Protective Effects of Methoxsalen Supplementation on Chronic Alcohol-induced Osteopenia and Steatosis in Rats
Abstract
:1. Introduction
2. Results
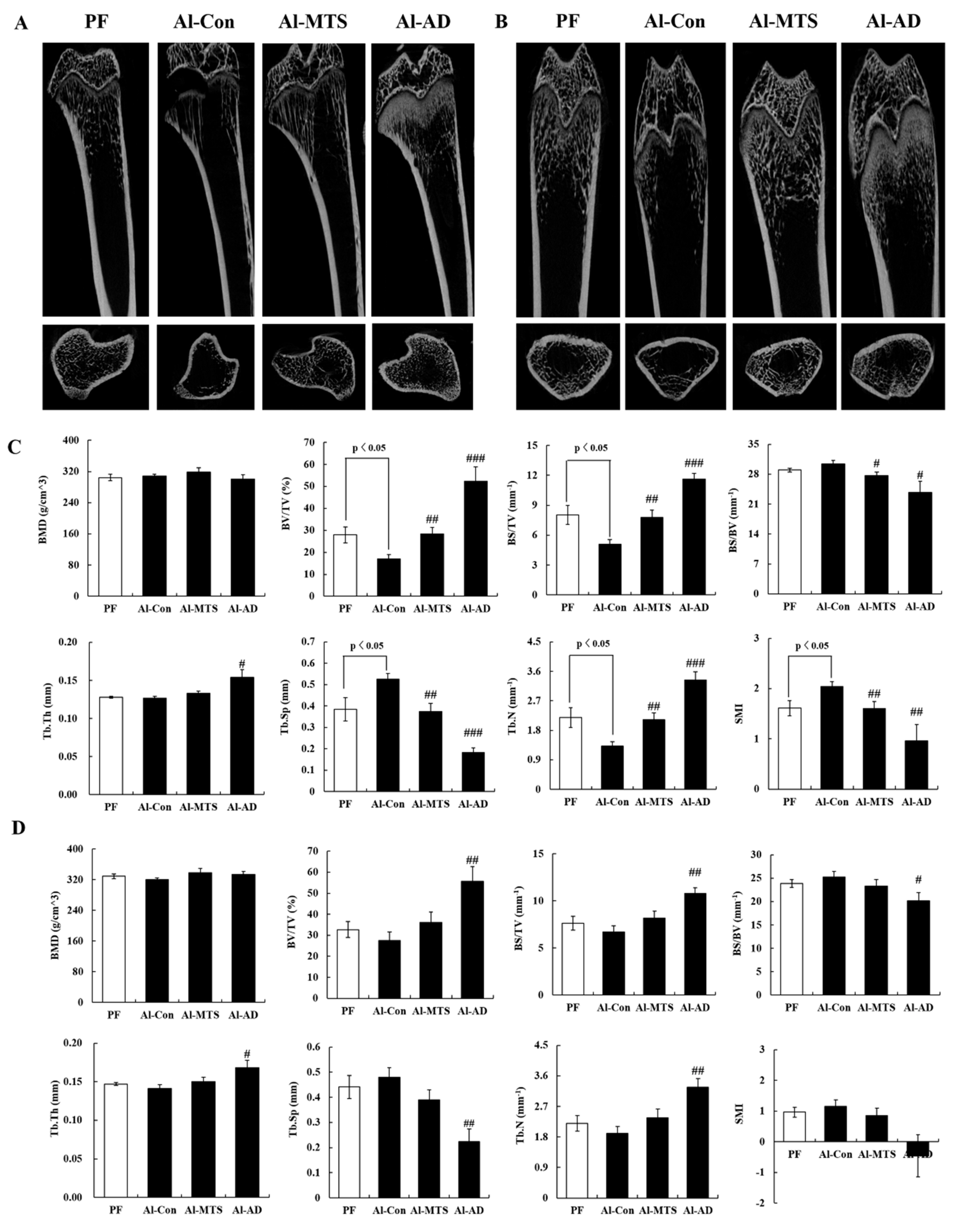
2.1. Bone Histomorphometric Parameters
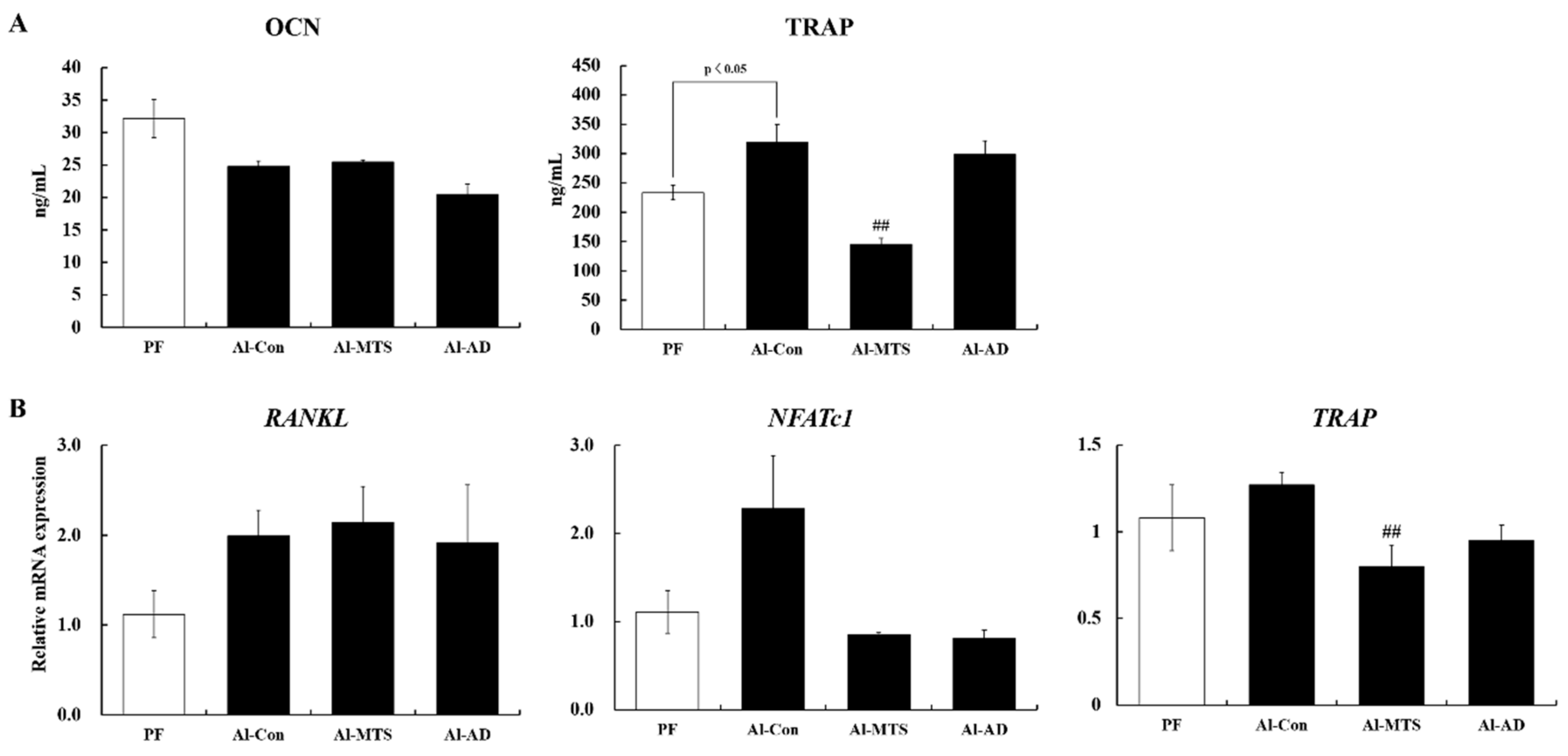
2.2. Serum Bone Turnover Markers and Bone Remodeling-Related Gene Expression
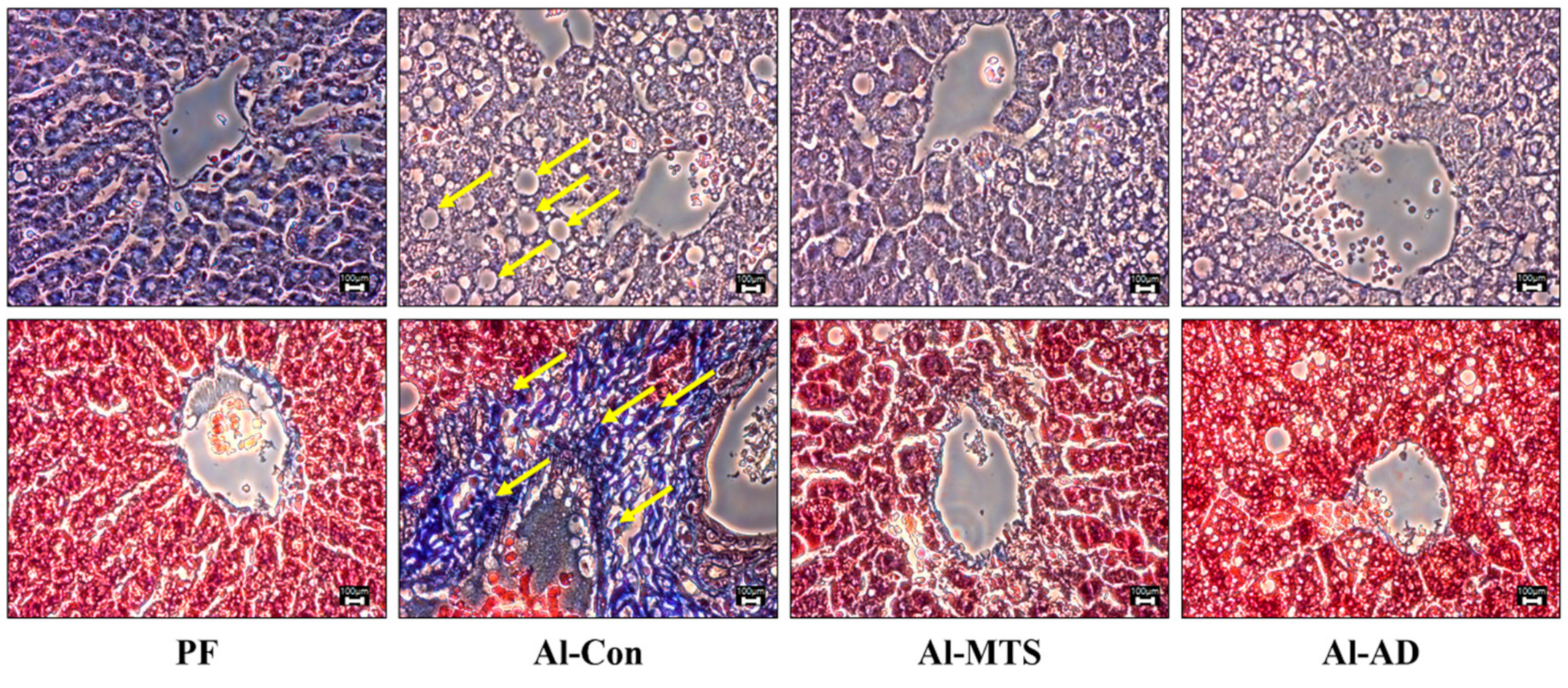
2.3. Hepatic Histology, Steatosis and Serum Alcohol Levels
2.4. Serum Hepatotoxicity and Inflammatory Markers
2.5. Hepatic Alcohol and Lipid Metabolic Enzyme Activities
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
- Group 1: pair-fed group (PF),
- Group 2: alcohol control group (Al-Con),
- Group 3: 0.005 % MTS (in diet) was supplemented with alcohol (Al-MTS) and
- Group 4: alendronate (500 μg/kg BW/day, oral) was administered alcohol (Al-AD).
4.2. Biomarkers In Serum
4.3. Micro-Computed Tomography (Micro-CT) Assay
4.4. Quantitative Real-Time PCR Analysis in Bone
4.5. Histological and Lipid Contents Analysis in Liver
4.6. Enzyme Activities in Liver
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rong, S.; Zhao, Y.; Bao, W.; Xiao, X.; Wang, D.; Nussler, A.K.; Yan, H.; Yao, P.; Liu, L. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine 2012, 19, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, Y.; Liu, Y.; Chen, H.; Shi, S.; Liu, Y. Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell. Mol. Life Sci. 2017, 74, 4443–4453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.P.; Miao, H.X.; Zheng, S.W.; Liu, W.L.; Chen, C.Q.; Zhong, H.B.; Li, S.F.; Fang, Y.P.; Sun, C.H. Risk factors for osteoporosis in liver cirrhosis patients measured by transient elastography. Medicine (Baltimore) 2018, 97, e10645. [Google Scholar] [CrossRef] [PubMed]
- González-Reimers, E.; García-Valdecasas-Campelo, E.; Santolaria-Fernández, F.; Milena-Abril, A.; Rodríguez-Rodríguez, E.; Martínez-Riera, A.; Pérez-Ramírez, A.; Alemán-Vallas, M.R. Rib fractures in chronic alcoholic men: Relationship with feeding habits, social problems, malnutrition, bone alterations, and liver dysfunction. Alcohol 2005, 37, 113–117. [Google Scholar] [CrossRef]
- Mercer, K.E.; Wynne, R.A.; Lazarenko, O.P.; Lumpkin, C.K.; Hogue, W.R.; Suva, L.J.; Chen, J.R.; Mason, A.Z.; Badger, T.M.; Ronis, M.J. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. J. Pharmacol. Exp. Ther. 2012, 343, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Leung, P.C.; Siu, W.S. Herbal treatment for osteoporosis: A current review. J. Tradit. Complement. Med. 2013, 3, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.K.; Ceska, O.; Warrington, P.J.; Ashwood-Smith, M.J. Increased furocoumarin content of celery during storage. J. Agric. Food Chem. 1985, 33, 1153–1157. [Google Scholar] [CrossRef]
- Melough, M.M.; Lee, S.G.; Cho, E.; Kim, K.; Provatas, A.A.; Perkins, C.; Park, M.K.; Qureshi, A.; Chun, O.K. Identification and quantitation of furocoumarins in popularly consumed foods in the US Using QuEChERS extraction coupled with UPLC-MS/MS analysis. J. Agric. Food Chem. 2017, 65, 5049–5055. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Czapski, J.; Czaczyk, K.; Biegańska-Marecik, R. The effect of pre-treatment and modified atmosphere packaging on contents of phenolic compounds and sensory and microbiological quality of shredded celeriac. J. Sci. Food Agric. 2014, 94, 1140–1148. [Google Scholar] [CrossRef]
- Engin, B.; Oguz, O. Evaluation of time-dependent response to psoralen plus UVA (PUVA) treatment with topical 8-methoxypsoralen (8-MOP) gel in palmoplantar dermatoses. Int. J. Dermatol. 2005, 44, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Woźniak, K.S.; Khan, M.T.H.; Orhan, I.E.; Sener, B.; Głowniak, K. An in vitro and in silico approach to cholinesterase inhibitory and antioxidant effects of the methanol extract, furanocoumarin fraction, and major coumarins of Angelica officinalis L. fruits. Phytochem. Lett. 2011, 4, 462–467. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Sakanaka, M.; Taniguchi, M.; Baba, K.; Kimura, Y. Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation. J. Nat. Med. 2014, 68, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Walasek, M.; Grzegorczyk, A.; Malm, A.; Skalicka-Woźniak, K. Bioactivity-guided isolation of antimicrobial coumarins from Heracleum mantegazzianum Sommier & Levier (Apiaceae) fruits by high-performance counter-current chromatography. Food Chem. 2015, 186, 133–138. [Google Scholar] [PubMed]
- Peng, Y.; Liu, W.; Xiong, J.; Gui, H.Y.; Feng, X.M.; Chen, R.N.; Hu, G.; Yang, J. Down regulation of differentiated embryonic chondrocytes 1 (DEC1) is involved in 8-methoxypsoralen-induced apoptosis in HepG2 cells. Toxicology 2012, 301, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.C.; Chou, C.T.; Liang, W.Z.; Kuo, C.C.; Hsu, S.S.; Wang, J.L.; Jan, C.R. Effect of methoxsalen on Ca2+ homeostasis and viability in human osteosarcoma cells. Chin. J. Physiol. 2017, 60, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, J.R.; Choi, R.Y.; Yee, S.T.; Hwang, Y.H.; Kim, M.J.; Lee, M.K. Methoxsalen supplementation attenuates bone loss and inflammatory response in ovariectomized mice. Chem. Biol. Interact. 2017, 278, 135–140. [Google Scholar] [CrossRef]
- Ham, J.R.; Choi, R.Y.; Lee, H.I.; Lee, M.K. Methoxsalen and bergapten prevent diabetes-induced osteoporosis by the suppression of osteoclastogenic gene expression in mice. Int. J. Mol. Sci. 2019, 20, 1298. [Google Scholar] [CrossRef] [Green Version]
- Maruel, D.B.; Boisseau, N.; Benhamou, C.L.; Jaffre, C. Alcohol and bone: review of dose effects and mechanisms. Osteoporosis Int. 2012, 23, 1–16. [Google Scholar] [CrossRef]
- Turner, R.T. Skeletal response to alcohol. Alcohol. Clin. Exp. Res. 2000, 24, 1693–1701. [Google Scholar] [CrossRef]
- McNerny, E.M.B.; Nickolas, T.L. Bone quality in chronic kidney disease: definitions and diagnostics. Curr. Osteoporosis Rep. 2017, 15, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wezeman, F.H.; Emanuele, M.A.; Moskal, S.F.; Steiner, J.; Lapaglia, N. Alendronate administration and skeletal response during chronic alcohol intake in the adolescent male rat. J. Bone Miner. Res. 2000, 15, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, T.; Rüegsegger, P. Quantification of bone microarchitecture with the structure model index. Comput. Methods Biomech. Biomed. Eng. 1997, 1, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.S.; Kim, C. Taurine chloramine inhibits osteoclastic differentiation and osteoclast marker expression in RAW 264.7 cells. Adv. Exp. Med. Biol. 2019, 1155, 61–70. [Google Scholar] [PubMed]
- Deyhim, F.; Garica, K.; Lopez, E.; Gonzalez, J.; Ino, S.; Garcia, M.; Patil, B.S. Citrus juice modulates bone strength in male senescent rat model of osteoporosis. Nutrition 2006, 22, 559–563. [Google Scholar] [CrossRef]
- Wang, W.; Wu, C.; Tian, B.; Liu, X.; Zhai, Z.; Qu, X.; Jiang, C.; Ouyang, Z.; Mao, Y.; Tang, T.; et al. The inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Sci. 2014, 15, 21913–21934. [Google Scholar] [CrossRef] [Green Version]
- Iitsuka, N.; Hie, M.; Nakanishi, A.; Tsukamoto, I. Ethanol increases osteoclastogenesis associated with the increased expression of RANK, PU. 1 and MITF in vitro and in vivo. Int. J. Mol. Med. 2012, 30, 165–172. [Google Scholar]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Bu, R.; Borysenko, C.W.; Li, Y.; Cao, L.; Sabokbar, A.; Blair, H.C. Expression and function of TNF-family proteins and receptors in human osteoblasts. Bone 2003, 33, 760–770. [Google Scholar] [CrossRef]
- Pietschmann, P.; Mechtcheriakova, D.; Meshcheryakova, A.; Föger-Samwald, U.; Ellinger, I. Immunology of osteoporosis: A mini-review. Gerontology 2016, 62, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Kawaratani, H.; Tsujimoto, T.; Douhara, A.; Takaya, H.; Moriya, K.; Namisaki, T.; Noguchi, R.; Yoshiji, H.; Fujimoto, M.; Fukui, H. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013, 2013, 495156–495165. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Hochrath, K.; Horvath, A.; Chen, P.; Seebauer, C.T.; Llorente, C.; Wang, L.; Alnouti, Y.; Fouts, D.E.; Stärkel, P.; et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018, 67, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.B.; Wu, C.; Dedousis, N.; Sipula, I.J.; Stefanovic-Racic, M.; Schoiswohl, G.; O’Donnell, C.P.; Alonso, L.C.; Kershaw, E.E.; Kelley, E.E.; et al. Adipose tissue-derived free fatty acids initiate myeloid cell accumulation in mouse liver in states of lipid oversupply. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E758CE770. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Villa, M.J.; Ojeda, M.L.; Rubio, J.M.; Murillo, M.L.; Sánchez, O.C. Beneficial role of dietary folic acid on cholesterol and bile acid metabolism in ethanol-fed rats. J. Stud. Alcohol Drugs 2000, 70, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, R.; Kager, L. The combustion of triolein-1-14C and its inhibition by alcohol in man. Life Sci. 1973, 13, 113–123. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [Green Version]
- Gaddini, G.W.; Turner, R.T.; Grant, K.A.; Iwaniec, U.T. Alcohol: a simple nutrient with complex actions on bone in the adult skeleton. Alcohol. Clin. Exp. Res. 2016, 40, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Zima, T. Alcohol abuse. EJIFCC. 2018, 29, 285–289. [Google Scholar]
- Kojima-Yuasa, A.; Goto, M.; Yoshikawa, E.; Morita, Y.; Sekiguchi, H.; Sutoh, K.; Usumi, K.; Matsui-Yuasa, I. Protective effects of hydrolyzed nucleoproteins from Salmon Milt against ethanol-induced liver injury in rats. Mar. Drugs 2016, 14, 232. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhuge, J.; Wang, X.; Bai, J.; Cederbaum, A.I. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology 2008, 47, 1483–1494. [Google Scholar] [CrossRef]
- Choi, R.Y.; Woo, M.J.; Ham, J.R.; Lee, M.K. Anti-steatotic and anti-inflammatory effects of Hovenia dulcis Thunb. extracts in chronic alcohol-fed rats. Biomed. Pharmacother. 2017, 90, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Giudetti, A.M.; Bellanti, F.; Priore, P.; Rollo, T.; Tamborra, R.; Siculella, L.; Vendemiale, G.; Altomare, E.; Gnoni, G.V. Oxidation of hepatic carnitine palmitoyl transferase-I (CPT-I) impairs fatty acid beta-oxidation in rats fed a methionine-choline deficient diet. PLoS ONE 2011, 6, e24084. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Seo, K.O.; Yun, K.W.; Kim, M.J.; Lee, M.K. Comparative study of the hepatoprotective efficacy of Artemisia iwayomogi and Artemisia capillaris on ethanol-administered mice. J. Food Sci. 2011, 76, 207–211. [Google Scholar] [CrossRef]
- Ham, J.R.; Lee, H.I.; Choi, R.Y.; Sim, M.O.; Seo, K.I.; Lee, M.K. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat diet-induced obese mice. Food Funct. 2016, 7, 689–697. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| PF | Al-Con | Al-MTS | Al-AD | |
|---|---|---|---|---|
| Ca (U/L) | 10.01 ± 0.25 | 9.35 ± 0.14 * | 9.71 ± 0.17 | 9.35 ± 0.24 |
| IP (U/L) | 8.14 ± 0.26 | 8.17 ± 0.26 | 7.80 ± 0.35 | 7.12 ± 0.45 |
| Alcohol (mg/L) | 33.75 ± 7.18 | 55.40 ± 5.09 * | 30.27 ± 3.66 ## | 28.12 ± 5.92 ## |
| Acetaldehyde (mg/L) | 41.85 ± 11.62 | 66.57 ± 19.37 | 28.22 ± 5.03 | 22.90 ± 3.45 |
| AST (U/L) | 94.20 ± 9.34 | 374.80 ± 93.30 * | 194.80 ± 24.63 | 319.83 ± 68.09 |
| ALT (U/L) | 30.00 ± 5.06 | 274.80 ± 87.44 * | 86.60 ± 15.14 | 116.40 ± 18.14 |
| TNF-α (pg/mL) | 208.67 ± 23.43 | 299.62 ± 19.86 * | 131.45 ± 34.99 ## | 237.33 ± 9.74 # |
| IL-6 (pg/mL) | 4.33 ± 0.16 | 3.92 ± 0.17 | 3.56 ± 0.21 | 3.40 ± 0.09 |
| PF | Al-Con | Al-MTS | Al-AD | |
|---|---|---|---|---|
| Hepatic lipid | ||||
| TG (mg/g) | 20.33 ± 1.33 | 30.01 ± 41.75 ** | 20.11 ± 0.84 ## | 29.94 ± 0.94 |
| Cholesterol (mg/g) | 3.90 ± 0.15 | 5.29 ± 0.23 ** | 4.78 ± 0.44 | 4.96 ± 0.50 |
| FFA (mmol/g) | 9.30 ± 0.92 | 11.61 ± 0.70 | 10.25 ± 0.49 | 11.40 ± 0.63 |
| Enzyme activity | ||||
| ADH1) | 384.77 ± 21.99 | 340.93 ± 8.61 | 347.45 ± 8.07 | 302.24 ± 15.16 # |
| ALDH1) | 661.77 ± 24.08 | 671.40 ± 25.19 | 634.03 ± 43.12 | 620.51 ± 30.78 |
| CYP2E12) | 17.99 ± 0.53 | 22.04 ± 1.35 * | 16.65 ± 1.03 # | 24.47 ± 1.35 |
| FAS3) | 4.11 ± 0.15 | 4.36 ± 0.08 | 2.72 ± 0.21 ### | 3.23 ± 0.35 |
| PAP2) | 41.26 ± 3.45 | 69.48 ± 5.61 ** | 45.34 ± 4.15 # | 71.94 ± 16.92 |
| β-oxidation2) | 1.33 ± 0.13 | 1.19 ± 0.10 | 1.57 ± 0.12 # | 1.55 ± 0.11 # |
| CPT2) | 11.32 ± 0.82 | 10.87 ± 0.33 | 12.54 ± 0.51 # | 11.79 ± 0.81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, J.R.; Choi, R.-Y.; Lee, H.-I.; Lee, M.-K. Protective Effects of Methoxsalen Supplementation on Chronic Alcohol-induced Osteopenia and Steatosis in Rats. Molecules 2020, 25, 1177. https://doi.org/10.3390/molecules25051177
Ham JR, Choi R-Y, Lee H-I, Lee M-K. Protective Effects of Methoxsalen Supplementation on Chronic Alcohol-induced Osteopenia and Steatosis in Rats. Molecules. 2020; 25(5):1177. https://doi.org/10.3390/molecules25051177
Chicago/Turabian StyleHam, Ju Ri, Ra-Yeong Choi, Hae-In Lee, and Mi-Kyung Lee. 2020. "Protective Effects of Methoxsalen Supplementation on Chronic Alcohol-induced Osteopenia and Steatosis in Rats" Molecules 25, no. 5: 1177. https://doi.org/10.3390/molecules25051177






