Poultry Farms as a Potential Source of Environmental Pollution by Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
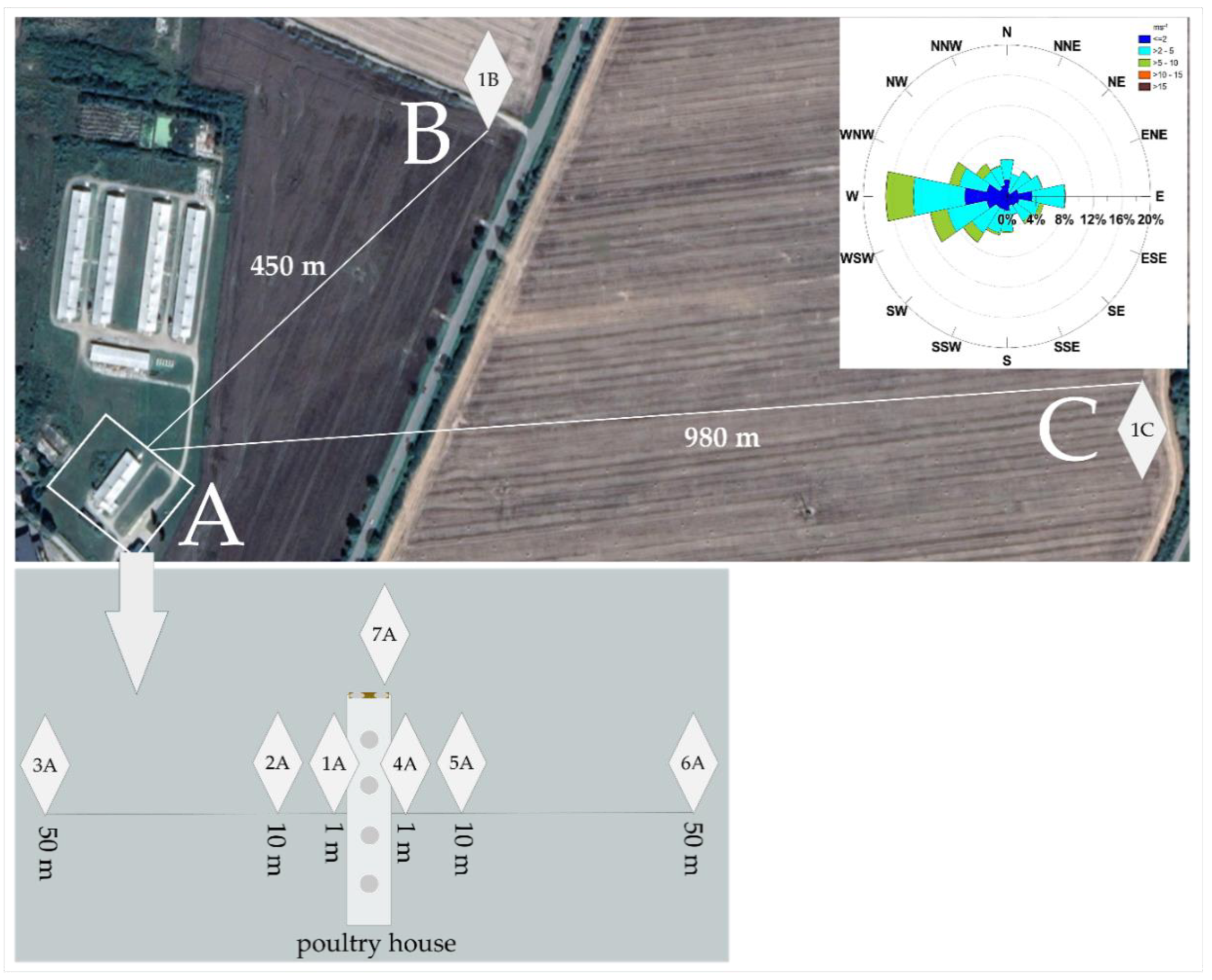
2.1. Study Area
2.1.1. Weather Conditions
2.1.2. Poultry House
2.2. Sample Handling
2.3. Analytical Procedure
2.3.1. Solvents and Standards
2.3.2. Sample Pretreatment
2.3.3. UPLC-MS/MS Analysis
2.3.4. Quality Assurance/Quality Control (QA/QC)
- calibration of the LC-MS/MS system,
- determination of the selected validation parameters based on calibration curves.
2.4. Antibiotic Resistance in Microorganisms Isolated from Soil
3. Results
3.1. Quality Assurance/Quality Control (QA/QC)
3.2. Analysis of Real Samples
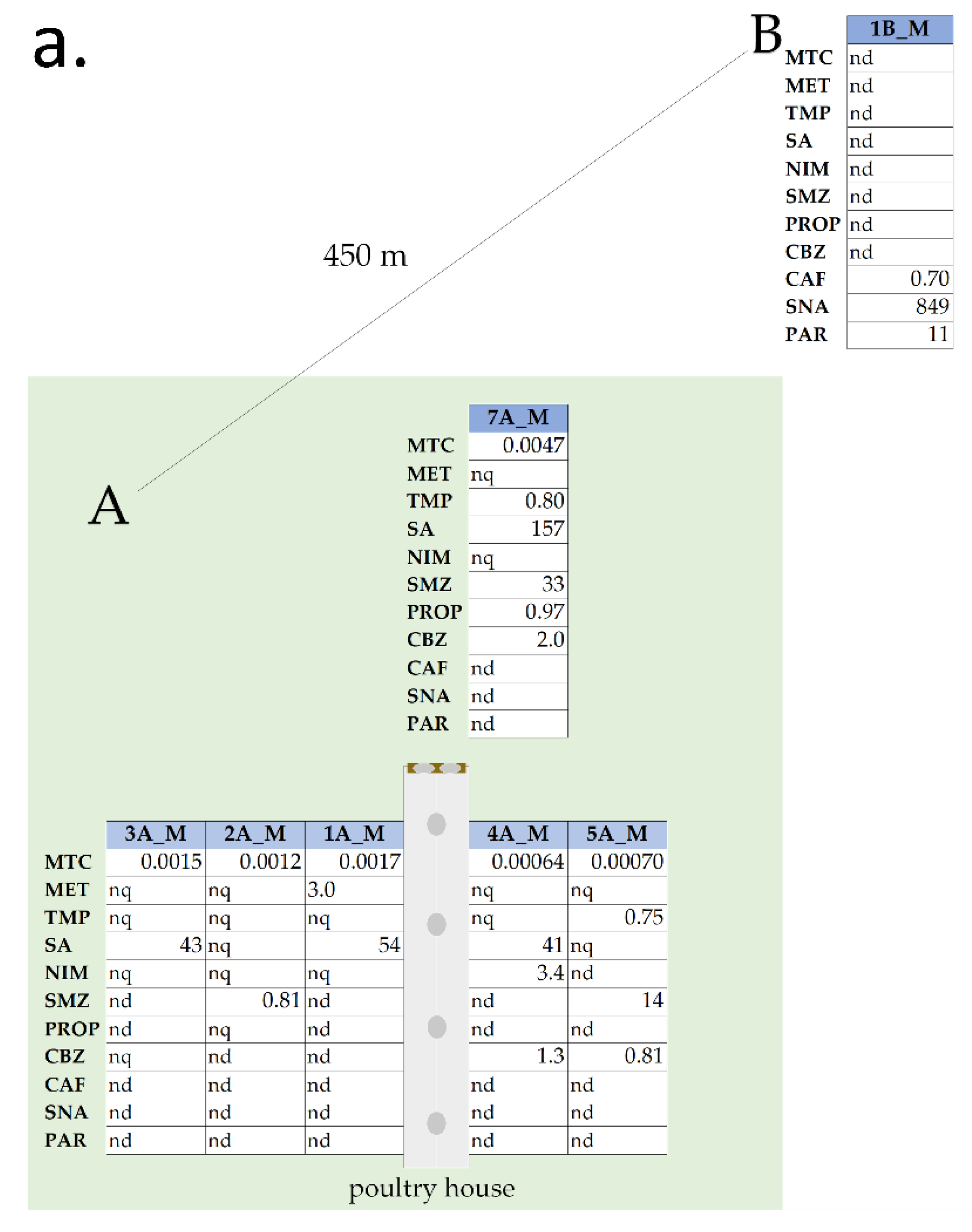
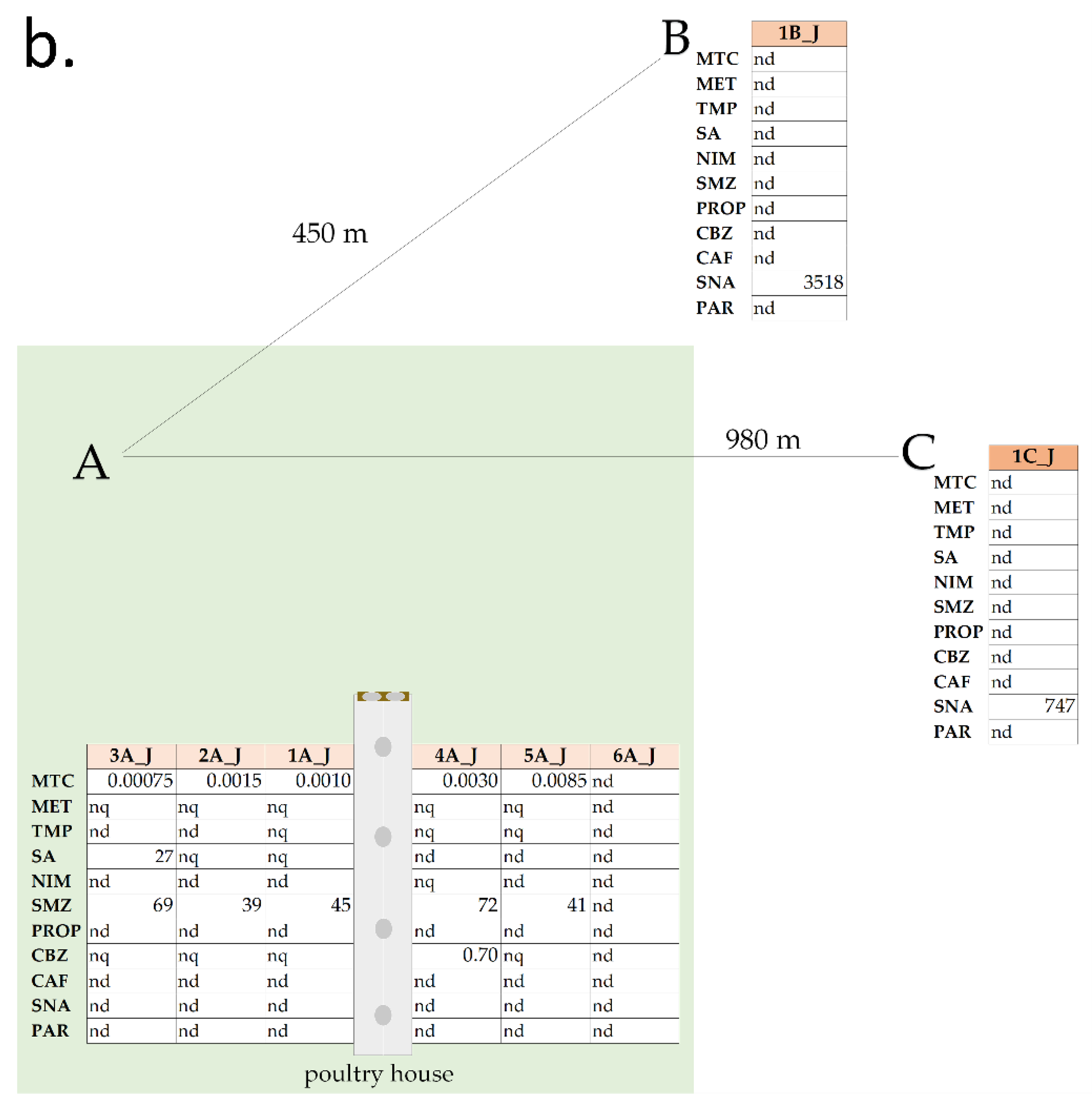
3.2.1. Area A
3.2.2. Areas B and C
3.3. Analysis of Microbial Resistance in Soil Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Parliament and the Council of the European Union. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing DIRECTIVE 2001/82/EC; European Parliament and the Council of the European Union: Luxembourg, 2018; Volume L4, pp. 43–167. [Google Scholar]
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563–564, 366–376. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Dahshan, H.; Abd-Elall, A.M.M.; Megahed, A.M.; Abd-El-Kader, M.A.; Nabawy, E.E. Veterinary antibiotic resistance, residues, and ecological risks in environmental samples obtained from poultry farms, Egypt. Environ. Monit. Assess. 2015, 187, 2. [Google Scholar] [CrossRef] [PubMed]
- Kaczala, F.; Blum, S.E. The Occurrence of Veterunary Pharmaceuticals in the Environment: A Review. Curr. Anal. Chem. 2016, 12, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furtula, V.; Farrell, E.G.; Diarrassouba, F.; Rempel, H.; Pritchard, J.; Diarra, M.S. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult. Sci. 2010, 89, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D. Poultry Development Review; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Braykov, N.P.; Eisenberg, J.N.S.; Grossman, M.; Zhang, L.; Vasco, K.; Cevallos, W.; Muñoz, D.; Acevedo, A.; Moser, K.A.; Marrs, C.F.; et al. Antibiotic Resistance in Animal and Environmental Samples Associated with Small-Scale Poultry Farming in Northwestern Ecuador. mSphere 2016, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Skóra, J.; Matusiak, K.; Wojewódzki, P.; Nowak, A.; Sulyok, M.; Ligocka, A.; Okrasa, M.; Hermann, J.; Gutarowska, B. Evaluation of microbiological and chemical contaminants in poultry farms. Int. J. Environ. Res. Public Health 2016, 13, 192. [Google Scholar] [CrossRef]
- Maheshwari, S. Environmental Impacts of Poultry Production. Poultry Fish. Wildl. Sci. 2013, 1, 1–2. [Google Scholar] [CrossRef]
- Viegas, S.; Faísca, V.M.; Dias, H.; Clérigo, A.; Carolino, E.; Viegas, C. Occupational exposure to poultry dust and effects on the respiratory system in workers. J. Toxicol. Environ. Heal.—Part A Curr. Issues 2013, 76, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Price, G.W.; Jamieson, R.; Burton, D.; Khosravi, K. Sorption and desorption of selected non-steroidal anti-inflammatory drugs in an agricultural loam-textured soil. Chemosphere 2017, 174, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef]
- AVEC. Annual Report of the Assiciation of Poultry Processors and Poultry Trade in the EU Countries; AVEC: Brussels, Belgium, 2018. [Google Scholar]
- Supreme Audit Office. Supervision of the Functioning of Animal Farm; Supreme Audit Office: Warsaw, Poland, 2014. [Google Scholar]
- Supreme Audit Office. Antibiotics Use in Animal Production in Lubuskie Voivodeship; Supreme Audit Office: Warsaw, Poland, 2017. [Google Scholar]
- Supreme Audit Office. Supervision of the Marketi ng and Use of Products Containing Anabolic, Hormonal, Incuring and Psychotropical Substances in Animal Treatment, Including Accompanying Products; Supreme Audit Office: Warsaw, Poland, 2018. [Google Scholar]
- Kasperowicz, A.; Wacław, B.; Wasilewski, T. Environment protection program for the Nidzica district for the years 2018–2021, with a perspective for 2022–2025. 2017. Available online: http://www.bip.powiatnidzicki.pl/system/obj/8640_8.3.1.Program_Ochrony_Srodowiska_Powiatu_Nidzickiego.pdf (accessed on 24 February 2020).
- Golovko, O.; Koba, O.; Kodesova, R.; Fedorova, G.; Kumar, V.; Grabic, R. Development of fast and robust multiresidual LC-MS/MS method for determination of pharmaceuticals in soils. Environ. Sci. Pollut. Res. 2016, 23, 14068–14077. [Google Scholar] [CrossRef]
- Baert, K.; de Baere, S.; Croubels, S.; de Backer, P. Pharmacokinetics and Oral Bioavailability of Sulfadiazineand Trimethoprim in Broiler Chickens. J. Vet. Pharmacol. Ther. 2003, 27, 301–309. [Google Scholar]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Wei, R.; Gec, F.; Zhangb, L.; Houb, X.; Caoa, Y.; Gongb, L.; Chenb, M.; Wangb, R.; Bao, E. Occurrence of 13 veterinary drugs in animal manure-amended soils in Eastern China. Chemosphere 2016, 144, 2377–2383. [Google Scholar] [CrossRef]
- An, J.; Chen, H.; Wei, S.; Gu, J. Antibiotic contamination in animal manure, soil, and sewage sludge in Shenyang, northeast China. Environ. Earth Sci. 2015, 74, 5077–5086. [Google Scholar] [CrossRef]
- Metoclopramide for Chickens and Ducks. Available online: http://www.poultrydvm.com/drugs/metoclopramide (accessed on 2 February 2020).
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Zaugg, S.; Zhang, X.; Sweedler, J.; Thormann, W. Determination of salicylate, gentisic acid and salicyluric acid in human urine by capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 752, 17–31. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Carrara, C.; Ptacek, C.J.; Robertson, W.D.; Blowes, D.W.; Moncur, M.C.; Sverko, E.; Backus, S. Fate of pharmaceutical and trace organic compounds in three septic system plumes, Ontario, Canada. Environ. Sci. Technol. 2008, 42, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Comeau, F.; Surette, C.; Brun, G.L.; Losier, R. The occurrence of acidic drugs and caffeine in sewage effluents and receiving waters from three coastal watersheds in Atlantic Canada. Sci. Total Environ. 2008, 396, 132–146. [Google Scholar] [CrossRef]
- Gibson, R.; Becerril-Bravo, E.; Silva-Castro, V.; Jiménez, B. Determination of acidic pharmaceuticals and potential endocrine disrupting compounds in wastewaters and spring waters by selective elution and analysis by gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1169, 31–39. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Gou, M.; Wang, J.T.; Chen, D.; He, J.Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef]
- Awad, Y.M.; Kim, S.-C.; El-Azeem, S.A.M.A.; Kim, K.-H.; Kim, K.-R.; Kim, K.; Jeon, C.; Lee, S.S.; Ok, Y.S. Veterinary antibiotics contamination in water, sediment, and soil near a swine manure composting facility. Environ. Earth Sci. 2014, 71, 1433–1440. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Parameters for the Monitored Ion Transition | ||||||
|---|---|---|---|---|---|---|
| Name | Short | Polarity | Parent Ion > Fragment Ion | Q1 Pre Bias [V] | CE [V] | Q3 Pre Bias [V] |
| acetylsalicylic acid | ASA | − | 179.10 > 136.95 | 13 | 11 | 24 |
| ampicillin | AMP | + | 349.9 > 106.05 | 20 | 25 | 20 |
| caffeine | CAF | + | 194.90 > 138.00 | 20 | 20 | 20 |
| carbamazepine | CBZ | + | 237.05 > 194.00 | 20 | 20 | 20 |
| ciprofloxacin | CIP | + | 322.00 > 314.10 | 20 | 21 | 20 |
| diclofenac | DIC | − | 294.05 > 249.90 | 15 | 12 | 16 |
| enrofloxacin | ENR | + | 360.10 > 316.10 | 20 | 20 | 20 |
| ibuprofen | IB | − | 205.00 > 161.20 | 14 | 9 | 16 |
| metoclopramide | NIM | − | 307.05 > 228.95 | 16 | 17 | 20 |
| metoprolol | MTC | + | 300.05 > 227.00 | 20 | 20 | 20 |
| nimesulide | MET | + | 268.15 > 116.00 | 20 | 20 | 20 |
| paracetamol | PAR | + | 152.00 > 110.10 | 17 | 18 | 21 |
| propranolol HCl | PROP | + | 260.10 > 116.10 | 20 | 19 | 20 |
| salicylic acid | SA | − | 137.30 > 93.15 | 10 | 17 | 20 |
| spectinomycin | SPEC | + | 332.80 > 98.10 | 16 | 29 | 14 |
| streptomycin | STREP | + | 582.00 > 263.20 | 28 | 33 | 11 |
| sulfacetamide Na | SFC | + | 214.95 > 92.05 | 11 | 23 | 21 |
| sulfacarbamide | SCA | + | 216.00 > 92.05 | 30 | 25 | 30 |
| sulfadiazine | SDA | + | 251.00 > 92.05 | 18 | 26 | 19 |
| sulfaguanidine | SGA | + | 214.95 > 92.05 | 15 | 26 | 21 |
| sulfanilamide | SNA | + | 172.9 > 86.15 | 19 | 16 | 13 |
| sulfamerazine | SMA | + | 264.95 > 92.05 | 13 | 29 | 14 |
| sulfamethazine | SMZ | + | 278.50 > 186.50 | 14 | 19 | 27 |
| sulfamethoxazole | SMX | + | 253.95 > 92.00 | 20 | 30 | 20 |
| sulfathiazole | STA | + | 255.90 > 156.00 | 20 | 15 | 20 |
| tetracycline | TET | + | 445.00 > 409.95 | 20 | 20 | 20 |
| trimethoprim | TMP | + | 291.05 > 230.10 | 20 | 25 | 20 |
| MS/MS Operation Parameters | ||||||
| Interface Temperature (°C) | 300 | |||||
| Desolvation LineTemperature (°C) | 250 | |||||
| Nebulizing Gas Flow (L/min) | 3 | |||||
| Heating Gas Flow (L/min) | 9 | |||||
| Heating Block (°C) | 350 | |||||
| Drying Gas Flow (L/min) | 10 | |||||
| Pharmaceuticals | Short | Coefficient of Calibration Curve (y = ax + b) | Linearity | Limits | Repeatability | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | Regression Coefficient R2 | min–max (ng/mL) | LOD (ng/mL) | LOQ (3 × LOD) (ng/mL) | MDL (ng/g) | MQL (ng/g) | CV (%) | U (k = 2) | ||
| acetylsalicylic acid | ASA | 107058 | −1301 | 0.9931 | 176.57–5000 | 58.86 | 176.57 | 14.72 | 44.15 | 2.5–2.8 | 0.95 |
| ampicillin | AMP | 663156 | 2850 | 0.9996 | 14.25–5000 | 4.75 | 14.25 | 1.19 | 3.56 | 1.1–3.9 | 0.78 |
| caffeine | CAF | 29004046 | 128181 | 0.999 | 3.9–500 | 0.75 | 2.25 | 0.19 | 0.56 | 0.79–3.9 | 0.98 |
| carbamazepine | CMZ | 17335651 | 9152 | 0.9991 | 8.8–500 | 2.93 | 8.80 | 0.73 | 2.20 | 1.0–11 | 0.65 |
| ciprofloxacin | CIP | 2687453 | 1143 | 0.9982 | 9.9–5000 | 3.3 | 9.9 | 0.8 | 2.5 | 1.9–11.0 | 1.4 |
| diclofenac | DIC | 6826340 | 130246 | 0.9984 | 8.86–1000 | 2.89 | 8.68 | 0.72 | 2.17 | 0.71–9.6 | 0.64 |
| enrofloxacin | ENR | 12634935 | −64163 | 0.9974 | 22.4–1000 | 7.5 | 22.4 | 1.9 | 5.6 | 1.4–5.1 | 1.4 |
| ibuprofen | IB | 10369 | 16715 | 0.9912 | 384.37–5000 | 316.8 | 950.4 | 79.2 | 237.6 | 2.2–3.8 | 1.1 |
| metoclopramide | MTC | 14227180430 | 1348134 | 0.998 | 0.39 × 10−3 | 0.13 × 10−3 | 0.39 × 10−3 | 0.03 × 10−3 | 0.09 × 10−3 | 0.24–8.4 | 0.39 |
| metoprolol | MET | 8726542 | 10467 | 0.9991 | 3.3–1000 | 1.1 | 3.3 | 0.3 | 0.8 | 0.41–4.4 | 1.5 |
| nimesulide | NIM | 134064871 | 3435909 | 0.9934 | 12.62–500 | 4.21 | 12.62 | 1.05 | 3.16 | 1.6–7.5 | 0.60 |
| paracetamol | PAR | 1696703 | 1073 | 0.9993 | 14.01–5000 | 4.67 | 14.01 | 1.17 | 3.50 | 0.76–9.9 | 0.87 |
| propranolol HCl | PROP | 12583700 | 38410 | 0.9993 | 2.8–1000 | 0.92 | 2.8 | 0.2 | 0.7 | 0.26–4.6 | 1.2 |
| salicylic acid | SA | 585092 | 90750 | 0.9964 | 49.06–5000 | 15.80 | 47.43 | 3.95 | 11.85 | 2.4–8.9 | 0.89 |
| sulfacetamide Na | SFC | 1590160 | 1393 | 0.9989 | 8.7–5000 | 2.9 | 8.7 | 0.7 | 2.2 | 1.2–7.6 | 1.4 |
| spectinomycin | SPEC | 55623 | −8960 | 0.998 | 173.2–5000 | 57.8 | 173.2 | 14.5 | 43.4 | 0.64–7.0 | 1.3 |
| streptomycin | STREP | 20759 | −23309 | 0.9983 | 533.4–5000 | 177.8 | 533.4 | 44.5 | 133.4 | 0.60–6.3 | 1.4 |
| sulfacarbamide | SFC | 41286 | −8452 | 0.9966 | 2.07–5000 | 0.69 | 2.07 | 0.17 | 0.52 | 2.1–3.3 | 0.40 |
| sulfadiazine | SDA | 5002325 | −12328 | 0.9995 | 19.39–1000 | 6.46 | 19.39 | 1.62 | 4.85 | 0.36–5.7 | 0.89 |
| sulfaguanidine | SGA | 1264343 | −1292 | 0.999 | 10.03–5000 | 3.34 | 10.03 | 0.84 | 2.51 | 0.80–12 | 0.87 |
| sulfamerazine | SMA | 7308819 | −14598 | 0.9998 | 8.45–1000 | 2.82 | 8.45 | 0.71 | 2.12 | 0.84–6.1 | 0.66 |
| sulfamethazine | SMZ | 583452 | 2984 | 0.9863 | 0.08–5000 | 0.03 | 0.08 | 0.01 | 0.02 | 1.0–8.8 | 0.75 |
| sulfamethoxazole | SMX | 8078106 | 2984 | 0.9995 | 0.38–1000 | 0.13 | 0.38 | 0.03 | 0.10 | 1.7–8.2 | 0.89 |
| sulfanilamide | SNA | 53617 | −25963 | 0.9973 | 1500.6–5000 | 502.9 | 1508.6 | 125.7 | 377.2 | 0.39–2.7 | 1.4 |
| sulfathiazole | STA | 4356784 | 615 | 0.9996 | 6.5–1000 | 2.2 | 6.5 | 0.6 | 1.7 | 0.64–6.0 | 1.1 |
| tetracycline | TET | 2063937 | −33350 | 0.9990 | 57.2–1000 | 19.1 | 57.2 | 4.8 | 14.3 | 0.50–10 | 1.6 |
| trimethoprim | TMP | 26514921 | 74017 | 0.9994 | 2.9–500 | 0.98 | 2.9 | 0.2 | 0.7 | 1.7–3.7 | 1.1 |
| Soil Samples Localization | A | B | C | |
|---|---|---|---|---|
| N. of Strains Tested | 66 | 15 | 4 | |
| Antibiotics | CIP | 29.0 ± 7.5 | 28.8 ±5.2 | 28.0 ± 4.0 |
| SPEC | 6.8 ± 8.0 | 6.2 ± 8.6 | 0.0 | |
| SMZ | 2.5 ± 7.1 | 0.0 | 0.0 | |
| TET | 13.1 ± 11.2 | 12.6 ± 10.1 | 9.5 ± 7.0 | |
| TMP | 2.7 ± 8.2 | 3.7 ± 9.8 | 0.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wychodnik, K.; Gałęzowska, G.; Rogowska, J.; Potrykus, M.; Plenis, A.; Wolska, L. Poultry Farms as a Potential Source of Environmental Pollution by Pharmaceuticals. Molecules 2020, 25, 1031. https://doi.org/10.3390/molecules25051031
Wychodnik K, Gałęzowska G, Rogowska J, Potrykus M, Plenis A, Wolska L. Poultry Farms as a Potential Source of Environmental Pollution by Pharmaceuticals. Molecules. 2020; 25(5):1031. https://doi.org/10.3390/molecules25051031
Chicago/Turabian StyleWychodnik, Katarzyna, Grażyna Gałęzowska, Justyna Rogowska, Marta Potrykus, Alina Plenis, and Lidia Wolska. 2020. "Poultry Farms as a Potential Source of Environmental Pollution by Pharmaceuticals" Molecules 25, no. 5: 1031. https://doi.org/10.3390/molecules25051031






