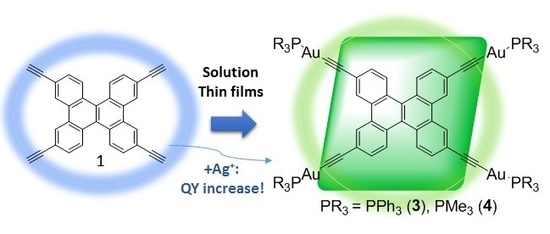
Luminescent Tetranuclear Gold(I) Dibenzo[g,p]chrysene Derivatives: Effect of the Environment on Photophysical Properties
Abstract
1. Introduction
2. Results and Discussion
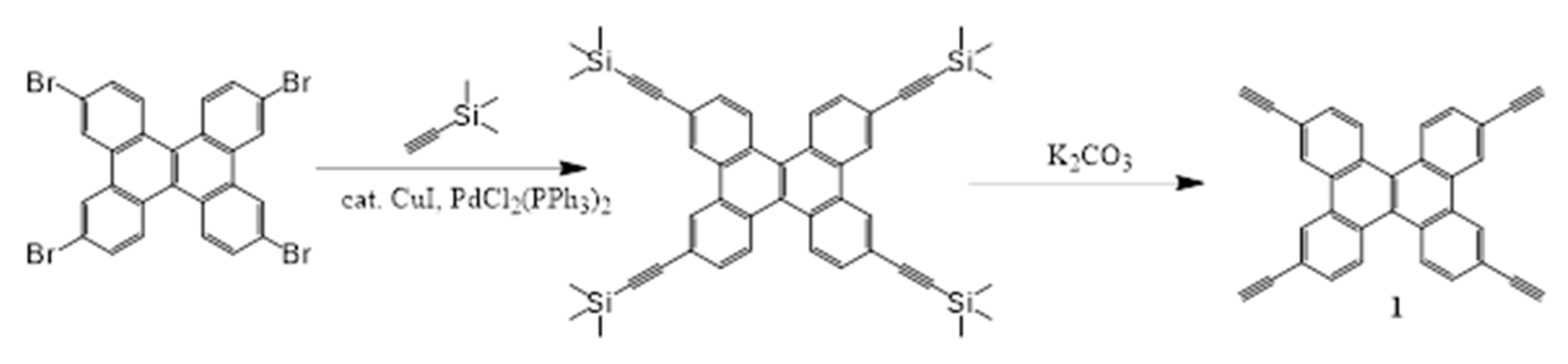
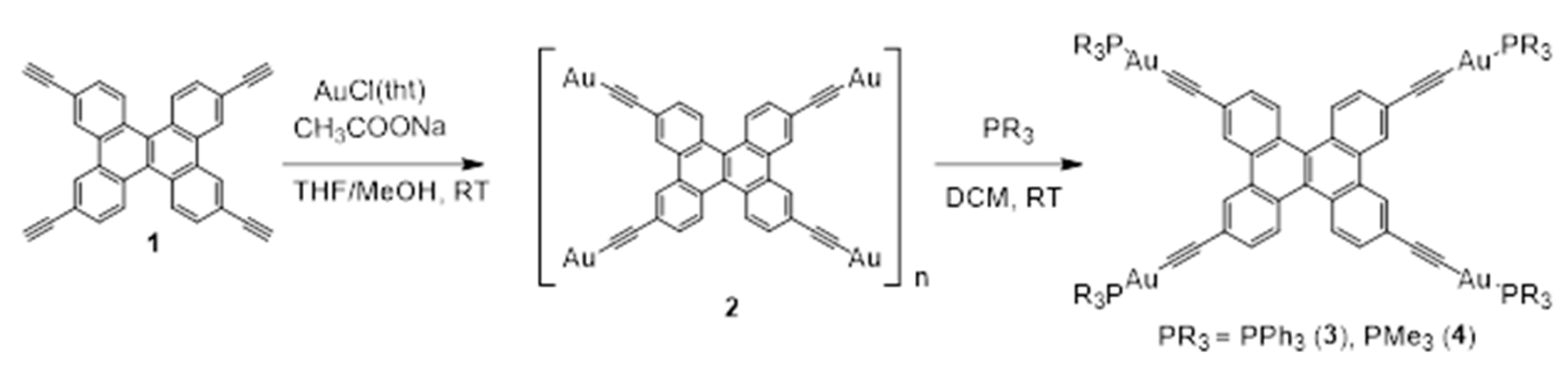
2.1. Synthesis and Characterization
2.2. Photophysical Characterization
3. Conclusions
4. Experimental Section
4.1. General Procedures
4.2. Physical Measurements
4.3. Synthesis of the Compounds
4.3.1. Synthesis of 1
4.3.2. Synthesis of 2
4.3.3. Synthesis of 3
4.3.4. Synthesis of 4
4.3.5. Preparation of Matrixes Including Compounds
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, G.; Phan, H.; Herng, T.S.; Gopalakrishna, T.Y.; Liu, C.; Zeng, W.; Ding, J.; Wu, J. Toward Stable Superbenzoquinone Diradicaloids. Angew. Chem. Int. Ed. 2017, 56, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Aratani, N.; Yamada, H. Semiconducting Self-Assembled Nanofibers Prepared from Photostable Octafluorinated Bisanthene Derivatives. Chem. A Eur. J. 2017, 23, 7000–7008. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Pfeffermann, M.; Skidin, D.; Wang, F.; Fu, Y.; Narita, A.; Tommasini, M.; Moresco, F.; Cuniberti, G.; Berger, R.; et al. Persulfurated Coronene: A New Generation of “Sulflower”. J. Am. Chem. Soc. 2017, 139, 2168–2171. [Google Scholar] [CrossRef] [PubMed]
- Tokuo, K.; Sakai, H.; Sakanoue, T.; Takenobu, T.; Araki, Y.; Wada, T.; Hasobe, T. Control of the electrochemical and photophysical properties of N-substituted benzo[ghi]perylene derivatives. Mater. Chem. Front. 2017, 1, 2299–2308. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Tang, X.; Zhao, Y.; Zhao, D.; Fan, J.; Liao, L.-S. Dibenzo[g,p]chrysene: A new platform for highly efficient red phosphorescent organic light-emitting diodes. Dyes Pigment. 2017, 146, 234–239. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Swager, T.M. Oxidative Cyclization of Bis(biaryl)acetylenes: Synthesis and Photophysics of Dibenzo[g,p]chrysene-Based Fluorescent Polymers. J. Am. Chem. Soc. 2001, 123, 12087–12088. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, Y.S.; Lee, H.W.; Jeong, S.; Kim, Y.K.; Yoon, S.S. Electroluminescent properties of diphenylamino-dibenzo[g,p]chrysene derivatives. Mol. Cryst. Liq. Cryst. 2016, 636, 99–106. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.H.; Kim, D.Y.; Kim, C.; Lee, H.W.; Lee, S.E.; Kim, Y.K.; Yoon, S.S. Blue organic light-emitting diodes based on diphenylamino dibenzo[g,p]chrysene derivatives. Thin Solid Film. 2017, 636, 8–14. [Google Scholar] [CrossRef]
- Ke, X.-S.; Hong, Y.; Tu, P.; He, Q.; Lynch, V.M.; Kim, D.; Sessler, J.L. Hetero Cu(III)–Pd(II) Complex of a Dibenzo[g,p]chrysene-Fused Bis-dicarbacorrole with Stable Organic Radical Character. J. Am. Chem. Soc. 2017, 139, 15232–15238. [Google Scholar] [CrossRef]
- Lima, J.C.; Rodríguez, L. Applications of gold(I) alkynyl systems: A growing field to explore. Chem. Soc. Rev. 2011, 40, 5442–5456. [Google Scholar] [CrossRef]
- Pujadas, M.; Rodríguez, L. Luminescent phosphine gold(I) alkynyl complexes. Highlights from 2010 to 2018. Coord. Chem. Rev. 2020, 408, 213179. [Google Scholar] [CrossRef]
- Belyaev, A.; Eskelinen, T.; Dau, T.M.; Ershova, Y.Y.; Tunik, S.P.; Melnikov, A.S.; Hirva, P.; Koshevoy, I.O. Cyanide-Assembled d 10 Coordination Polymers and Cycles: Excited State Metallophilic Modulation of Solid-State Luminescence. Chem. - A Eur. J. 2018, 24, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-H.; Wong, C.-Y.; Yip, J.H.K. Ligand Perturbations on Fluorescence of Dinuclear Platinum Complexes of 5,12-Diethynyltetracene: A Spectroscopic and Computational Study. Organometallics 2013, 32, 1620–1629. [Google Scholar] [CrossRef]
- Lentijo, S.; Aullón, G.; Miguel, J.A.; Espinet, P. Highly fluorescent complexes with gold, palladium or platinum linked to perylene through a tetrafluorophenyl group. Dalton Trans. 2013, 42, 6353. [Google Scholar] [CrossRef]
- Chan, K.T.; Tong, G.S.M.; To, W.-P.; Yang, C.; Du, L.; Phillips, D.L.; Che, C.-M. The interplay between fluorescence and phosphorescence with luminescent gold and gold complexes bearing heterocyclic arylacetylide ligands. Chem. Sci. 2017, 8, 2352–2364. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.J.; Rome, B.; Aguiló, E.; Arcau, J.; Puttreddy, R.; Rissanen, K.; Lima, J.C.; Rodríguez, L. A coumarin based gold(I)-alkynyl complex: A new class of supramolecular hydrogelators. Org. Biomol. Chem. 2015, 13, 2026–2033. [Google Scholar] [CrossRef]
- Arcau, J.; Andermark, V.; Aguiló, E.; Gandioso, A.; Moro, A.; Cetina, M.; Lima, J.C.; Rissanen, K.; Ott, I.; Rodríguez, L. Luminescent alkynyl-gold coumarin derivatives and their biological activity. Dalton Trans. 2014, 43, 4426–4436. [Google Scholar] [CrossRef]
- Ferrer, M.; Mounir, M.; Rodríguez, L.; Rossell, O.; Coco, S.; Gómez-Sal, P.; Martín, A. Effect of the organic fragment on the mesogenic properties of a series of organogold(I) isocyanide complexes. X-ray crystal structure of [Au(CCC5H4N)(CNC6H4O(O)CC6H4OC10H21)]. J. Organomet. Chem. 2005, 690, 2200–2208. [Google Scholar] [CrossRef]
- Ferrer, M.; Gutiérrez, A.; Rodríguez, L.; Rossell, O.; Lima, J.C.; Font-Bardia, M.; Solans, X. Study of the Effect of the Phosphane Bridging Chain Nature on the Structural and Photophysical Properties of a Series of Gold(I) Ethynylpyridine Complexes. Eur. J. Inorg. Chem. 2008, 2008, 2899–2909. [Google Scholar] [CrossRef]
- Makowiecki, J.; Piosik, E.; Neunert, G.; Stolarski, R.; Piecek, W.; Martynski, T. Molecular organization of perylene derivatives in Langmuir–Blodgett multilayers. Opt. Mater. (Amst). 2015, 46, 555–560. [Google Scholar] [CrossRef]
- Ito, F.; Yamamoto, K.; Kogasaka, Y.; Katoh, R. Intermolecular Dynamics of Perylene in Polymer Matrices during the Drop-Casting Process Probed by Fluorescence and Droplet Mass Changes. Langmuir 2018, 34, 8281–8287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Peng, X.; Qi, Q.; Li, Y.; Lai, W.-Y.; Huang, W. Stimuli-responsive circularly polarized luminescence from an achiral perylenyl dyad. Org. Biomol. Chem. 2017, 15, 8463–8470. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.; Rodríguez, L. Highlights on Gold TADF Complexes. Inorganics 2019, 7, 124. [Google Scholar] [CrossRef]
- Pinto, A.; Hernández, G.; Gavara, R.; Aguiló, E.; Moro, A.J.; Aullón, G.; Malfois, M.; Lima, J.C.; Rodríguez, L. Supramolecular tripodal Au(I) assemblies in water. Interactions with a pyrene fluorescent probe. New J. Chem. 2019, 43, 8279–8289. [Google Scholar] [CrossRef]
- Pinto, A.; Svahn, N.; Lima, J.C.; Rodríguez, L. Aggregation induced emission of gold(I) complexes in water or water mixtures. Dalton Trans. 2017, 46, 11125–11139. [Google Scholar] [CrossRef] [PubMed]
- Aguiló, E.; Moro, A.J.; Gavara, R.; Alfonso, I.; Pérez, Y.; Zaccaria, F.; Guerra, C.F.; Malfois, M.; Baucells, C.; Ferrer, M.; et al. Reversible Self-Assembly of Water-Soluble Gold(I) Complexes. Inorg. Chem. 2018, 57, 1017–1028. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |
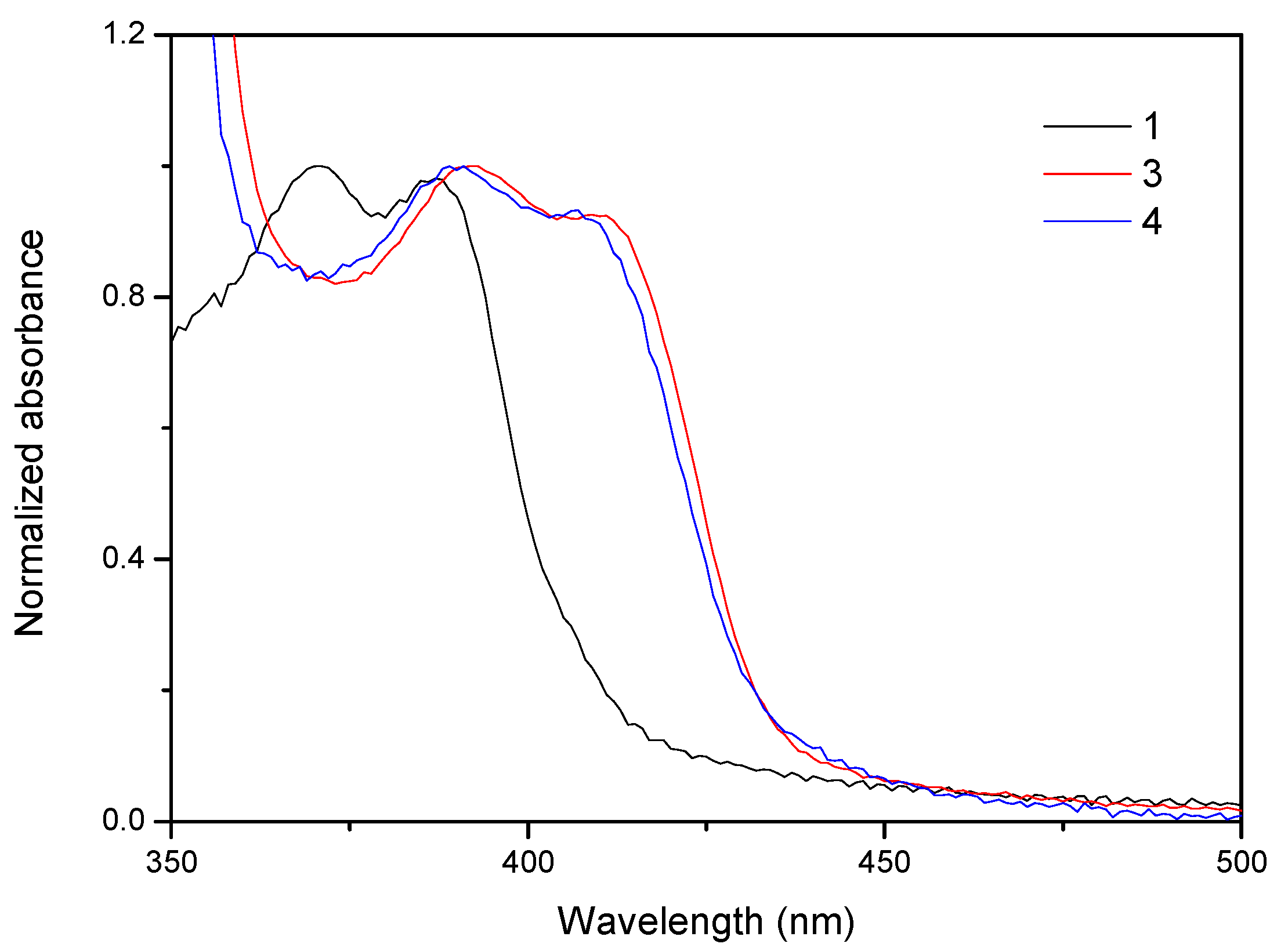
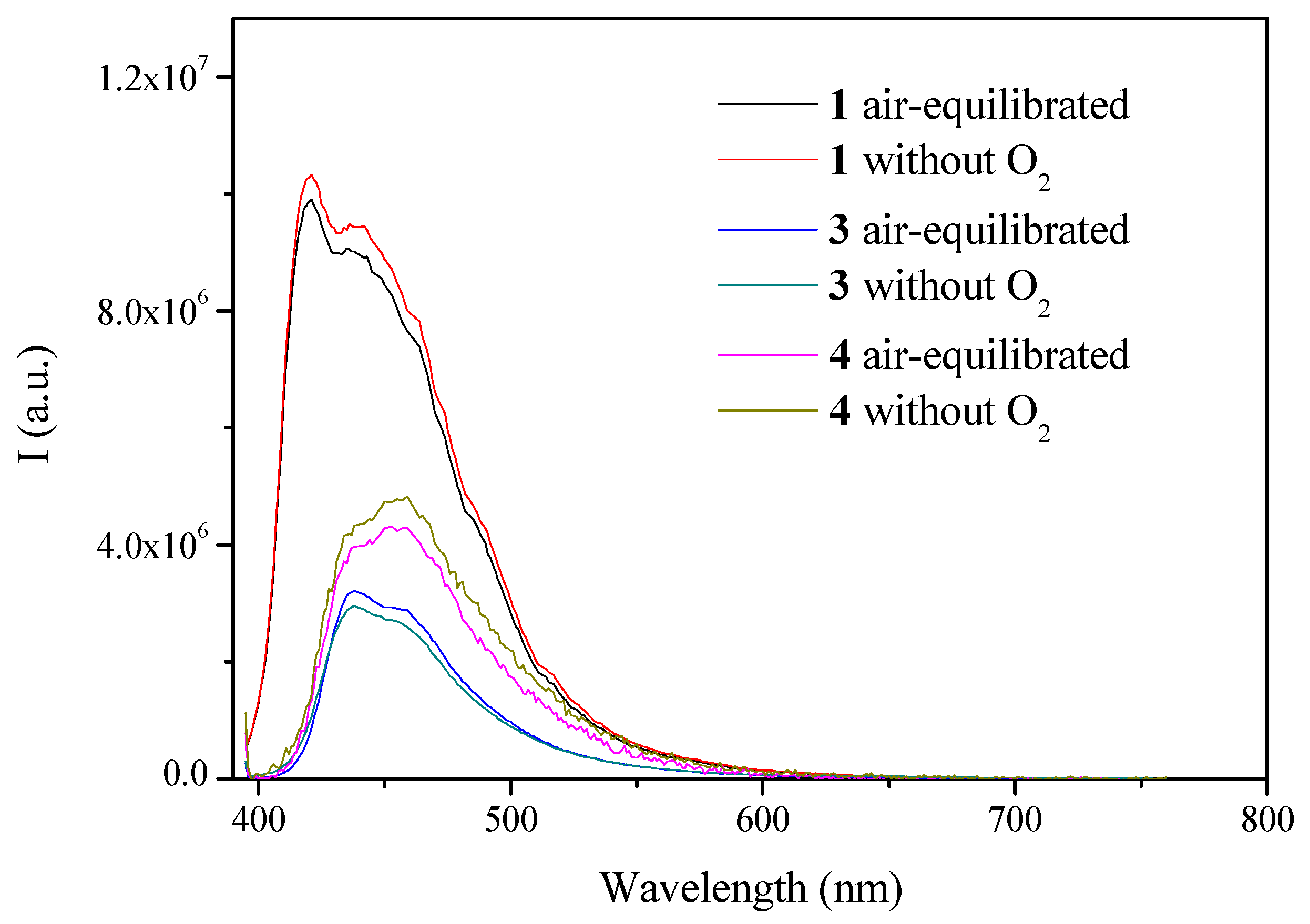
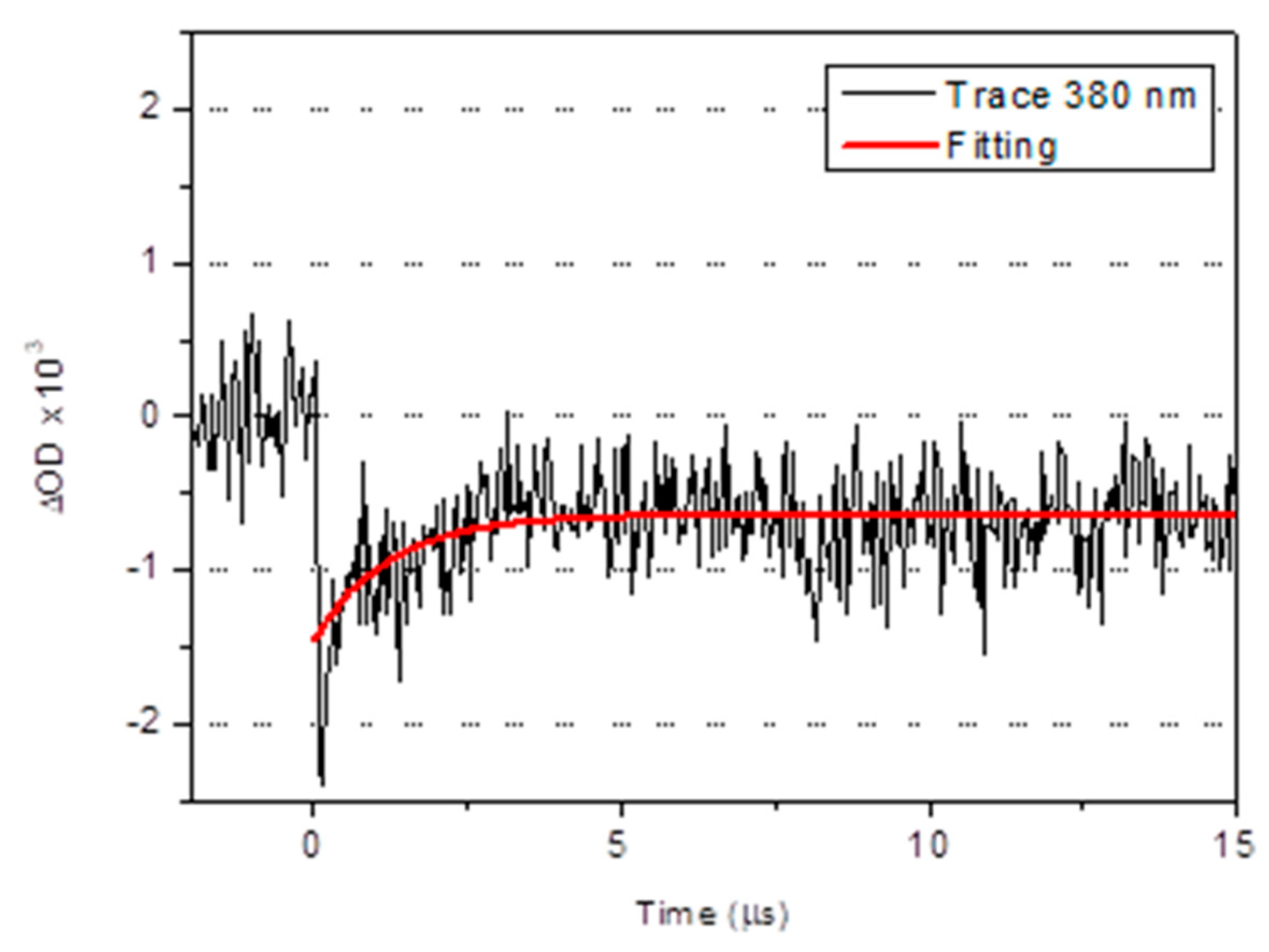
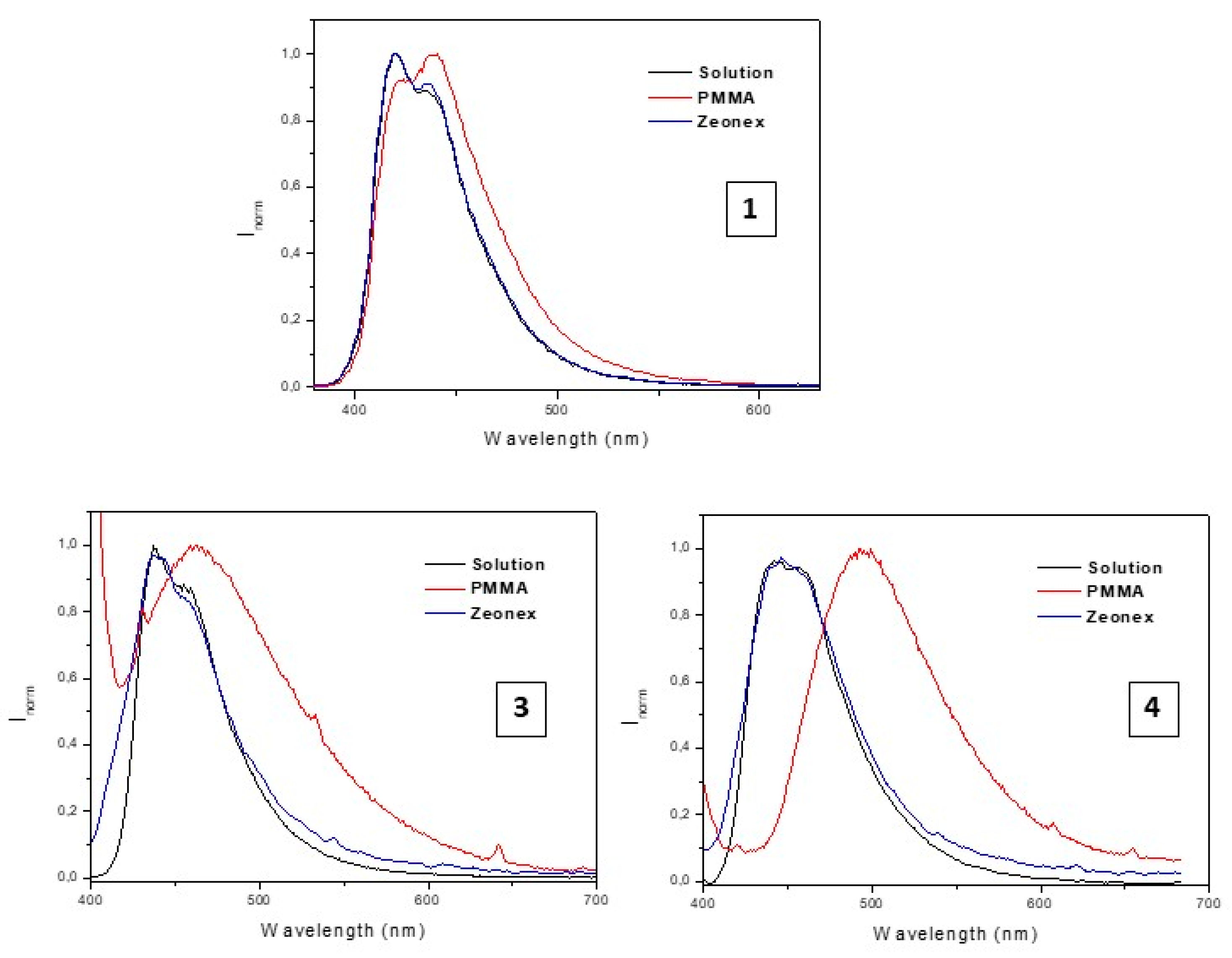
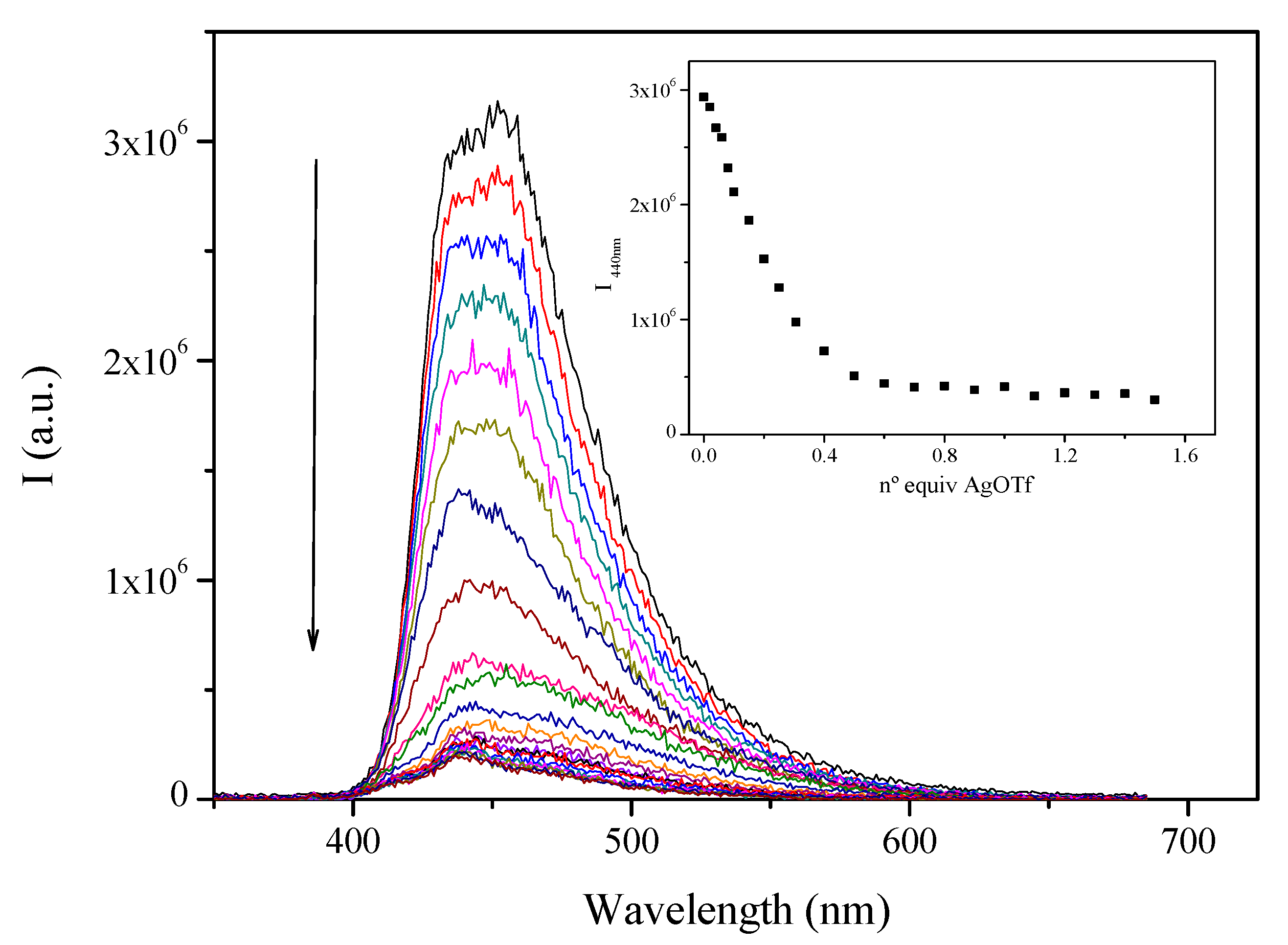

| Compound | Absorption λmax (nm) (104 ε (M−1 cm−1) | Emission (Solution, λmax (nm)) | τ |
|---|---|---|---|
| 1 | 371 (2.0), 388 (1.9) | 424, 442 | 3.65 ns |
| 3 | 390 (5.4), 411 (5.0) | 444, 460 | 3.90 ns |
| 4 | 393 (3.3), 413 (3.0) | 444, 460 | 1.22 ns |
| Compound | Solution | PMMA | Zeonex | |||
|---|---|---|---|---|---|---|
| Air | N2 | Air | N2 | Air | N2 | |
| 1 | 0.22 | 0.27 | 0.20 | 0.20 | 0.22 | 0.22 |
| 3 | 0.05 | 0.05 | 0.06 | 0.06 | 0.07 | 0.07 |
| 4 | 0.06 | 0.07 | 0.07 | 0.07 | 0.03 | 0.04 |
| 3·AgOTf | 0.007 | 0.007 | 0.012 | 0.015 | 0.04 | 0.04 |
| 4·AgOTf | 0.03 | 0.03 | 0.05 | 0.06 | 0.05 | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caparrós, F.J.; Outis, M.; Jung, Y.; Choi, H.; Lima, J.C.; Rodríguez, L. Luminescent Tetranuclear Gold(I) Dibenzo[g,p]chrysene Derivatives: Effect of the Environment on Photophysical Properties. Molecules 2020, 25, 949. https://doi.org/10.3390/molecules25040949
Caparrós FJ, Outis M, Jung Y, Choi H, Lima JC, Rodríguez L. Luminescent Tetranuclear Gold(I) Dibenzo[g,p]chrysene Derivatives: Effect of the Environment on Photophysical Properties. Molecules. 2020; 25(4):949. https://doi.org/10.3390/molecules25040949
Chicago/Turabian StyleCaparrós, Francisco J., Mani Outis, Yongsik Jung, Hyeonho Choi, João Carlos Lima, and Laura Rodríguez. 2020. "Luminescent Tetranuclear Gold(I) Dibenzo[g,p]chrysene Derivatives: Effect of the Environment on Photophysical Properties" Molecules 25, no. 4: 949. https://doi.org/10.3390/molecules25040949
APA StyleCaparrós, F. J., Outis, M., Jung, Y., Choi, H., Lima, J. C., & Rodríguez, L. (2020). Luminescent Tetranuclear Gold(I) Dibenzo[g,p]chrysene Derivatives: Effect of the Environment on Photophysical Properties. Molecules, 25(4), 949. https://doi.org/10.3390/molecules25040949