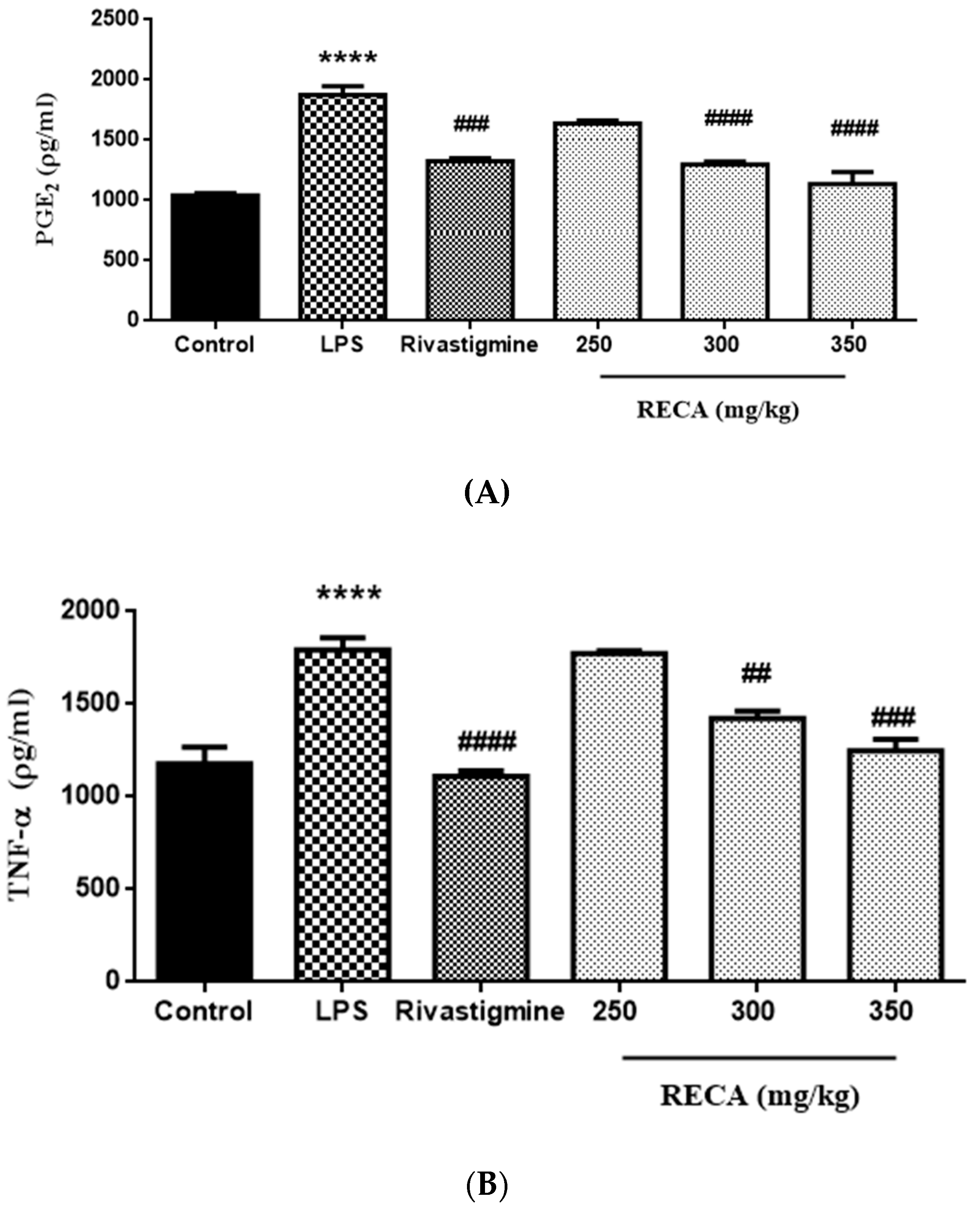
2.7. Inhibitory Effect of RECA on Anti-Inflammatory Activities In Vivo
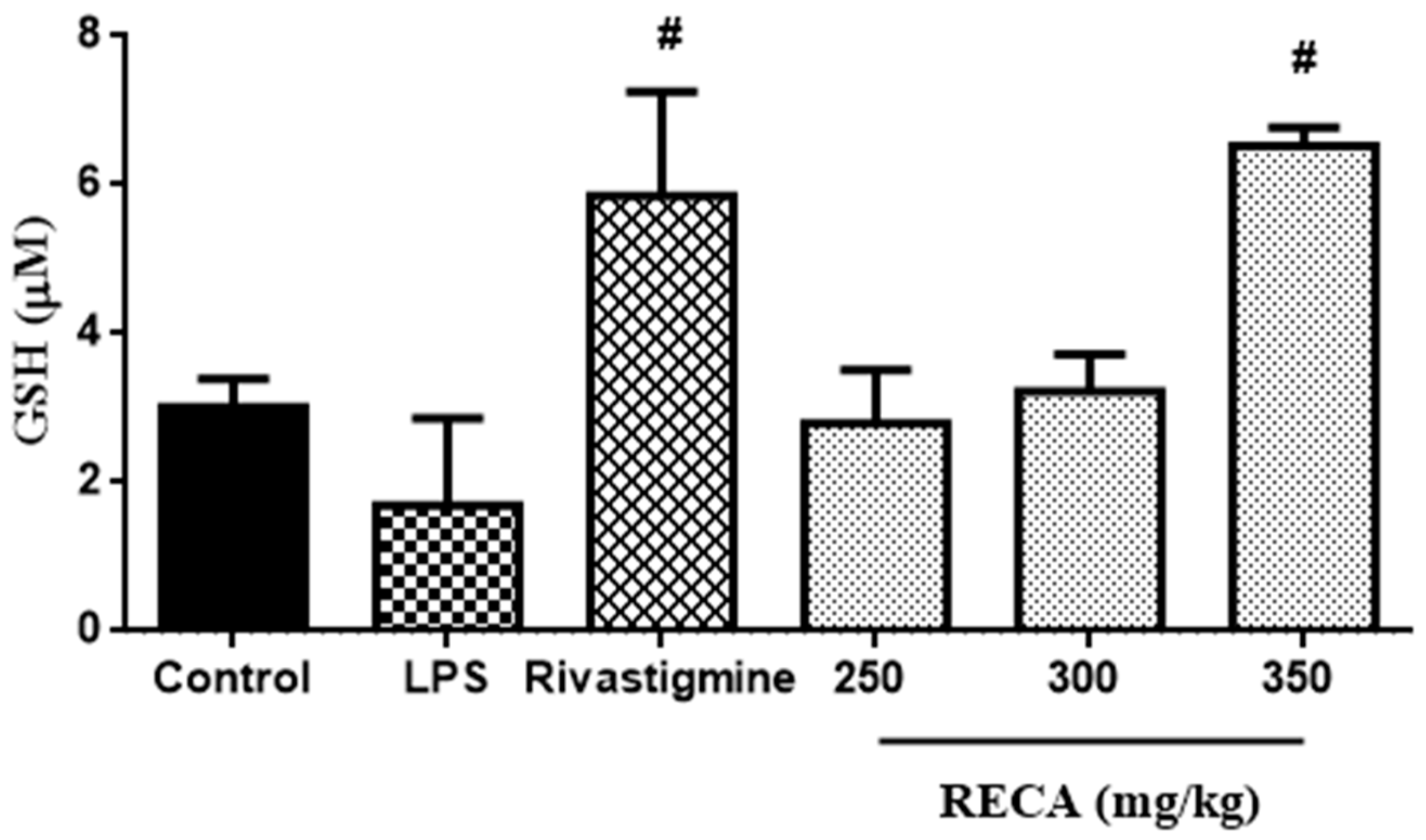
2.8. Inhibitory Effect of RECA on Antioxidant Activity In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Preparation and Extraction
4.3. Fingerprint Chromatogram Assessment
4.4. Cell Culture
4.5. Determination of Cell Viability Assay
4.6. Determination of Anti-Acetylcholinesterase Activity
4.7. Determination of Anti-Inflammatory Activities
4.7.1. Nitrite Production
4.7.2. Prostaglandin E2 (PGE2) and Tumor Necrosis Factor Alpha (TNF-α) Levels
4.8. Determination of Antioxidant Activities
4.8.1. Intracellular Reactive Oxygen Species (ROS) Production
4.8.2. Intracellular Reduced Glutathione (GSH) Level
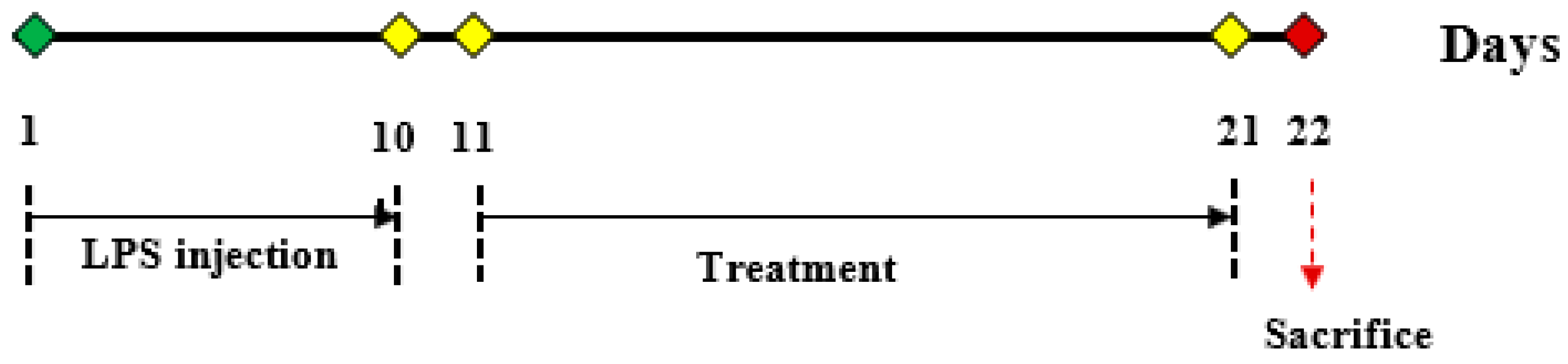
4.9. Animal Study Design
4.9.1. Vehicles
4.9.2. Biochemical Analyses
4.9.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Helzner, E.P.; Scarmeas, N.; Cosentino, S.; Tang, M.X.; Schupf, N.; Stern, Y. Survival in Alzheimer disease: A multiethnic, population-based study of incident cases. Neurology 2008, 71, 1489–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luca, M.; Luca, A.; Calandra, C. The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxid. Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Barone, E. Editorial (Thematic Issue: Oxidative Stress and Alzheimer Disease: Where Do We Stand?). Curr. Alzheimer Res. 2016, 13, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Overk, C.R.; Felder, C.C.; Tu, Y.; Schober, D.A.; Bales, K.R.; Wuu, J.; Mufson, E.J. Cortical M1 receptor concentration increases without a concomitant change in function in Alzheimer’s disease. J. Chem. Neuroanat. 2010, 40, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors and memory. Chem. Biol. Interact. 2010, 187, 403–408. [Google Scholar] [CrossRef]
- Hung, S.Y.; Fu, W.M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 47. [Google Scholar] [CrossRef]
- Rosales-Corral, S.; Tan, D.-X.; Manchester, L.; Reiter, R.J. Diabetes and Alzheimer Disease, Two Overlapping Pathologies with the Same Background: Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Huang, G.J.; Huang, S.S.; Chiu, C.S.; Chen, H.J.; Hou, W.C.; Sheu, M.J.; Lin, Y.C.; Shie, P.H. Antinociceptive activities and the mechanisms of anti-inflammation of asiatic acid in mice. Evid. Based Complement. Altern. Med. 2011, 2011, 1. [Google Scholar] [CrossRef] [Green Version]
- Osborn, G.G.; Saunders, A.V. Current treatments for patients with Alzheimer disease. J. Am. Osteopath. Assoc. 2010, 110, S16–S26. [Google Scholar]
- Cooper, E.L.; Ma, M.J. Alzheimer Disease: Clues from traditional and complementary medicine. J. Tradit. Complement. Med. 2017, 7, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of era or an endless frontier? Biomeditsinskaya Khimiya 2011, 57, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, S.K.; Yun, H.J.; Yi, H.S.; Noh, E.K.; Park, S.D. Evodiamine and rutaecarpine inhibit migration by LIGHT via suppression of NADPH oxidase activation. J. Cell. Biochem. 2009, 107, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Lee, T.F.; Chen, C.F.; Wang, L.C.H. Thermoregulatory effects of alkaloids isolated from Wu-chu-yu in afebrile and febrile rats. Pharmacol. Biochem. Behav. 1995, 50, 293–298. [Google Scholar] [CrossRef]
- Liu, A.-J.; Wang, S.-H.; Hou, S.-Y.; Lin, C.-J.; Chiu, W.-T.; Hsiao, S.-H.; Chen, T.-H.; Shih, C.-M. Evodiamine Induces Transient Receptor Potential Vanilloid-1-Mediated Protective Autophagy in U87-MG Astrocytes. Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Kudoh, C.; Arita, R.; Honda, M.; Kishi, T.; Komatsu, Y.; Asou, H.; Mimura, M. Effect of ninjin’yoeito, a Kampo (traditional Japanese) medicine, on cognitive impairment and depression in patients with Alzheimer’s disease: 2 years of observation. Psychogeriatrics 2016, 16, 85–92. [Google Scholar] [CrossRef]
- Chanana, P.; Kumar, A. Possible Involvement of Nitric Oxide Modulatory Mechanisms in the Neuroprotective Effect of Centella asiatica Against Sleep Deprivation Induced Anxiety Like Behaviour, Oxidative Damage and Neuroinflammation. Phyther. Res. 2016, 30, 671–680. [Google Scholar] [CrossRef]
- Hashim, P. Centella asiatica in food and beverage applications and its potential antioxidant and neuroprotective effect. Int. Food Res. J. 2011, 18, 1215. [Google Scholar]
- Shinomol, G.K.; Muralidhara, M.S.; Bharath, M. Exploring the Role of “Brahmi” (Bacopa monnieri and Centella asiatica) in Brain Function and Therapy. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 33–49. [Google Scholar]
- Orhan, I.E. Centella asiatica (L.) Urban: From traditional medicine to modern medicine with neuroprotective potential. Evid. Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Bangar, O.; Naithani, V. Medicinal plants with a potential to treat Alzheimer and associated symptoms. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 84. [Google Scholar] [CrossRef]
- Gohil, K.; Patel, J.; Gajjar, A. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.; Dubery, I. Identification and quantification of triterpenoid centelloids in Centella asiatica (L.) Urban by densitometric TLC. JPC. Planar Chromatogr. Mod. TLC 2011, 24, 82–87. [Google Scholar] [CrossRef]
- Won, J.H.; Shin, J.S.; Park, H.J.; Jung, H.J.; Koh, D.J.; Jo, B.G.; Lee, J.Y.; Yun, K.; Lee, K.T. Anti-inflammatory effects of madecassic acid via the suppression of NF-κB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med. 2010, 76, 251–257. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, J.Y.; Son, D.J.; Park, E.K.; Song, M.J.; Hellström, M.; Hong, J.T. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int. J. Mol. Sci. 2017, 18, 738. [Google Scholar] [CrossRef]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2018, 17, 161–194. [Google Scholar] [CrossRef]
- Edward, M.J. American Herbal Products Association’s Botanical Safety Handbook edited by M. McGuffin, C.; Hobbs, R. Upton, and A. Goldberg, CRC Press, Boca Raton, FL, 231 pages, 1997. $$39.95. J. Toxicol. Cutan. Ocul. Toxicol. 2008, 19, 167–168. [Google Scholar] [CrossRef]
- Randriamampionona, D.; Diallo, B.; Rakotoniriana, F.; Rabemanantsoa, C.; Cheuk, K.; Corbisier, A.M.; Mahillon, J.; Ratsimamanga, S.; El Jaziri, M. Comparative analysis of active constituents in Centella asiatica samples from Madagascar: Application for ex situ conservation and clonal propagation. Fitoterapia 2007, 78, 481–489. [Google Scholar] [CrossRef]
- Alqahtani, A.; Tongkao-On, W.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K.; Li, G.Q. Seasonal Variation of Triterpenes and Phenolic Compounds in Australian Centella asiatica (L.) Urb. Phytochem. Anal. 2015, 26, 436–443. [Google Scholar] [CrossRef]
- Rafi, M.; Handayani, F.; Darusman, L.K.; Rohaeti, E.; Wahyu, Y.; Sulistiyani; Honda, K.; Putri, S.P. A combination of simultaneous quantification of four triterpenes and fingerprint analysis using HPLC for rapid identification of Centella asiatica from its related plants and classification based on cultivation ages. Ind. Crops Prod. 2018, 122, 93–97. [Google Scholar] [CrossRef]
- Geran, R.I. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rept. 1972, 3, 17–27. [Google Scholar]
- Micheau, J.; Marighetto, A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behav. Brain Res. 2011, 221, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-M.; Mo, M.-S.; Xu, P.-Y. Progress in mechanisms of acetylcholinesterase inhibitors and memantine for the treatment of Alzheimer’s disease. Neuroimmunol Neuroinflammation 2015, 2, 274. [Google Scholar]
- Waldemar, G.; Dubois, B.; Emre, M.; Georges, J.; McKeith, I.G.; Rossor, M.; Scheltens, P.; Tariska, P.; Winblad, B. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur. J. Neurol. 2007, 14, e1–e26. [Google Scholar] [CrossRef] [PubMed]
- Barragán Martínez, D.; García Soldevilla, M.A.; Parra Santiago, A.; Tejeiro Martínez, J. Alzheimer’s disease. Medicine 2019, 12, 4338–4341. [Google Scholar]
- Agholme, L.; Lindström, T.; Kgedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [Green Version]
- Jämsä, A.; Hasslund, K.; Cowburn, R.F.; Bäckström, A.; Vasänge, M. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem. Biophys. Res. Commun. 2004, 319, 993–1000. [Google Scholar] [CrossRef]
- Pahlman, S.; Ruusala, A.I.; Abrahamsson, L.M.; Mattsson, M.E.; Esscher, T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: A comparison with phorbolester-induced differentiation. Cell. Differ. 1984, 14, 135–144. [Google Scholar] [CrossRef]
- Presgraves, S.P.; Ahmed, T.; Borwege, S.; Joyce, J.N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 2003, 5, 579–598. [Google Scholar] [CrossRef]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Paul, P.; Lee, S.; Qiao, L.; Josifi, E.; Tiao, J.R.; Chung, D.H. PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem. Biophys. Res. Commun. 2012, 424, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 53193. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Ahmad, I.; Shahid, M.N.; Gill, M.S.A.; Nadeem, M.F.; Mahmood, W.; Rashid, I. In vitro acetylcholinesterase and butyrylcholinesterase inhibitory potentials of Jatropha gossypifolia plant extracts. Acta Pol. Pharm. Drug Res. 2016, 73, 419–423. [Google Scholar]
- Orhan, I.E.; Atasu, E.; Senol, F.S.; Ozturk, N.; Demirci, B.; Das, K.; Sekeroglu, N. Comparative studies on Turkish and Indian Centella asiatica (L.) Urban (gotu kola) samples for their enzyme inhibitory and antioxidant effects and phytochemical characterization. Ind. Crops Prod. 2013, 47, 316–322. [Google Scholar] [CrossRef]
- Niamnuy, C.; Charoenchaitrakool, M.; Mayachiew, P.; Devahastin, S. Bioactive Compounds and Bioactivities of Centella asiatica (L.) Urban Prepared by Different Drying Methods and Conditions. Dry. Technol. 2013, 31, 2007–2015. [Google Scholar] [CrossRef]
- Cuello, A.C. Early and Late CNS Inflammation in Alzheimer’s Disease: Two Extremes of a Continuum? Trends Pharmacol. Sci. 2017, 38, 956–966. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New Roles for the Synaptic Stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Dheen, S.T.; Kaur, C.; Ling, E.-A. Microglial Activation and its Implications in the Brain Diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Dilshara, M.G.; Lee, K.T.; Lee, C.M.; Choi, Y.H.; Lee, H.J.; Choi, I.W.; Kim, G.Y. New compound, 5-O-isoferuloyl-2-deoxy-D-ribono-γ-lacton from Clematis mandshurica: Anti-inflammatory effects in lipopolysaccharide-stimulated BV2 microglial cells. Int. Immunopharmacol. 2015, 24, 14–23. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Cai, X.; Weng, Y.; Wang, Y.; Shen, Q.; Shi, X. Milk Fat Globule-Epidermal Growth Factor-Factor 8 Reverses Lipopolysaccharide-Induced Microglial Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Chun, H.S. Melatonin Attenuates Manganese and Lipopolysaccharide-Induced Inflammatory Activation of BV2 Microglia. Neurochem. Res. 2017, 42, 656–666. [Google Scholar] [CrossRef]
- Sasmita, A.O.; Ling, A.P.K.; Voon, K.G.L.; Koh, R.Y.; Wong, Y.P. Madecassoside activates anti-neuroinflammatory mechanisms by inhibiting lipopolysaccharide-induced microglial inflammation. Int. J. Mol. Med. 2018, 41, 3033–3040. [Google Scholar] [CrossRef]
- Naidoo, D.B.; Chuturgoon, A.A.; Phulukdaree, A.; Guruprasad, K.P.; Satyamoorthy, K.; Sewram, V. Withania somnifera modulates cancer cachexia associated inflammatory cytokines and cell death in leukaemic THP-1 cells and peripheral blood mononuclear cells (PBMC’s). BMC Complement. Altern. Med. 2018, 18, 126. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Peng, Z.; Li, B.; Xia, J.; Wang, Z.; Yuan, J.; Fang, L.; Lu, X. Isoflurane attenuates lipopolysaccharide-induced acute lung injury by inhibiting ROS-mediated NLRP3 inflammasome activation. Am. J. Transl. Res. 2016, 8, 2033. [Google Scholar]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, R.; Wan Yaacob, W.M.H.; Othman, Z.; Long, I.; Ahmad, A.H.; Al-Rahbi, B. Lipopolysaccharide-Induced Memory Impairment in Rats: A Model of Alzheimer’s Disease. Physiol. Res 2017, 66, 553–565. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurul Huda, M.; Vasudevan, M.; Lim, S.; Sharmili, V.; Abu Bakar, A.M.; Kalavathy, R. Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 2017, 84, 488–495. [Google Scholar]
- Lykhmus, O.; Mishra, N.; Koval, L.; Kalashnyk, O.; Gergalova, G.; Uspenska, K.; Komisarenko, S.; Soreq, H.; Skok, M. Molecular Mechanisms Regulating LPS-Induced Inflammation in the Brain. Front. Mol. Neurosci. 2016, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykhmus, O.; Voytenko, L.; Koval, L.; Mykhalskiy, S.; Kholin, V.; Peschana, K.; Zouridakis, M.; Tzartos, S.; Komisarenko, S.; Skok, M. α7 nicotinic acetylcholine receptor-specific antibody induces inflammation and amyloid β 42 accumulation in the mouse brain to impair memory. PLoS ONE 2015, 10, e0122706. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Iarlori, C.; Gambi, F.; Feliciani, C.; Salone, A.; Toma, L.; DeLuca, G.; Salvatore, M.; Conti, P.; Gambi, D. Treatment with an acetylcholinesterase inhibitor in Alzheimer patients modulates the expression and production of the pro-inflammatory and anti-inflammatory cytokines. J. Neuroimmunol. 2004, 148, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, Z.Z.; Shamsuddin, N.; Mukhtar, S.M.; James, R.J.; Adenan, M.I. Anti-Oxidant Activities of Raw-Extract Centella Asiatica (RECA) on Lipopolysaccharide (LPS)-Induced Neuroinflammation Sprague Dawley Rats. Int. J. Eng. Technol. 2018, 7, 96–101. [Google Scholar]
- Cacciatore, I.; Baldassarre, L.; Fornasari, E.; Mollica, A.; Pinnen, F. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxid. Med. Cell. Longev. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Mischley, L.K.; Lau, R.C.; Shankland, E.G.; Wilbur, T.K.; Padowski, J.M. Phase IIb study of intranasal glutathione in Parkinson’s disease. J. Parkinson’s Dis. 2017, 7, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Richie, J.P.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251–263. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Rupa, P.; Matsui, T.; Tanaka, M.; Konishi, T.; Sauchi, Y.; Sato, K.; Ono, S.; Mine, Y. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J. Agric. Food Chem. 2014, 62, 9499–9506. [Google Scholar] [CrossRef]
- Allen, J.; Bradley, R.D. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J. Altern. Complement. Med. 2011, 17, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S.L.; Lagopoulos, J.; Hickie, I.B.; Diamond, K.; Graeber, M.B.; Lewis, S.J.G.; Naismith, S.L. Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimer’s Dement. 2014, 10, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Shukla, D.; Tripathi, M.; Ersland, L. Cognitive Improvement with Glutathione Supplement in Alzheimer’s Disease: A Way Forward. J. Alzheimer’s Dis. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafamantanana, M.H.; Rozet, E.; Raoelison, G.E.; Cheuk, K.; Ratsimamanga, S.U.; Hubert, P.; Quetin-Leclercq, J. An improved HPLC-UV method for the simultaneous quantification of triterpenic glycosides and aglycones in leaves of Centella asiatica (L.) Urb (APIACEAE). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Types of Triterpenes | Retention Time (min) | Concentration of Triterpenes in RECA ± SEM (mg/g) | % of Dry Plant |
|---|---|---|---|
| Madecassoside | 9.077 | 231.44 ± 7.05 | 3.70 ± 0.11 |
| Asiaticoside | 11.393 | 223.21 ± 5.63 | 3.57 ± 0.09 |
| Madecassic acid | 22.207 | 118.95 ± 8.47 | 1.91 ± 0.14 |
| Asiatic acid | 25.417 | 108.37 ± 2.25 | 1.73 ± 0.04 |
| Time (min) | Pump A, Water (%) | Pump B, Acetonitrile (%) |
|---|---|---|
| 0 | 80 | 20 |
| 15 | 65 | 35 |
| 30 | 35 | 65 |
| 35 | 20 | 80 |
| 40 | 20 | 80 |
| 45 | 80 | 20 |
| 55 | 80 | 20 |
| Animal Group | Treatment |
|---|---|
| (i) | Saline (ip) + distilled water |
| (ii) | RECA (250 mg/kg, PO) + LPS (250 µg/kg, ip) |
| (iii) | RECA (300 mg/kg, PO) + LPS (250 µg/kg, ip) |
| (iv) | RECA (350 mg/kg, PO) + LPS (250 µg/kg, ip) |
| (v) | LPS-treated (250 µg/kg, ip) |
| (vi) | Rivastigmine (5 mg/kg, PO) + LPS (250 µg/kg, ip) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafiz, Z.Z.; Amin, M.‘A.M.; Johari James, R.M.; Teh, L.K.; Salleh, M.Z.; Adenan, M.I. Inhibitory Effects of Raw-Extract Centella asiatica (RECA) on Acetylcholinesterase, Inflammations, and Oxidative Stress Activities via In Vitro and In Vivo. Molecules 2020, 25, 892. https://doi.org/10.3390/molecules25040892
Hafiz ZZ, Amin M‘AM, Johari James RM, Teh LK, Salleh MZ, Adenan MI. Inhibitory Effects of Raw-Extract Centella asiatica (RECA) on Acetylcholinesterase, Inflammations, and Oxidative Stress Activities via In Vitro and In Vivo. Molecules. 2020; 25(4):892. https://doi.org/10.3390/molecules25040892
Chicago/Turabian StyleHafiz, Zetty Zulikha, Muhammad ‘Afif Mohd Amin, Richard Muhammad Johari James, Lay Kek Teh, Mohd Zaki Salleh, and Mohd Ilham Adenan. 2020. "Inhibitory Effects of Raw-Extract Centella asiatica (RECA) on Acetylcholinesterase, Inflammations, and Oxidative Stress Activities via In Vitro and In Vivo" Molecules 25, no. 4: 892. https://doi.org/10.3390/molecules25040892
APA StyleHafiz, Z. Z., Amin, M. ‘A. M., Johari James, R. M., Teh, L. K., Salleh, M. Z., & Adenan, M. I. (2020). Inhibitory Effects of Raw-Extract Centella asiatica (RECA) on Acetylcholinesterase, Inflammations, and Oxidative Stress Activities via In Vitro and In Vivo. Molecules, 25(4), 892. https://doi.org/10.3390/molecules25040892





