Antioxidant Properties of Camphene-Based Thiosemicarbazones: Experimental and Theoretical Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Radical Scavenging Activity
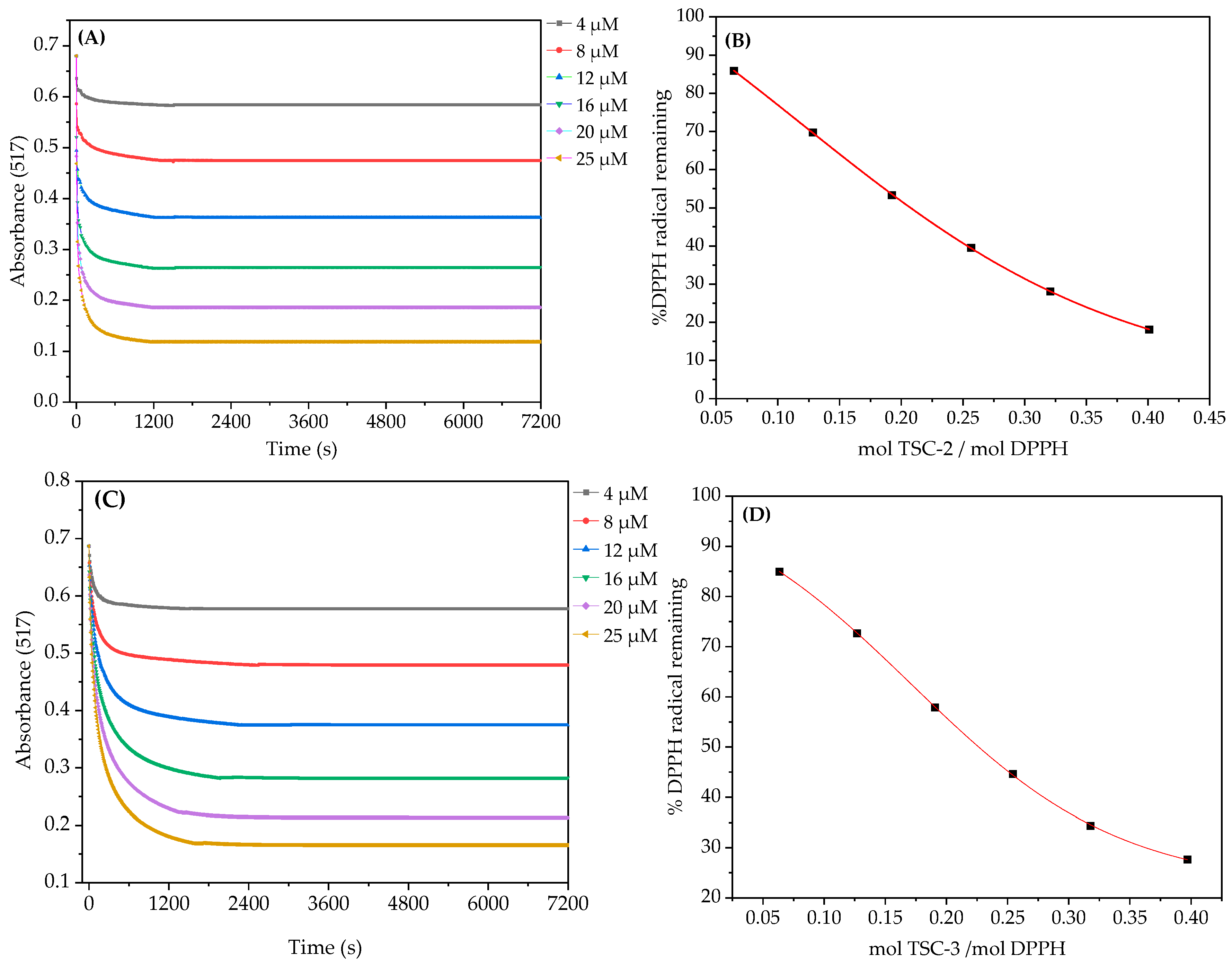
2.1.1. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
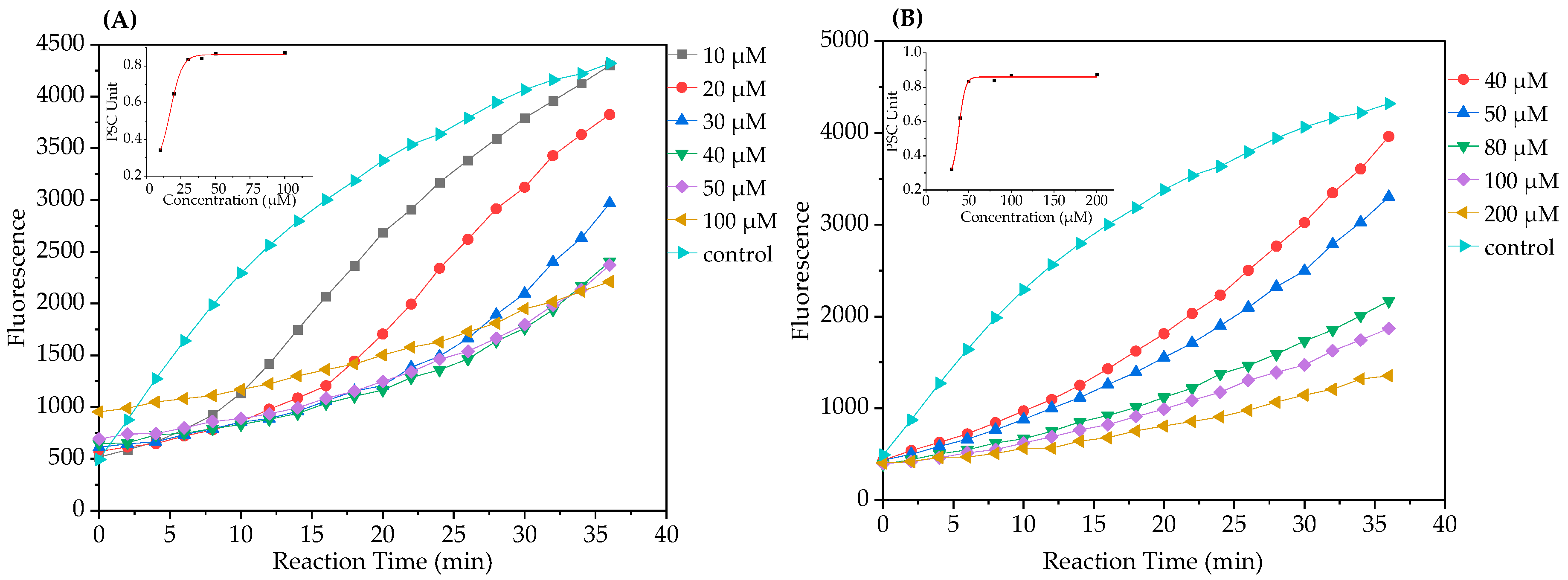
2.1.2. Peroxyl Radical Scavenging Capacity Assay
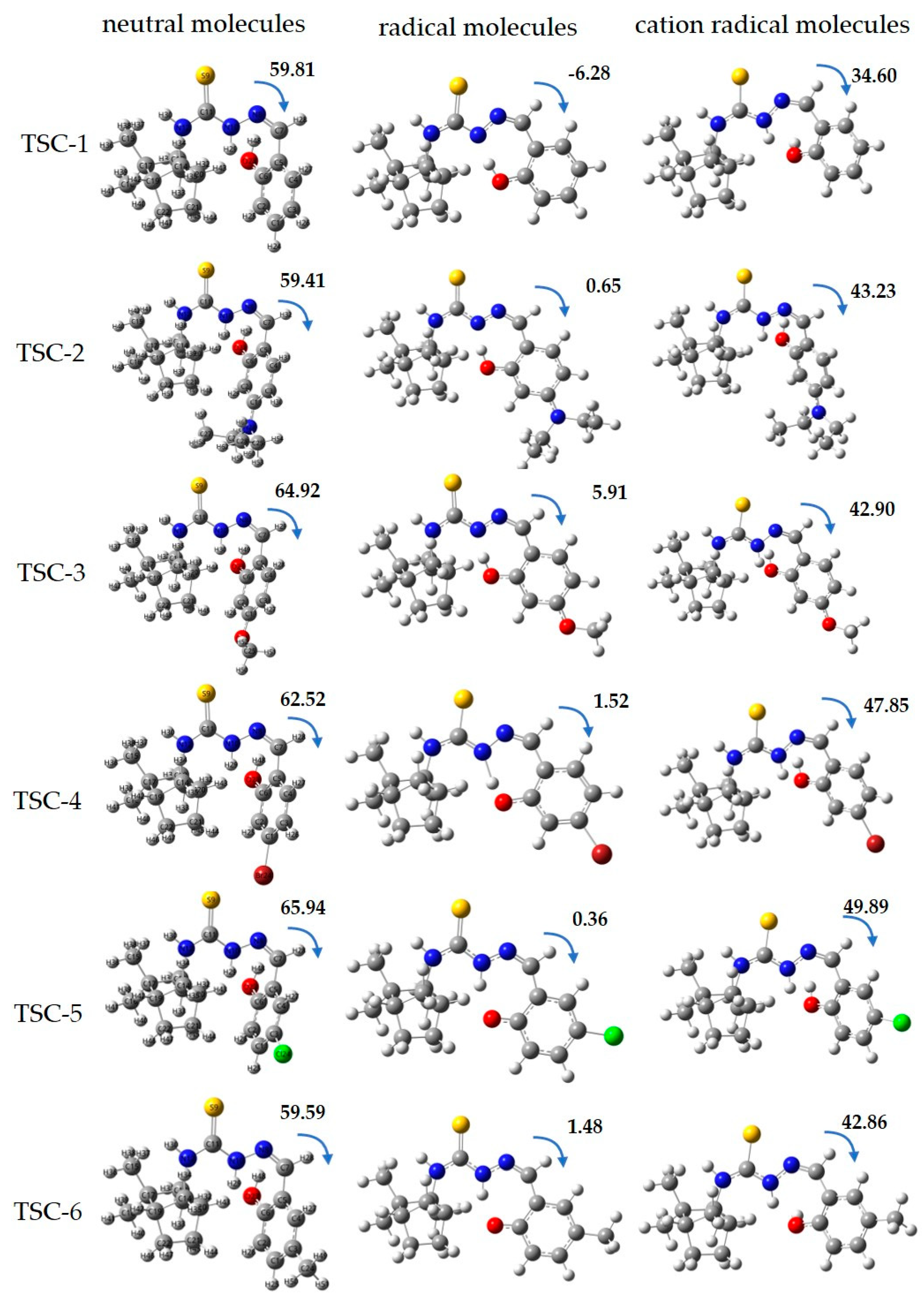
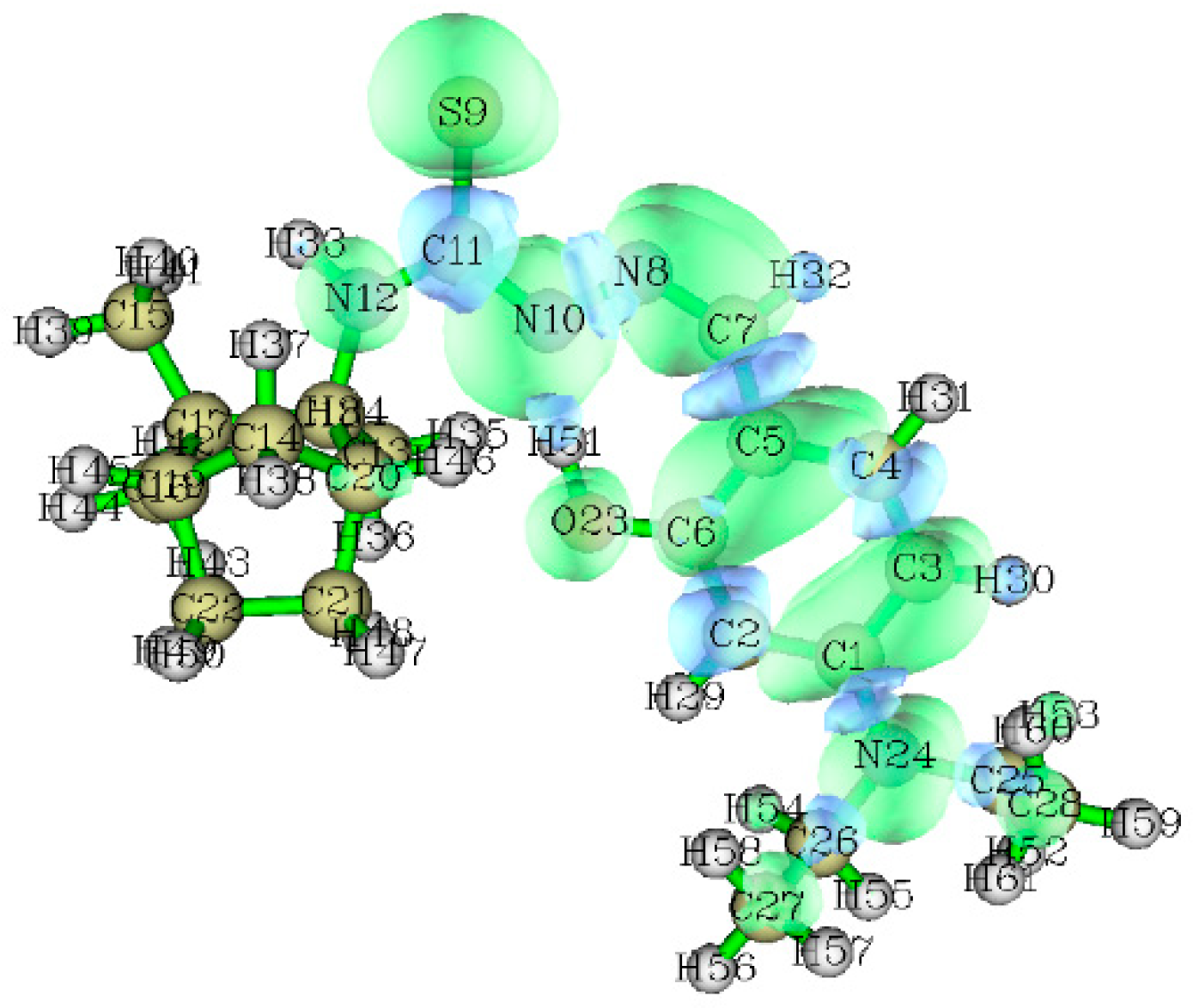
2.2. Theoretical Calculations
3. Materials and Methods
3.1. Synthesis of the Camphene-Based Thiosemicarbazones (TSC-1~6)
3.2. DPPH Radical Scavenging Assay
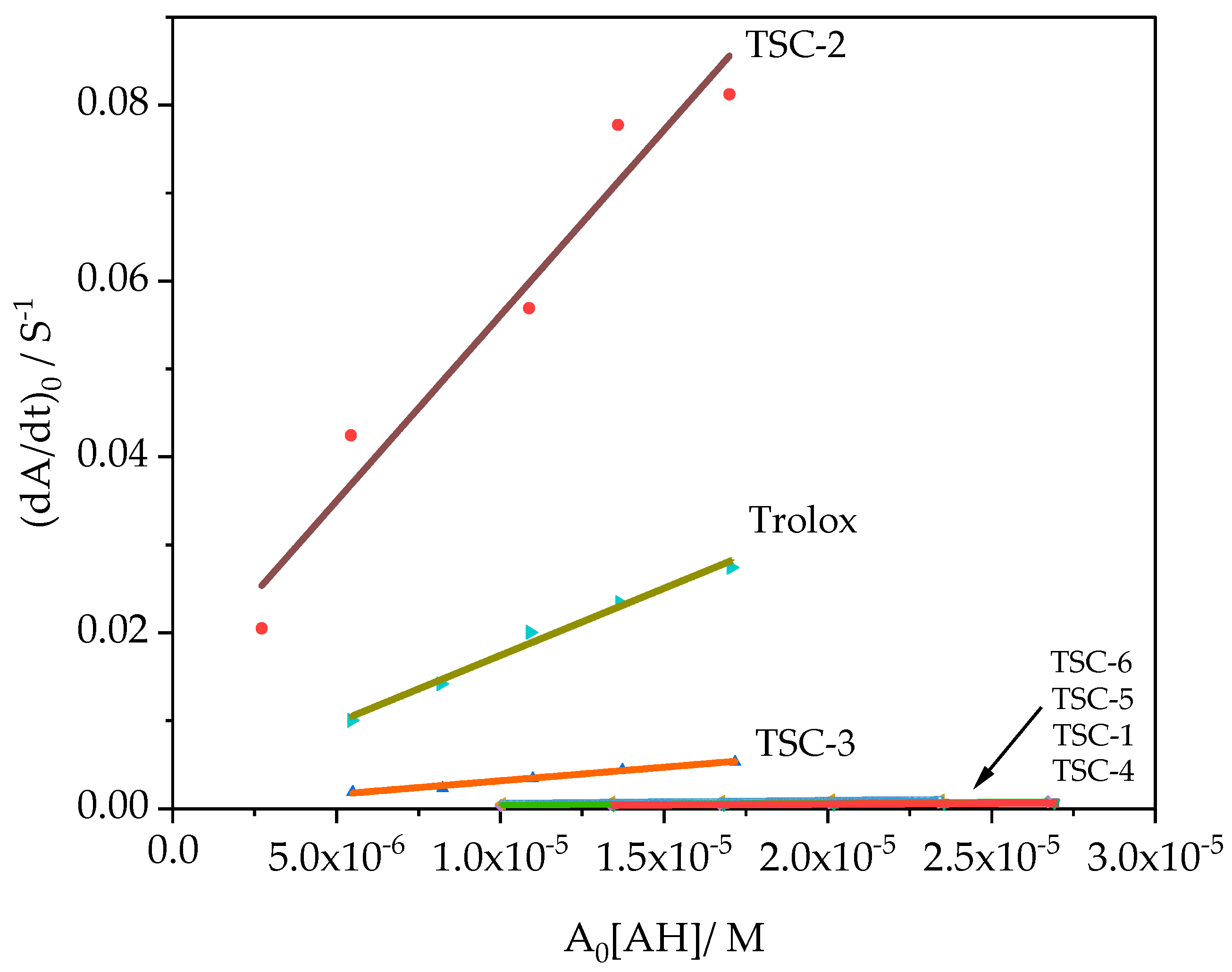
3.3. Scavenging DPPH Kinetics Assay
3.4. Peroxyl Radical Scavenging Capacity (PSC) Assay
3.5. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.; Thompson, A.; Agarwal, A.; Plessis, S.S.d. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. (Ijeb) 2010, 5, 425–435. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. Ijbs 2008, 4, 89–96. [Google Scholar]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of SE-glutathione peroxidase, catalase, and CU/ZN-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Andreescu, S.; Hepel, M. Preface. In Oxidative Stress: Diagnostics, Prevention, and Therapy; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. ix–x. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.M.; Moo-Huchin, M.I.; Estrada-León, R.J.; Cuevas-Glory, L.; Estrada-Mota, I.A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Yazdi, S.A.; Mirzaahmadi, A.; Khandar, A.A.; Eigner, V.; Dušek, M.; Lotfipour, F.; Mahdavi, M.; Soltani, S.; Dehghan, G. Synthesis, characterization and in vitro biological activities of new water-soluble copper(II), zinc(II), and nickel(II) complexes with sulfonato-substituted Schiff base ligand. Inorg. Chim. Acta 2017, 458, 171–180. [Google Scholar] [CrossRef]
- Hosseini-Yazdi, S.A.; Mirzaahmadi, A.; Khandar, A.A.; Eigner, V.; Dušek, M.; Mahdavi, M.; Soltani, S.; Lotfipour, F.; White, J. Reactions of copper(II), nickel(II), and zinc(II) acetates with a new water-soluble 4-phenylthiosemicarbazone Schiff base ligand: Synthesis, characterization, unexpected cyclization, antimicrobial, antioxidant, and anticancer activities. Polyhedron 2017, 124, 156–165. [Google Scholar] [CrossRef]
- Brodowska, K.; Correia, I.; Garribba, E.; Marques, F.; Klewicka, E.; Łodyga-Chruscińska, E.; Pessoa, J.C.; Dzeikala, A.; Chrusciński, L. Coordination ability and biological activity of a naringenin thiosemicarbazone. J. Inorg. Biochem. 2016, 165, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Subhashree, G.R.; Haribabu, J.; Saranya, S.; Yuvaraj, P.; Anantha Krishnan, D.; Karvembu, R.; Gayathri, D. In vitro antioxidant, antiinflammatory and in silico molecular docking studies of thiosemicarbazones. J. Mol. Struct. 2017, 1145, 160–169. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Le, T.H.; Bui, T.T.T. Antioxidant activities of thiosemicarbazones from substituted benzaldehydes and N-(tetra-O-acetyl-β-D-galactopyranosyl)thiosemicarbazide. Eur. J. Med. Chem. 2013, 60, 199–207. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Shan, Y.; Liu, F.; Xu, X.; Yang, Y.; Zhang, Q.; Zhang, Y.; Kuang, H.; Wang, Z.; et al. Design, synthesis and anticancer activity of novel nopinone-based thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 2360–2363. [Google Scholar] [CrossRef]
- Pavan, F.R.; Maia, P.I.d.S.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti–Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef]
- Cai, P.; Xiong, Y.; Yao, Y.; Chen, W.; Dong, X. Synthesis, screening and biological activity of potent thiosemicarbazone compounds as a tyrosinase inhibitor. New J. Chem. 2019, 43, 14102–14111. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants - Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr. 2009, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, I.; Hadzopoulou-Cladaras, M. Camphene, a Plant Derived Monoterpene, Exerts Its Hypolipidemic Action by Affecting SREBP-1 and MTP Expression. PLoS ONE 2016, 11, e0147117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavrakov, G.; Valcheva, V.; Philipova, I.; Doytchinova, I. Novel camphane-based anti-tuberculosis agents with nanomolar activity. Eur. J. Med. Chem. 2013, 70, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kokina, T.E.; Sheludyakova, L.A.; Eremina, Y.A.; Vorontsova, E.V.; Glinskaya, L.A.; Piryazev, D.A.; Lider, E.V.; Tkachev, A.V.; Larionov, S.V. Complexes of Cu(I) and Pd(II) with (+)-Camphor and (-)-Cavrone Thiosemicarbazones: Synthesis, Structure, and Cytotoxicity of the Pd(II) Complex. Russ. J. Gen. Chem. 2017, 87, 2332–2342. [Google Scholar] [CrossRef]
- Souza, M.R.P.; Coelho, N.P.; Baldin, V.P.; Scodro, R.B.L.; Cardoso, R.F.; da Silva, C.C.; Vandresen, F. Synthesis of novel (-)-Camphene-based thiosemicarbazones and evaluation of anti-Mycobacterium tuberculosis activity. Nat. Prod. Res. 2018, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Kuang, H.; Zhang, Y.; Gu, W.; Zhu, Y.; Wang, S. Synthesis and antitumor activity of 2-isocamphanyl thiosemicarbazone derivatives via ROS-enhanced mitochondrial damage. Chem. Biol. Drug Des. 2019, 94, 1281–1291. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and effective evaluation of the antioxidant capacity of propolis extracts using DPPH bleaching kinetic profiles, FT-IR and UV–Vis spectroscopic data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Rapid Peroxyl Radical Scavenging Capacity (PSC) Assay for Assessing both Hydrophilic and Lipophilic Antioxidants. J. Agric. Food Chem. 2005, 53, 6572–6580. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; You, L.; Yang, X.; Yang, J.; Chen, F.; Jiang, Y.; Yang, B. Identification of phenolics in litchi and evaluation of anticancer cell proliferation activity and intracellular antioxidant activity. Free Radic. Biol. Med. 2015, 84, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Amić, A.; Lučić, B.; Stepanić, V.; Marković, Z.; Marković, S.; Marković, J.M.D.; Amić, D. Free radical scavenging potency of quercetin catecholic colonic metabolites: Thermodynamics of 2H+/2e− processes. Food Chem. 2017, 218, 144–151. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Gaussian, version 09. Software for Theoretical Calculation. Gaussian Inc.: Wallingford, CT, USA, 2019.
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Wang, G.; Xue, Y.; An, L.; Zheng, Y.; Dou, Y.; Zhang, L.; Liu, Y. Theoretical study on the structural and antioxidant properties of some recently synthesised 2,4,5-trimethoxy chalcones. Food Chem. 2015, 171, 89–97. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas phase and in solvent. Theor. Chem. Acc. 2004, 111, 210–216. [Google Scholar] [CrossRef]
- Jin, R.; Li, J. Theoretical Study of the Radical Scavenging Activity of Shikonin and Its Derivatives. Chin. J. Chem. 2012, 30, 84–90. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Zhang, L.; An, L.; Chen, R.; Liu, Y.; Luo, Q.; Li, Y.; Wang, H.; Xue, Y. Computational study on the antioxidant property of coumarin-fused coumarins. Food Chem. 2020, 304, 125446. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.U.; Barbosa da Silva, A.P.; Ueda-Nakamura, T.; Dias Filho, B.P.; Conceicao da Silva, C.; Nakamura, C.V. Effects of a thiosemicarbazide camphene derivative on Trichophyton mentagrophytes. Molecules 2009, 14, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Moore, J.; Yu, L. High-Throughput Relative DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lwt - Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- CAMPOS, A.; Duran, N.; Lopez-Alarcon, C.; Lissi, E. Kinetic and stoichiometric evaluation of free radicals scavengers activities based on diphenyl-picryl hydrazyyl (DPPH) consumption. J. Chil. Chem. Soc. 2012, 57, 1381–1384. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.; Kantham, S.; Rao, V.M.; Palanivelu, M.K.; Pham, H.L.; Shaw, P.N.; McGeary, R.P.; Ross, B.P. Metal chelation, radical scavenging and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Nicklisch, S.C.T.; Spahn, J.E.; Zhou, H.; Gruian, C.M.; Waite, J.H. Redox Capacity of an Extracellular Matrix Protein Associated with Adhesion in Mytilus californianus. Biochemistry 2016, 55, 2022–2030. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.C.; Dao, D.Q.; Nguyen, M.T.; Nam, P.C. A DFT analysis on the radical scavenging activity of oxygenated terpenoids present in the extract of the buds of Cleistocalyx operculatus. Rsc Adv. 2017, 7, 39686–39698. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
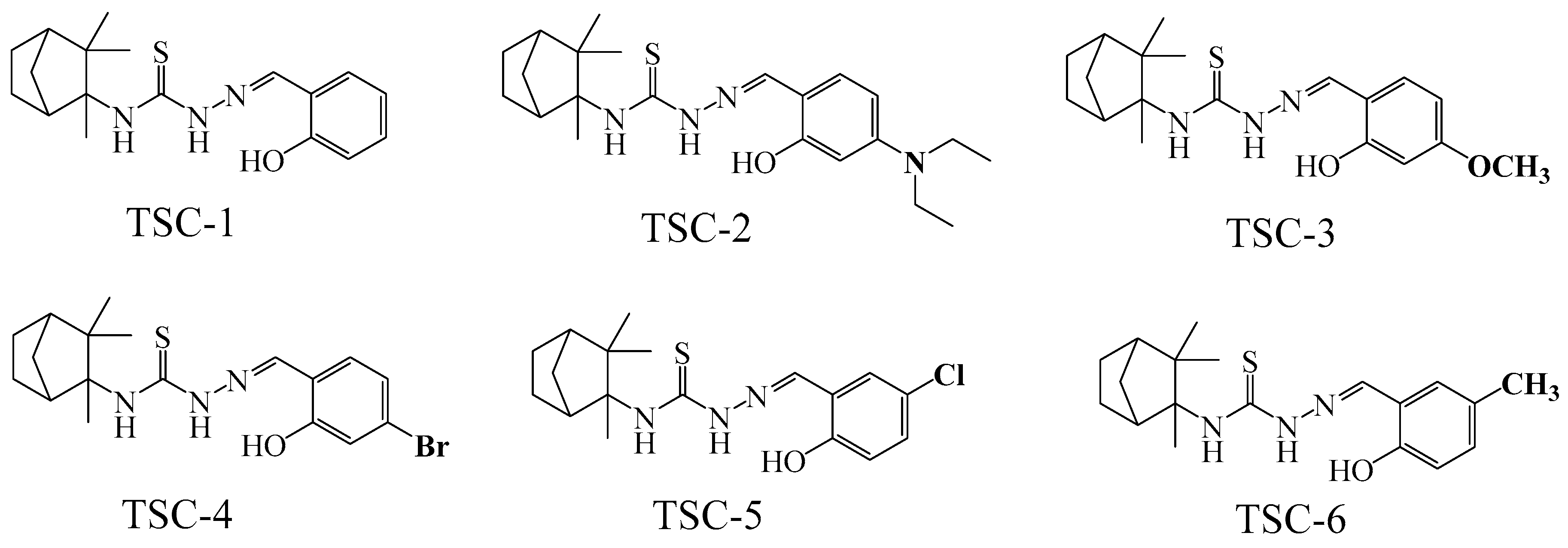


| Compounds | DPPH Assay | PSC Assay | ||||
|---|---|---|---|---|---|---|
| EC50(µM) at Fixed Time 1 | EC50 mol/mol DPPH Kinetic | Stoichiometric Factor (n) 2 | Bimolecular Rate Constant 3 kb (M−1 s−1) | EC50 (µM) | PSC Value (µmol of Trolox equiv/µmol) | |
| TSC-1 | 0.411 | 0.308 ± 0.003 | 1.29 ± 0.23 | 16.16 ± 1.15 | 62.26 ± 1.65 | 0.34 |
| TSC-2 | 0.208 | 0.208 ± 0.004 | 2.27 ± 0.13 | 4218.08 ± 551.26 | 16.78 ± 0.12 | 1.27 |
| TSC-3 | 0.246 | 0.226 ± 0.002 | 2.27 ± 0.20 | 308.71 ± 19.91 | 37.78 ± 0.13 | 0.56 |
| TSC-4 | 0.475 | 0.398 ± 0.006 | 1.21 ± 0.16 | 16.61 ± 0.75 | 34.62 ± 0.58 | 0.62 |
| TSC-5 | 0.393 | 0.341 ± 0.015 | 1.33 ± 0.23 | 22.87 ± 2.32 | 43.33 ± 0.99 | 0.49 |
| TSC-6 | 0.297 | 0.302 ± 0.015 | 1.44 ± 0.30 | 45.61 ± 4.13 | 48.38 ± 0.12 | 0.44 |
| Trolox | 0.241 | 0.249 ± 0.002 | 1.86 ± 0.12 | 3527.11 ± 103.95 | 21.38 ± 0.25 | 1 |
| Compounds | BDE 1 (kcal/mol) | kb (M−1 s−1) 2 | HOMO-LUMO Energy Gap (eV) | AIE (eV) 3 | ||
|---|---|---|---|---|---|---|
| NH | NH-N=CH | OH | ||||
| TSC-1 | 98.57 | 84.63 | 84.63 | 16.16 | 4.53 | 5.45 |
| TSC-2 | 98.49 | 80.86 | 81.24 | 4218 | 4.26 | 5.41 |
| TSC-3 | 98.59 | 85.33 | 85.92 | 308.71 | 4.67 | 5.49 |
| TSC-4 | 98.84 | 86.48 | 83.53 | 16.61 | 4.48 | 5.56 |
| TSC-5 | 98.51 | 86.54 | 85.47 | 22.87 | 4.53 | 5.56 |
| TSC-6 | 98.41 | 85.99 | 83.51 | 45.61 | 4.54 | 5.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Liu, H.; Xia, D.; Wang, S. Antioxidant Properties of Camphene-Based Thiosemicarbazones: Experimental and Theoretical Evaluation. Molecules 2020, 25, 1192. https://doi.org/10.3390/molecules25051192
Yang L, Liu H, Xia D, Wang S. Antioxidant Properties of Camphene-Based Thiosemicarbazones: Experimental and Theoretical Evaluation. Molecules. 2020; 25(5):1192. https://doi.org/10.3390/molecules25051192
Chicago/Turabian StyleYang, Lijuan, Haochuang Liu, Dasha Xia, and Shifa Wang. 2020. "Antioxidant Properties of Camphene-Based Thiosemicarbazones: Experimental and Theoretical Evaluation" Molecules 25, no. 5: 1192. https://doi.org/10.3390/molecules25051192





