Activation of JNK and p38 in MCF-7 Cells and the In Vitro Anticancer Activity of Alnus hirsuta Extract
Abstract
:1. Introduction
2. Results
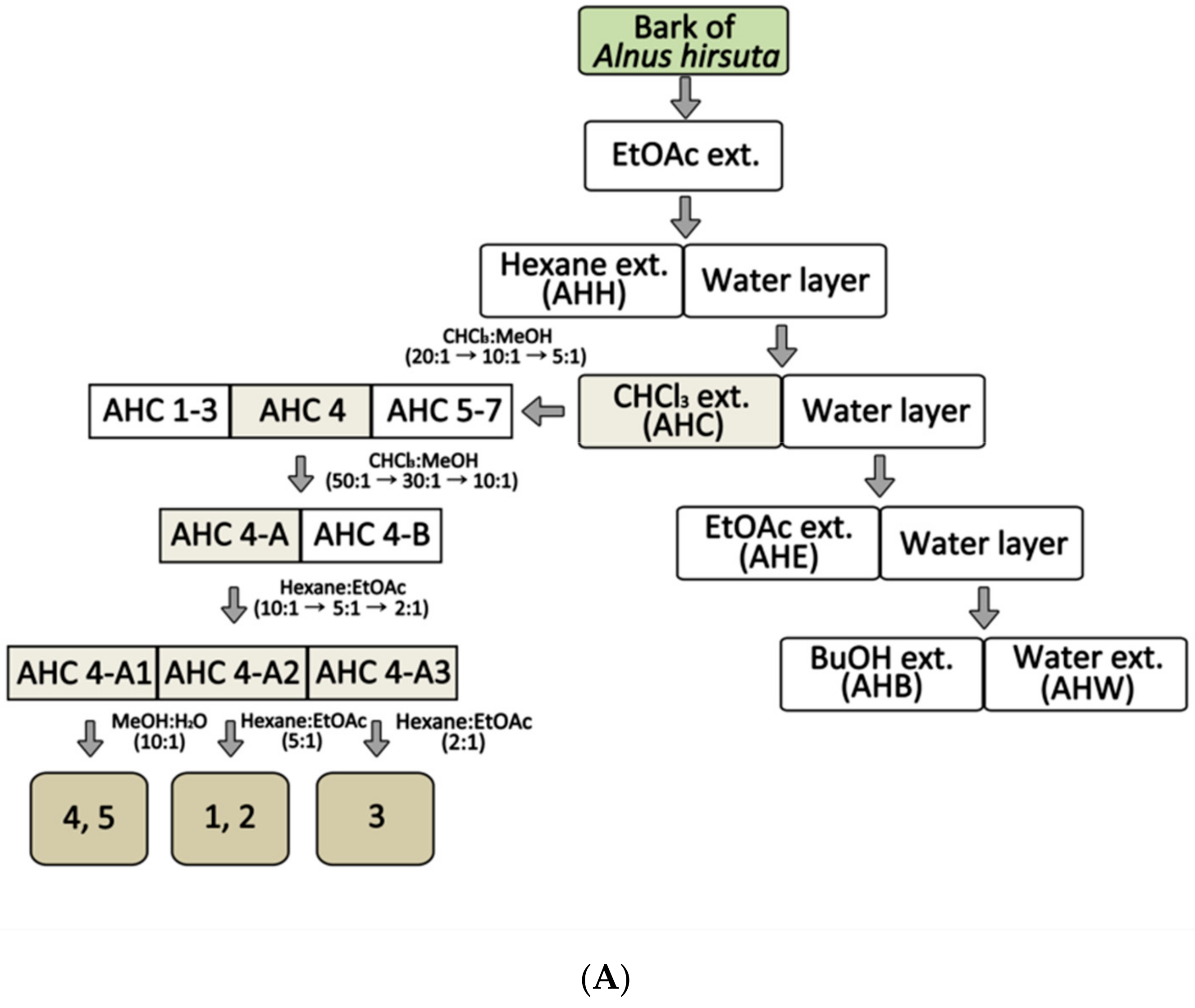
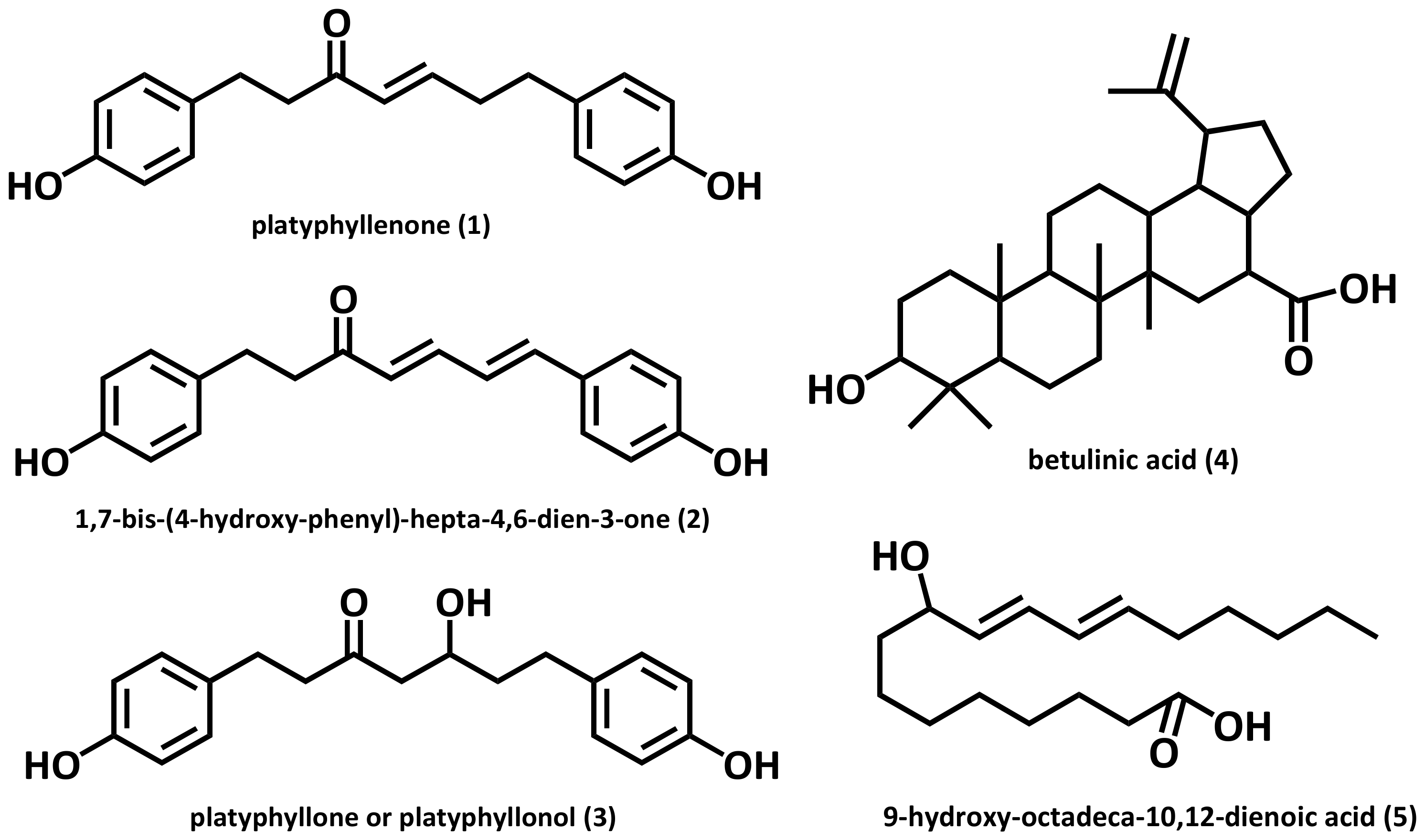
2.1. Solvent Extracts of Alnus hirsuta and the Constituents of the AHC Extract
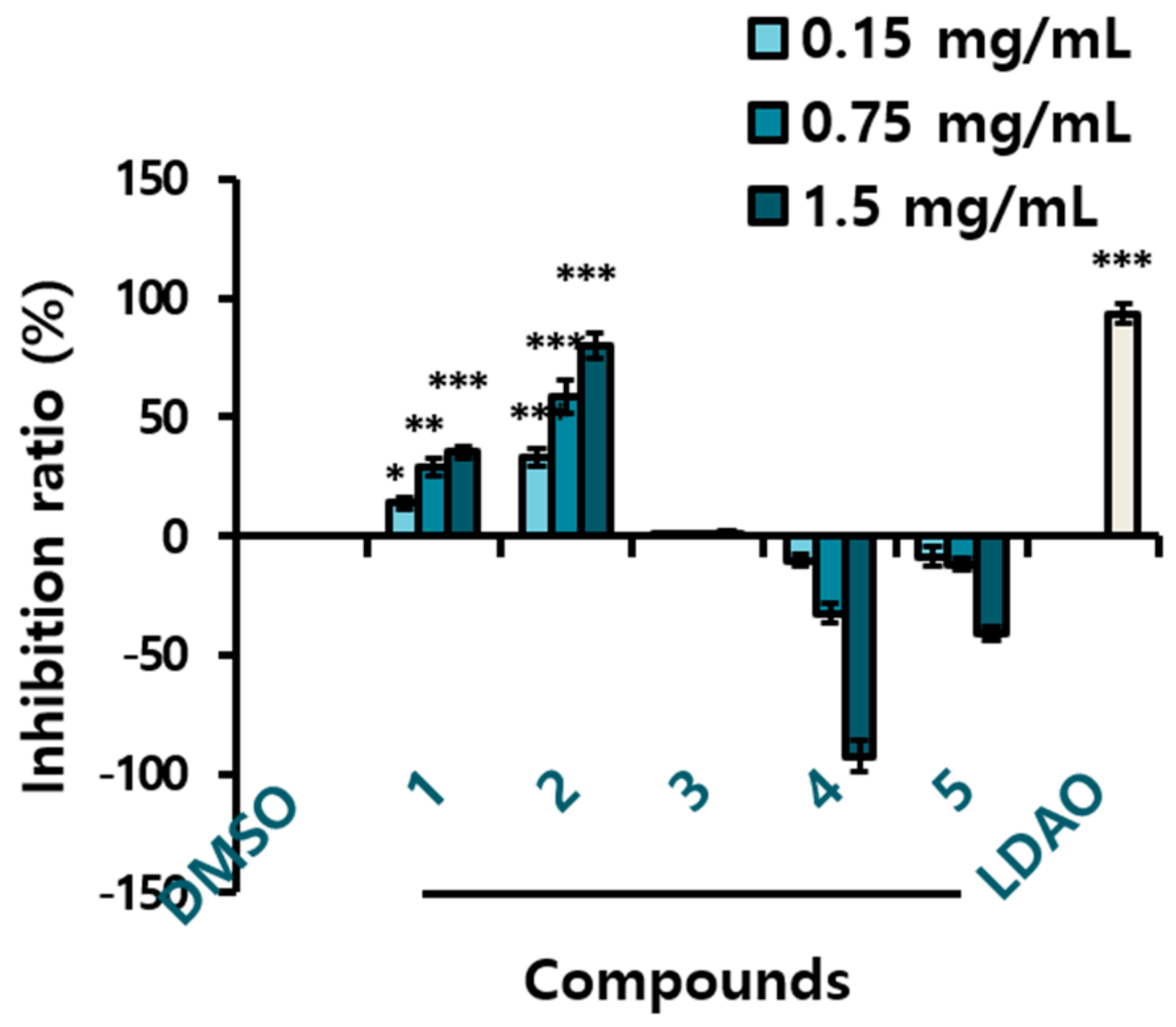
2.2. Yeast Inhibitory Activity of the AHC Extract and the Compounds
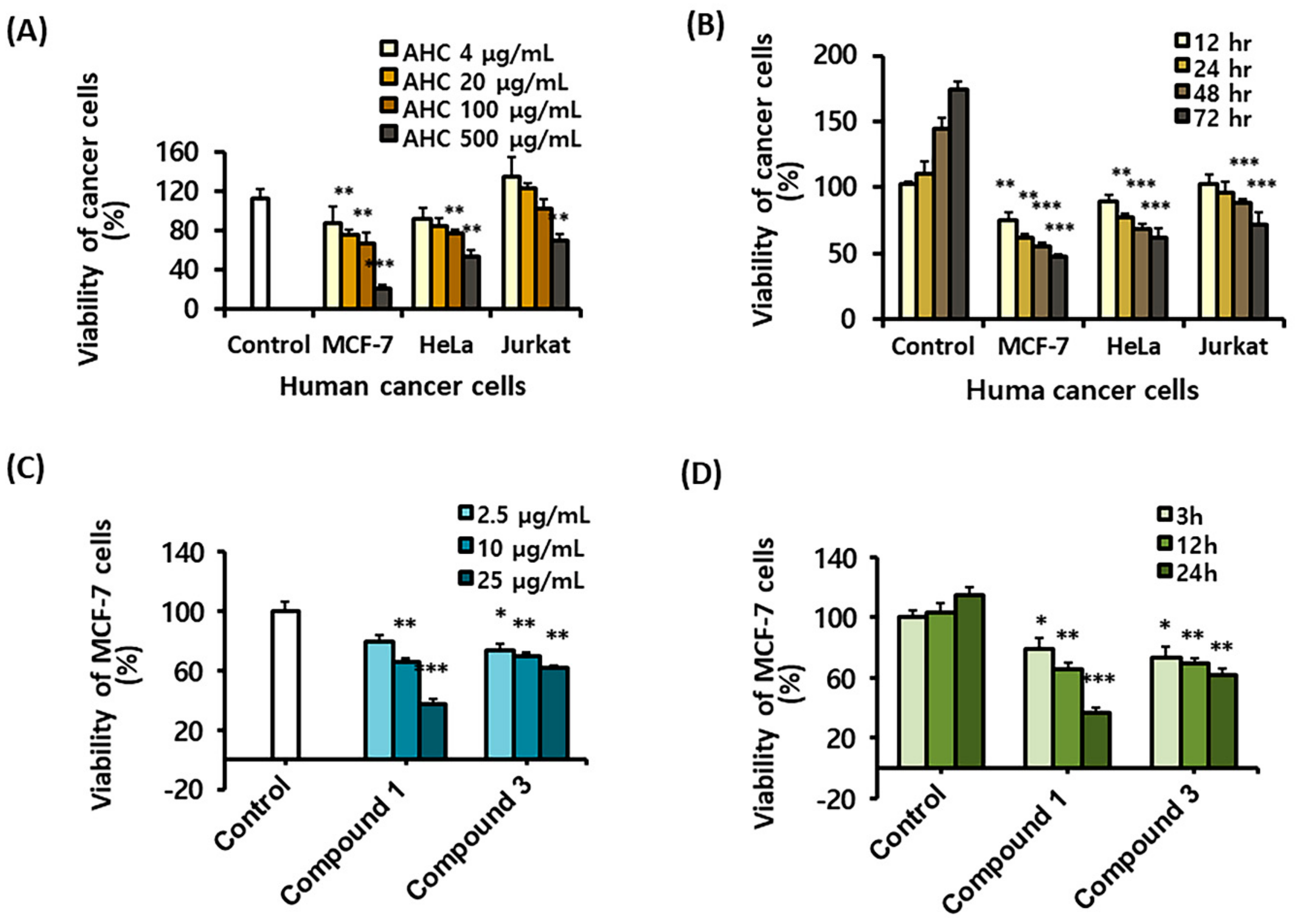
2.3. Cytotoxic Effects of the AHC Extract and the Compounds Against Cancer Cells
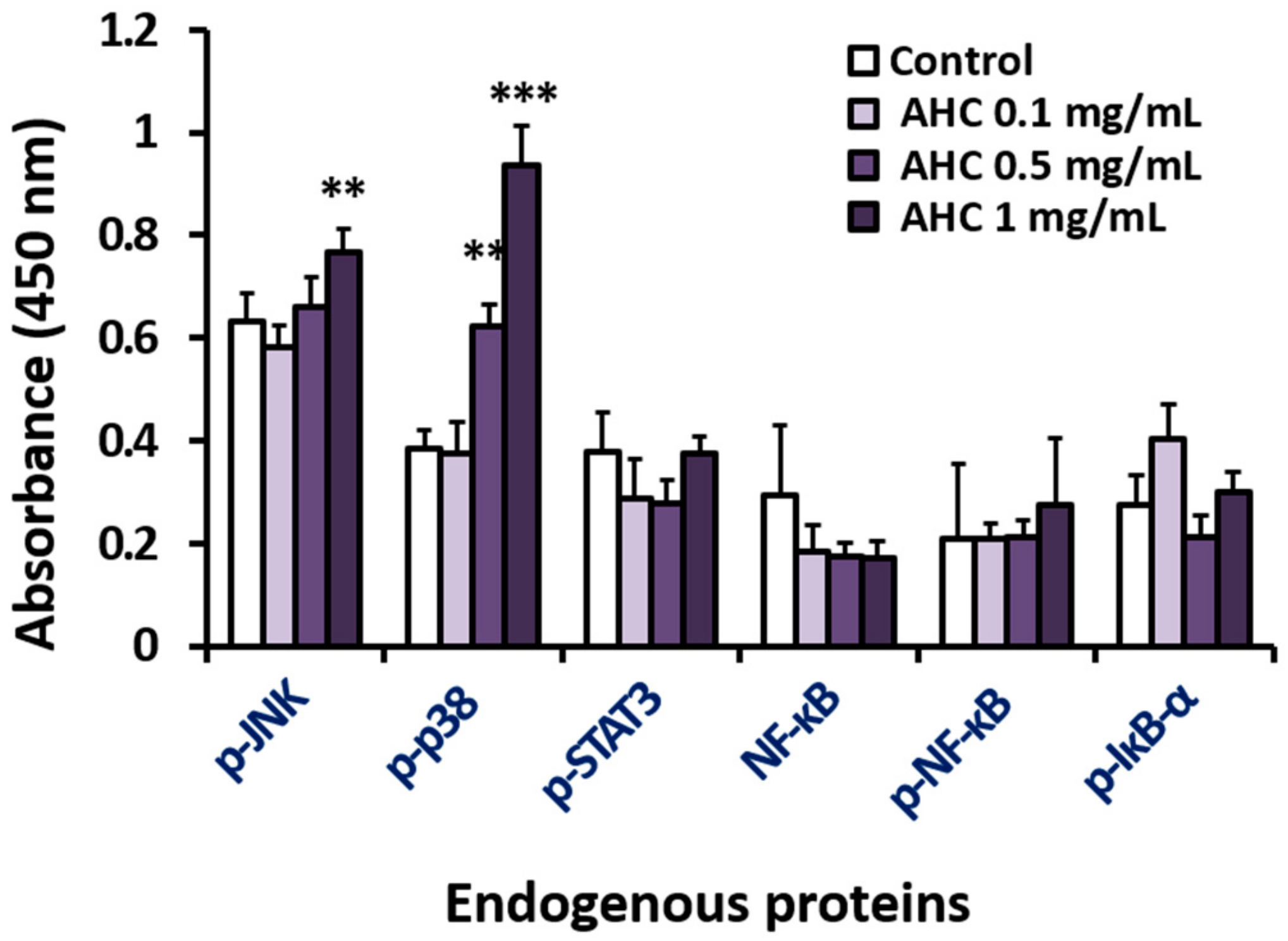
2.4. Effect of the AHC Extract on JNK and p38 Pathways
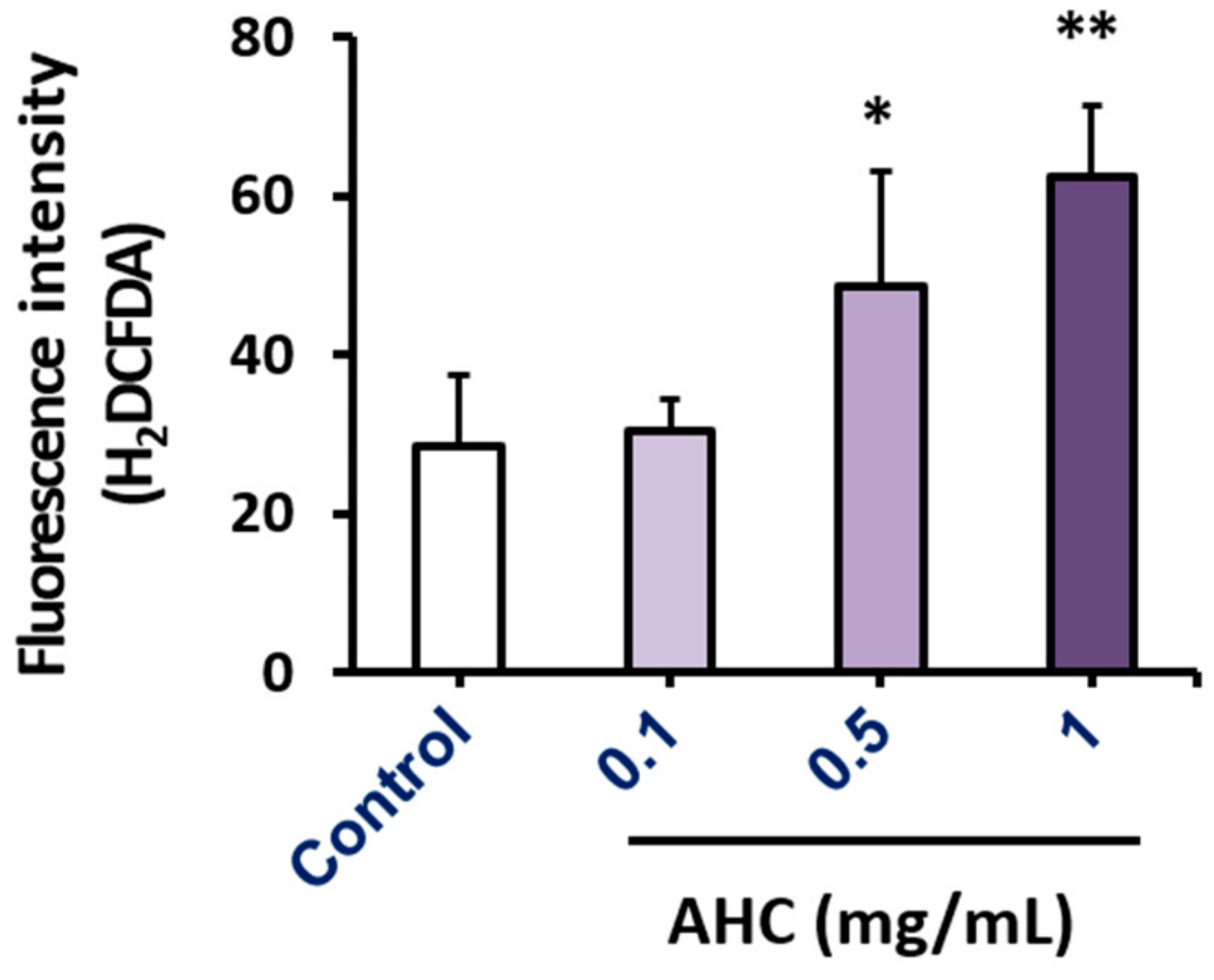
2.5. Effect of the AHC Extract on ROS Production
2.6. Apoptotic Activity of the AHC Extract
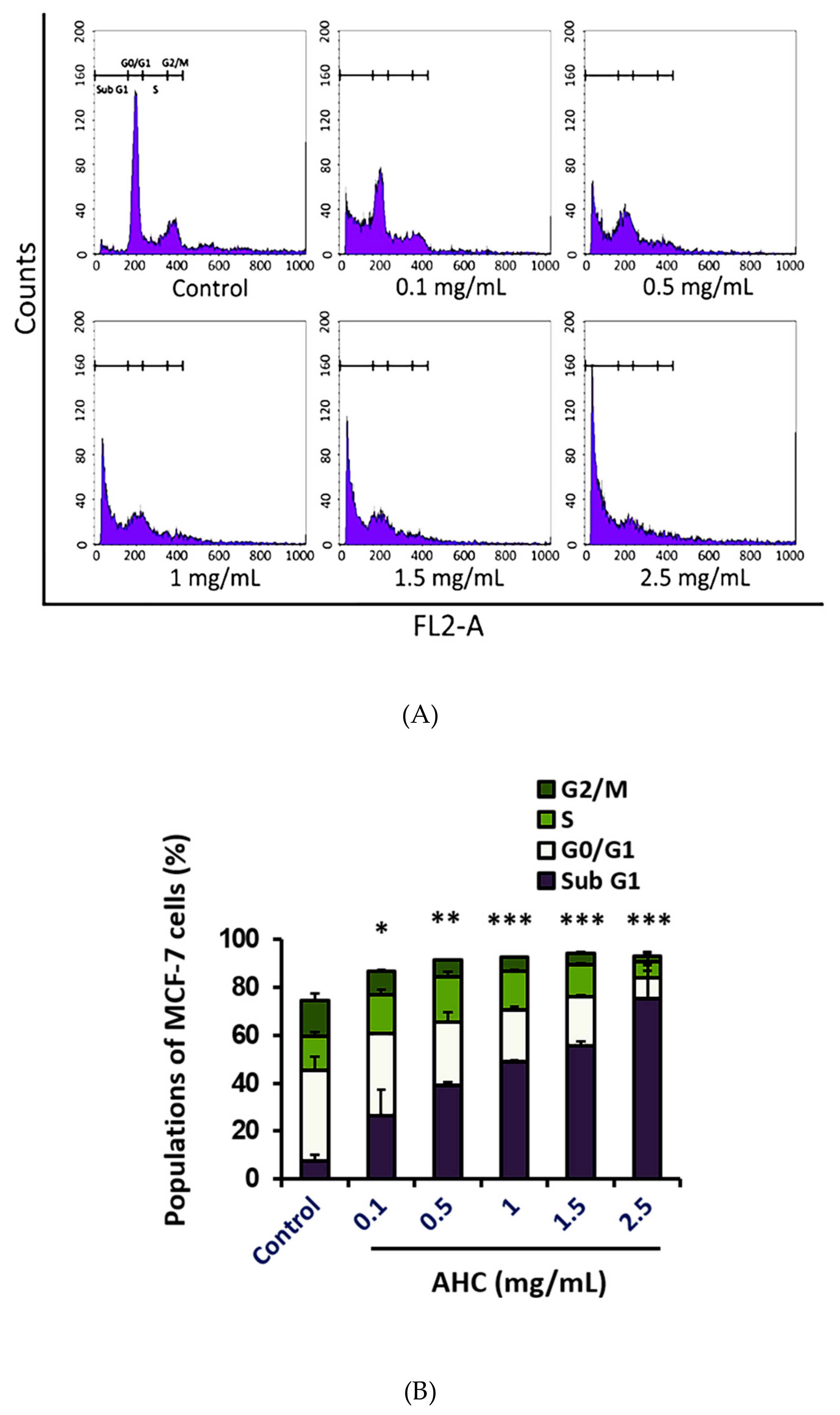
2.7. Cell Cycle Arrest Effect by the AHC Extract
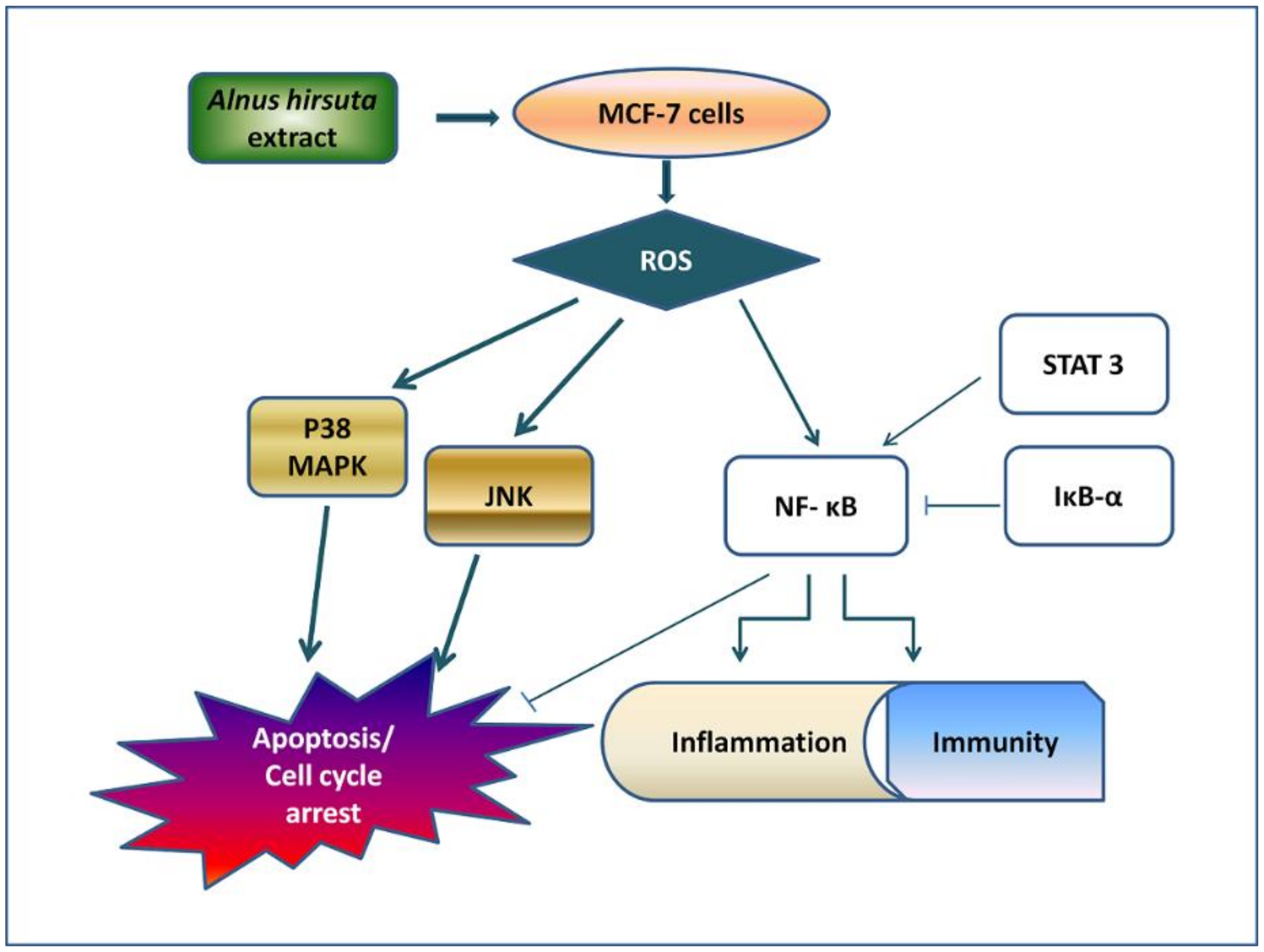
3. Discussion
4. Materials and Methods
4.1. Plant Materials: Extraction and Isolation
4.2. Evaluation of Yeast Suppression Effects of the Extracts and Compounds
4.3. Cytotoxicity Determination of the Extracts and the Compounds
4.4. Detection of Endogenous Proteins in the AHC Extract-Treated MCF-7 Cells
4.5. Measurement of ROS Generation in MCF-7 Cells by the AHC Extract
4.6. Evaluation of Apoptotic Effect of the AHC Extract on MCF-7 Cells
4.7. Cell Cycle Analysis of MCF-7 Cells Treated with the AHC Extract
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, T.D.; Khamis, H.J.; Tyczynski, J.E. Comparison of male and female breast cancer incidence trends, tumor characteristics, and survival. Ann. Epidemiol. 2005, 15, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Lukong, K.E. Understanding breast cancer–The long and winding road. BBA Clin. 2017, 7, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, J.H.; Seo, H.J.; Kim, K.H.; Kim, J.I.; An, C.H.; Park, W.C.; Song, B.J.; Oh, S.J.; Jung, S.S. Clinical Significance of Epstein-Barr Virus Expression in Breast Cancer. J. Korean Breast Cancer Soc. 2004, 7, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131. [Google Scholar] [CrossRef] [Green Version]
- COMŞA, Ş.; Cimpean, A.M.; Raica, M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer. Res. 2015, 35, 3147–3154. [Google Scholar]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [Green Version]
- Low, H.B.; Zhang, Y. Regulatory roles of MAPK phosphatases in cancer. Immune Netw. 2016, 16, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838. [Google Scholar] [CrossRef] [Green Version]
- Avisetti, D.R.; Babu, K.S.; Kalivendi, S.V. Activation of p38/JNK pathway is responsible for embelin induced apoptosis in lung cancer cells: Transitional role of reactive oxygen species. PLoS ONE 2014, 9, e87050. [Google Scholar] [CrossRef]
- Park, B.-W.; Heo, M.-K.; Kim, K.-S.; Ko, S.-S.; Kim, S.-I.; Lee, K.-S. Mitogen-activated Protein Kinases Activities and c-erbB-2 Expression in Breast Cancer Carcinogenesis and Progression. Ann. Surg. Treatment Res. 2003, 64, 6–13. [Google Scholar]
- Cellurale, C.; Girnius, N.; Jiang, F.; Cavanagh-Kyros, J.; Lu, S.; Garlick, D.S.; Mercurio, A.M.; Davis, R.J. Role of JNK in mammary gland development and breast cancer. Cancer Res. 2012, 72, 472–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, B.; Konstantinovsky, S.; Kleinberg, L.; Nguyen, M.T.; Bassarova, A.; Kvalheim, G.; Nesland, J.M.; Reich, R. The mitogen-activated protein kinases (MAPK) p38 and JNK are markers of tumor progression in breast carcinoma. Gynecol. Oncol. 2006, 102, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, H.; Ma, N.; Wang, Y. Phosphorylated-p38 mitogen-activated protein kinase expression is associated with clinical factors in invasive breast cancer. Springerplus 2016, 5, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Zhi, H.; Lepp, A.; Wang, P.; Huang, J.; Basir, Z.; Chitambar, C.R.; Myers, C.R.; Chen, G. p38γ mitogen-activated protein kinase (MAPK) confers breast cancer hormone sensitivity by switching estrogen receptor (ER) signaling from classical to nonclassical pathway via stimulating ER phosphorylation and c-Jun transcription. J. Biol. Chem. 2012, 287, 14681–14691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Hung, M.-C. Regulation of the activity of p38 mitogen-activated protein kinase by Akt in cancer and adenoviral protein E1A-mediated sensitization to apoptosis. Mol. Cell. Biol. 2003, 23, 6836–6848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, B.; Li, H.; Zhang, M.; Xu, J.; Lu, Y.; Zheng, Y.; Qian, J.; Chang, J.T.; Yang, J.; Yi, Q. p38 MAPK inhibits breast cancer metastasis through regulation of stromal expansion. Int. J. Cancer 2015, 136, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, T.M.; Rincon, M. Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B.; Kidd, V.J.; Mak, T.W.; Ingram, A.J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 2002, 277, 12710–12717. [Google Scholar] [CrossRef] [Green Version]
- Cheol Park, Y.H.C.; Lee, W.H. Involvement of ERK and JNK Pathways in Apoptosis by Esculetin, a Coumarin Derivative, in Human Leukemic U937 Cells. Cancer Prev. Res. 2008, 13, 262–270. [Google Scholar]
- Chen, C.-Y.; Chen, K.-C.; Yang, T.-Y.; Liu, H.-C.; Hsu, S.-L. Gallic acid induces a reactive oxygen species-provoked c-Jun NH2-terminal kinase-dependent apoptosis in lung fibroblasts. Evid.-Based Complementary Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, S.; Lin, A. Apoptosis in cancer Carcinogenesis. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; He, T.; Chang, Y.; Zhao, Y.; Chen, X.; Bai, S.; Wang, L.; Shen, M.; She, G. The genus Alnus, a comprehensive outline of its chemical constituents and biological activities. Molecules 2017, 22, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roze, L.; Bikovens, O.; Telysheva, G. Determination and separation of diarylheptanoids from alder growing in Latvia. Environment. Technology. Resources (Latvia) 2011. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.I.; Rovelo, R.; Verjan, J.G.; Illescas, O.; Baeza, A.E.; De La Fuente, M.; Avila, I.; Navarrete, A. Anti-inflammatory activities, triterpenoids, and diarylheptanoids of Alnus acuminata ssp. arguta. Pharm. Biol. 2011, 49, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Kim, K.H.; Kwon, J.H.; Kim, S.B.; Kim, H.W.; Lee, M.W. Cytotoxic activities of diarylheptanoids from Alnus japonica. Arch. Pharmacal Res. 2008, 31, 1287. [Google Scholar] [CrossRef]
- Hu, W.; Wang, M.-H. Antioxidative activity and anti-inflammatory effects of diarylheptanoids isolated from Alnus hirsuta. J. Wood Sci. 2011, 57, 323–330. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Saha, A.; Kuzuhara, T.; Asakawa, Y. Bioactivity guided isolation of anticancer constituents from leaves of Alnus sieboldiana (Betulaceae). Phytomedicine 2011, 18, 491–498. [Google Scholar] [CrossRef]
- Sati, S.C.; Sati, N.; Sati, O. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn. Rev. 2011, 5, 174. [Google Scholar] [CrossRef] [Green Version]
- Tung, N.H.; Kim, S.K.; Ra, J.C.; Zhao, Y.-Z.; Sohn, D.H.; Kim, Y.H. Antioxidative and hepatoprotective diarylheptanoids from the bark of Alnus japonica. Planta Med. 2010, 76, 626–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynes, M.A. Solid-phase immunoassays. Curr. Protoc. Toxicol. 2005, 23, 18.17.11–18.17.19. [Google Scholar] [CrossRef] [PubMed]
- Lequin, R.M. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.S.; Tezuka, Y.; Awale, S.; Banskota, A.H.; Kadota, S. Six New Diarylheptanoids from the Seeds of Alpinia b lepharocalyx. J. Nat. Prod. 2001, 64, 289–293. [Google Scholar] [CrossRef]
- Dai, Y.; Thuong, P.T.; Hung, T.M.; Jin, W.; Cui, Z.; Bae, K.-H. Constituents and their DPPH Scavenging Activities from the Leaves of Alnus hirsuta (Spach) Rupr. Korean J. Med. Crop Sci. 2005, 13, 85–90. [Google Scholar]
- Hendry, D.Y.; Gueritte-Voegelein, F.; Insel, P.A.; Ferry, N.; Bouguet, J.; Potier, P.; Sevenet, T.; Hanoune, J. Isolation and characterization of 9-hydroxy-10-trans, 12-cis-octadecadienoic acid, a novel regulator of platelet adenylate cyclase from Glechoma hederacea L. Labiatae. Eur. J. Biochem. 1987, 170, 389–394. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Fouad, M.A.; Abdel-Lateff, A.; Okino, T.; Mohamed, G.A. Alnuheptanoid A: A new diarylheptanoid derivative from Alnus japonica. Nat. Prod. Res. 2014, 28, 1765–1771. [Google Scholar] [CrossRef]
- Ra, J.C.; Kim, Y.H.; Kwon, H.J.; Nguyen, H.T. Diaryl hepatonoid-based compounds useful as virus inhibitors. US Patent US 2011/0105738A1, 5 May 2011. [Google Scholar]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Dinić, J.; Novaković, M.; Podolski-Renić, A.; Stojković, S.; Mandić, B.; Tešević, V.; Vajs, V.; Isaković, A.; Pešić, M. Antioxidative activity of diarylheptanoids from the bark of black alder (Alnus glutinosa) and their interaction with anticancer drugs. Planta Med. 2014, 80, 1088–1096. [Google Scholar] [CrossRef]
- Lv, H.; She, G. Naturally Occurring Diarylheptanoids-A Supplementary Version. Rec. Nat. Prod. 2012, 6, 321–333. [Google Scholar]
- Burhans, W.C.; Weinberger, M.; Marchetti, M.A.; Ramachandran, L.; D’Urso, G.; Huberman, J.A. Apoptosis-like yeast cell death in response to DNA damage and replication defects. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 532, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.D.; Graça, J.; Mendes, V.; Chaves, S.R.; Amorim, M.A.; Mendes, M.V.; Moradas-Ferreira, P.; Côrte-Real, M.; Costa, V. Activation of the Hog1p kinase in Isc1p-deficient yeast cells is associated with mitochondrial dysfunction, oxidative stress sensitivity and premature aging. Mech. Ageing Dev. 2012, 133, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, D.R.; O’Neill, L.A.; Shields, D.C. The evolution of the MAP kinase pathways: Coduplication of interacting proteins leads to new signaling cascades. J. Mol. Evol. 1999, 49, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Fukazawa, T.; Sugiyama, Y.; Yagami, K.-I.; Urano, T.; Tanikawa, T. The advantage of enzyme-linked immunosorbent assay (ELISA) as a method of microbiological monitoring for rat virus (RV). Exp. Anim. 1991, 40, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Cusabio. Four Types of ELISA. Available online: https://www.cusabio.com/c-20659.html (accessed on 27 February 2018).
- Swanink, C.; Meis, J.; Rijs, A.; Donnelly, J.P.; Verweij, P.E. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J. Clin. Microbiol. 1997, 35, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Selman, L.; Henriksen, M.; Brandt, J.; Palarasah, Y.; Waters, A.; Beales, P.; Holmskov, U.; Jørgensen, T.; Nielsen, C.; Skjodt, K. An enzyme-linked immunosorbent assay (ELISA) for quantification of human collectin 11 (CL-11, CL-K1). J. Immunol. Methods 2012, 375, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Porsch-Özcürümez, M.; Kischel, N.; Priebe, H.; Splettstösser, W.; Finke, E.-J.; Grunow, R. Comparison of enzyme-linked immunosorbent assay, Western blotting, microagglutination, indirect immunofluorescence assay, and flow cytometry for serological diagnosis of tularemia. Clin. Diagn. Lab. Immunol. 2004, 11, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.W. Development of an enzyme-linked immunosorbent assay (ELISA) for the detection of pistachio residues in processed foods. Food Sci. Technol. 2010, 11, 1–150. [Google Scholar]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, Y.S.; Lee, H.-K.; Song, H.; Cho, D.-H.; Lee, W.J.; Hur, D.Y. Endoplasmic reticulum stress-mediated apoptosis of EBV-transformed B cells by cross-linking of CD70 is dependent upon generation of reactive oxygen species and activation of p38 MAPK and JNK pathway. J. Immunol. 2010, 185, 7274–7284. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Juan, G.; Li, X.; Gorczyca, W.; Murakami, T.; Traganos, F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis). Cytom. J. Int. Soc. Anal. Cytol. 1997, 27, 1–20. [Google Scholar] [CrossRef]
- Visconti, R.; Della Monica, R.; Grieco, D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016, 35, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, R.; dos Santos, S.; Andrade, L.; Severino, P.; Carvalho, A. Natural Products as Treatment against Cancer: A Historical and Current Vision. Clin. Oncol 2019, 4, 1–5. [Google Scholar]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.-M.; Shen, Z.-Z.; Fontana, J.A.; Barsky, S.H. Genistein’s “ER-dependent and independent” actions are mediated through ER pathways in ER-positive breast carcinoma cell lines. Anticancer. Res. 2000, 20, 2409–2416. [Google Scholar]
- Maminta, M.; Molteni, A.; Rosen, S. Stable expression of the human estrogen receptor in HeLa cells by infection: effect of estrogen on cell proliferation and c-myc expression. Mol. Cell. Endocrinol. 1991, 78, 61–69. [Google Scholar] [CrossRef]
- Yang, E.-J.; An, J.-H.; Son, Y.K.; Yeo, J.-H.; Song, K.-S. The cytotoxic constituents of Betula platyphylla and their effects on human lung A549 cancer cells. Nat. Prod. Sci. 2018, 24, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.-C.; Yu, C.-T.R.; Tsai, C.-H.; Tsai, J.-R.; Chen, J.-C.; Wu, C.-C.; Wu, W.-J.; Hsu, S.-L. An epidermal growth factor inhibitor, Gefitinib, induces apoptosis through a p53-dependent upregulation of pro-apoptotic molecules and downregulation of anti-apoptotic molecules in human lung adenocarcinoma A549 cells. Eur. J. Pharmacol. 2008, 600, 37–44. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davarinejad, H. Quantifications of Western Blots with ImageJ. Available online: https://imagej.nih.gov/ij/disclaimer.html (accessed on 27 February 2019).
- Lin, H.; Carlson, D.M.; St George, J.A.; Plopper, C.G.; Wu, R. An ELISA method for the quantitation of tracheal mucins from human and nonhuman primates. Am. J. Respir. Cell Mol. Biol. 1989, 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shigeyuki, H.; Michalek, S.M.; Mitsuo, T.; Ichijiro, M.; McGhee, J.R. An enzyme-linked immunosorbent assay (ELISA) for quantification of antibodies to Streptococcus mutans surface antigens. Mol. Immunol. 1983, 20, 453–464. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lin, S.-C.; Yang, S.-Y.; Pan, M.-Y.; Lin, Y.-W.; Hsu, C.-Y.; Wei, Y.-H.; Chang, J.-S.; Chang, C.-C. Prodigiosin-induced cytotoxicity involves RAD51 down-regulation through the JNK and p38 MAPK pathways in human breast carcinoma cell lines. Toxicol. Lett. 2012, 212, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, M.; Kubota, Y.; Nakamura, T.; Ichikawa, K. Regulation of stress-activated MAP kinase pathways during cell fate decisions. Nagoya J. Med. Sci. 2011, 73, 1–14. [Google Scholar] [PubMed]
- Koul, H.K.; Pal, M.; Koul, S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef]
- Kang, N.; Wang, M.-M.; Wang, Y.-H.; Zhang, Z.-N.; Cao, H.-R.; Lv, Y.-H.; Yang, Y.; Fan, P.-H.; Qiu, F.; Gao, X.-M. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Girnius, N.; Edwards, Y.J.; Garlick, D.S.; Davis, R.J. The cJUN NH2-terminal kinase (JNK) signaling pathway promotes genome stability and prevents tumor initiation. Elife 2018, 7, e36389. [Google Scholar] [CrossRef]
- Liu, J.; Wu, N.; Ma, L.-N.; Zhong, J.-T.; Liu, G.; Zheng, L.-H.; Lin, X.-K. p38 MAPK signaling mediates mitochondrial apoptosis in cancer cells induced by oleanolic acid. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 4519–4525. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Nikulenkov, F.; Zawacka-Pankau, J.; Li, H.; Gabdoulline, R.; Xu, J.; Eriksson, S.; Hedström, E.; Issaeva, N.; Kel, A. ROS-dependent activation of JNK converts p53 into an efficient inhibitor of oncogenes leading to robust apoptosis. Cell Death Differ. 2014, 21, 612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, R.P.; Zhang, R.; Batra, S.; Shi, Y.; Srivastava, S.K. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis 2009, 30, 1744–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, W.-H.; Hsieh, Y.-S.; Kuo, H.-C.; Teng, C.-Y.; Huang, H.-I.; Wang, C.-J.; Yang, S.-F.; Liou, Y.-S.; Kuo, W.-H. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch. Toxicol. 2007, 81, 719–728. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Tao, Z.; Zhao, L.; Zhu, Z.; Wu, W.; He, Y.; Chen, H.; Zheng, B.; Huang, X. Curcumin derivative WZ35 inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.T.; Huang, H.C.; Lin, J.K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010, 49, 141–151. [Google Scholar] [CrossRef]
- Santabarbara-Ruiz, P.; Lopez-Santillan, M.; Martinez-Rodriguez, I.; Binagui-Casas, A.; Perez, L.; Milán, M.; Corominas, M.; Serras, F. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLoS Genet. 2015, 11, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Yu, X.; Yang, H.; Wu, X. Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. PLoS ONE 2013, 8, e66781. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |


| Position | Compound 1 | Compound 2 | Compound 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δH | Multiplicity | δC | δH | Multiplicity | δC | δH | Multiplicity | δC | |
| 1 | 2.74 (t, J = 7.7 Hz) | CH2 | 30.7 | 2.83 (t, J = 15.5 Hz) | CH2 | 31.0 | 2.73 (m, J = 12.0 Hz) | CH2 | 29.9 |
| 2 | 2.78 (t, J = 7.7 Hz) | CH2 | 42.8 | 2.90 (t, J = 14.5 Hz) | CH2 | 43.3 | 2.71 (m, J = 11.5 Hz) | CH2 | 46.5 |
| 3 | - | C | 203.0 | - | C | 203.1 | - | C | 212.1 |
| 4 | 6.03 (d, J = 16.0 Hz) | CH | 131.7 | 6.26 (d, J = 15.5 Hz) | CH | 146.4 | 2.61 (m, H-4) | CH2 | 51.3 |
| 5 | 6.84 (dt, J = 16.0 Hz) | CH | 149.4 | 7.37 (d, J = 13.0 Hz) | CH | 143.8 | 4.0 (J = 13.0 Hz) | CH | 68.4 |
| 6 | 2.42 (t, J = 7.0 Hz) | CH2 | 35.8 | 6.96 (d, J = 15.5 Hz) | CH | 125.1 | 1.66 (dd, J = 9.0 Hz) | CH2 | 40.0 |
| 7 | 2.43 (t, J = 7.5 Hz) | CH2 | 34.6 | 6.84 (t, J = 15.5 Hz) | CH | 129.0 | 2.51 (m, H-7) | CH2 | 32.3 |
| 1′ | - | C | 133.1 | - | C | 133.5 | - | C | 133.4 |
| 2′, 6′ | 6.96 (d, J = 6.0 Hz) | CH2 | 130.5 | 7.03 (d, J = 8.5 Hz) | CH2 | 129.4, 129.0 | 6.99 (d, J = 8.5Hz) | CH2 | 130.4 |
| 3′, 5′ | 6.69 (d, J = 8.5 Hz) | CH2 | 116.3 | 6.69 (d, J = 8.5 Hz) | CH2 | 116.3, 116.3 | 6.69 (dd, J = 8.5Hz) | CH2 | 116.2 |
| 4′ | - | C | 156.7 | - | C | 156.8 | - | C | 156.5 |
| 1″ | - | C | 133.3 | - | C | 127.5 | - | C | 134.2 |
| 2″, 6″ | 6.98 (d, J = 6.0 Hz) | CH2 | 130.5 | 7.39 (d, J = 4.0 Hz) | CH2 | 130.5, 130.3 | 6.98 (d, J = 8.5Hz) | CH2 | 130.4 |
| 3″, 5″ | 6.70 (d, J = 8.5 Hz) | CH2 | 116.3 | 6.77 (d, J = 8.5 Hz) | CH2 | 116.9, 116.9 | 6.68 (dd, J = 9.0Hz) | CH2 | 116.3 |
| 4″ | - | C | 156.7 | - | C | 160.3 | - | C | 156.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, M.; Sung, C.K.; Im, Y.J.; Chun, C. Activation of JNK and p38 in MCF-7 Cells and the In Vitro Anticancer Activity of Alnus hirsuta Extract. Molecules 2020, 25, 1073. https://doi.org/10.3390/molecules25051073
Ryu M, Sung CK, Im YJ, Chun C. Activation of JNK and p38 in MCF-7 Cells and the In Vitro Anticancer Activity of Alnus hirsuta Extract. Molecules. 2020; 25(5):1073. https://doi.org/10.3390/molecules25051073
Chicago/Turabian StyleRyu, Mina, Chung Ki Sung, Young Jun Im, and ChangJu Chun. 2020. "Activation of JNK and p38 in MCF-7 Cells and the In Vitro Anticancer Activity of Alnus hirsuta Extract" Molecules 25, no. 5: 1073. https://doi.org/10.3390/molecules25051073
APA StyleRyu, M., Sung, C. K., Im, Y. J., & Chun, C. (2020). Activation of JNK and p38 in MCF-7 Cells and the In Vitro Anticancer Activity of Alnus hirsuta Extract. Molecules, 25(5), 1073. https://doi.org/10.3390/molecules25051073






