Structure and Aggregation Mechanisms in Amyloids
Abstract
:1. Background
2. Protein Aggregation
3. Amyloid Fibrils
3.1. The Tinctorial Properties of Amyloid Fibrils
3.2. Structure of Amyloid Fibrils at the Subunit Level
3.3. The Cross-α Amyloid-Like Fibril
3.4. Hetero-Amyloid Fibrils
3.5. The Major Differences between the Techniques that Inform on Amyloid Structure
4. Toxic Species in Amyloid Diseases
5. Kinetics and Thermodynamics of Amyloid Fibril Formation
5.1. Aggregation Via a Nucleation-Dependent Mechanism
5.1.1. Primary Nucleation Mechanisms
5.1.2. Secondary Nucleation Mechanisms
5.2. Aggregation Via a Nucleation-Independent Mechanism
5.3. The Energy Landscape View of Protein Aggregation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C. World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018; pp. 6–7. [Google Scholar]
- Westermark, P.; Sletten, K.; Johansson, B.; Cornwell, G.G. Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc. Natl. Acad. Sci. USA 1990, 87, 2843–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banypersad, S.M.; Moon, J.C.; Whelan, C.; Hawkins, P.N.; Wechalekar, A.D. Updates in cardiac amyloidosis: A review. J. Am. Heart Assoc. 2012, 1, e000364. [Google Scholar] [CrossRef] [Green Version]
- Sipe, J.D. Amyloidosis. Annu. Rev. Biochem. 1992, 61, 947–975. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Mejía, L.M.; Ramirez-Alvarado, M. Systemic Amyloidoses. Annu. Rev. Biochem. 2013, 82, 745–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintas, A.; Saraiva, M.J.M.; Brito, R.M.M. The amyloidogenic potential of transthyretin variants correlates with their tendency to aggregate in solution. Febs Lett. 1997, 418, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Jesus, C.S.H.; Almeida, Z.L.; Vaz, D.C.; Faria, T.Q.; Brito, R.M.M. A New Folding Kinetic Mechanism for Human Transthyretin and the Influence of the Amyloidogenic V30M Mutation. Int. J. Mol. Sci. 2016, 17, 1428. [Google Scholar] [CrossRef] [Green Version]
- Takano, K.; Funahashi, J.; Yutani, K. The stability and folding process of amyloidogenic mutant human lysozymes. Eur. J. Biochem. 2001, 268, 155–159. [Google Scholar] [CrossRef]
- Isaacson, R.L.; Weeds, A.G.; Fersht, A.R. Equilibria and kinetics of folding of gelsolin domain 2 and mutants involved in familial amyloidosis-Finnish type. Proc. Natl. Acad. Sci. USA 1999, 96, 11247–11252. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.A.; Lazo, N.D.; Lomakin, A.; Condron, M.M.; Arai, H.; Yamin, G.; Rigby, A.C.; Teplow, D.B. Familial Alzheimer’s disease mutations alter the stability of the amyloid beta-protein monomer folding nucleus. Proc. Natl. Acad. Sci. USA 2007, 104, 16522–16527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, C.M. Protein misfolding, evolution and disease. Trends Biochem. Sci. 1999, 24, 329–332. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid—From bacteria to humans. Trends Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef]
- Pham, C.L.L.; Kwan, A.H.; Sunde, M. Functional amyloid: Widespread in Nature, diverse in purpose. Essays Biochem. 2014, 56, 207–219. [Google Scholar]
- Otzen, D.; Riek, R. Functional Amyloids. Cold Spring Harb. Perspect. Biol. 2019, a033860. [Google Scholar] [CrossRef]
- Avni, A.; Swasthi, H.M.; Majumdar, A.; Mukhopadhyay, S. Intrinsically disordered proteins in the formation of functional amyloids from bacteria to humans. In Progress in Molecular Biology and Translational Science; Academic Press, Elsevier: Cambridge, MA, USA, 2019; Volume 166, pp. 109–143. [Google Scholar]
- Sipe, J.D.; Cohen, A.S. Review: History of the Amyloid Fibril. J. Struct. Biol. 2000, 130, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Makin, O.S.; Serpell, L.C. Structures for amyloid fibrils. FEBS J. 2005, 272, 5950–5961. [Google Scholar] [CrossRef]
- Close, W.; Neumann, M.; Schmidt, A.; Hora, M.; Annamalai, K.; Schmidt, M.; Reif, B.; Schmidt, V.; Grigorieff, N.; Fändrich, M. Physical basis of amyloid fibril polymorphism. Nat. Commun. 2018, 9, 699. [Google Scholar] [CrossRef]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.Ø.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalandoni-Buan, A.C.; Decena-Soliven, A.L.A.; Cao, E.P.; Barraquio, V.L.; Barraquio, W.L. Characterization and Identification of Congo Red Decolorizing Bacteria from Monocultures and Consortia. Philipp. J. Sci. 2010, 139, 71–78. [Google Scholar]
- Nilsson, M.R. Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Jacob, R.F.; Mason, R.P. Quantifying Amyloid β-Peptide (Aβ) Aggregation Using the Congo Red-Aβ (CR–Aβ) Spectrophotometric Assay. Anal. Biochem. 1999, 266, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Eisert, R.; Felau, L.; Brown, L.R. Methods for enhancing the accuracy and reproducibility of Congo red and thioflavin T assays. Anal. Biochem. 2006, 353, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Howie, A.J.; Brewer, D.B. Optical properties of amyloid stained by Congo red: History and mechanisms. Micron 2009, 40, 285–301. [Google Scholar] [CrossRef]
- Sen, S.; Basdemir, G. Diagnosis of renal amyloidosis using Congo red fluorescence. Pathol. Int. 2003, 53, 534–538. [Google Scholar] [CrossRef]
- Giorgadze, T.A.; Shiina, N.; Baloch, Z.W.; Tomaszewski, J.E.; Gupta, P.K. Improved detection of amyloid in fat pad aspiration: An evaluation of Congo red stain by fluorescent microscopy. Diagn. Cytopathol. 2004, 31, 300–306. [Google Scholar] [CrossRef]
- Levine, H. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of Amyloid Fibril Formation by Polyphenols: Structural Similarity and Aromatic Interactions as a Common Inhibition Mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef]
- Turnell, W.G.; Finch, J.T. Binding of the dye congo red to the amyloid protein pig insulin reveals a novel homology amongst amyloid-forming peptide sequences. J. Mol. Biol. 1992, 227, 1205–1223. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Randolph, T.W.; Manning, M.C.; Stevens, F.J.; Carpenter, J.F. Congo red populates partially unfolded states of an amyloidogenic protein to enhance aggregation and amyloid fibril formation. J. Biol. Chem. 2003, 278, 10842–10850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caughey, B.; Ernst, D.; Race, R.E. Congo red inhibition of scrapie agent replication. J. Virol. 1993, 67, 6270–6272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, A.; Yankner, B.A. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. USA 1994, 91, 12243–12247. [Google Scholar] [CrossRef] [Green Version]
- Chander, H.; Chauhan, A.; Chauhan, V. Binding of Proteases to Fibrillar Amyloid-β Protein and its Inhibition by Congo Red. J. Alzheimer’s Dis. 2007, 12, 261–269. [Google Scholar] [CrossRef]
- Vassar, P.S.; Culling, C.F. Fluorescent stains, with special reference to amyloid and connective tissues. Arch. Pathol. 1959, 68, 487–498. [Google Scholar]
- Kelényi, G. Thioflavin S fluorescent and Congo red anisotropic stainings in the histologic demonstration of amyloid. Acta Neuropathol. 1967, 7, 336–348. [Google Scholar] [CrossRef]
- Younan, N.D.; Viles, J.H. A Comparison of Three Fluorophores for the Detection of Amyloid Fibers and Prefibrillar Oligomeric Assemblies. ThT (Thioflavin T); ANS (1-Anilinonaphthalene-8-sulfonic Acid); and bisANS (4,4′-Dianilino-1,1′-binaphthyl-5,5′-disulfonic Acid). Biochemistry 2015, 54, 4297–4306. [Google Scholar] [CrossRef]
- Nagarajan, S.; Lapidus, L.J. Fluorescent Probe DCVJ Shows High Sensitivity for Characterization of Amyloid β-Peptide Early in the Lag Phase. ChemBioChem 2017, 18, 2205–2211. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.; Sjölander, D.; Hammarström, P. Spectroscopic characterization of diverse amyloid fibrils in vitro by the fluorescent dye Nile red. Mol. Biosyst. 2011, 7, 1232–1240. [Google Scholar] [CrossRef]
- Kovalska, V.; Chernii, S.; Losytskyy, M.; Tretyakova, I.; Dovbii, Y.; Gorski, A.; Chernii, V.; Czerwieniec, R.; Yarmoluk, S. Design of functionalized β-ketoenole derivatives as efficient fluorescent dyes for detection of amyloid fibrils. New J. Chem. 2018, 42, 13308–13318. [Google Scholar] [CrossRef] [Green Version]
- Fanni, A.M.; Monge, F.A.; Lin, C.-Y.; Thapa, A.; Bhaskar, K.; Whitten, D.G.; Chi, E.Y. High Selectivity and Sensitivity of Oligomeric p -Phenylene Ethynylenes for Detecting Fibrillar and Prefibrillar Amyloid Protein Aggregates. ACS Chem. Neurosci. 2019, 10, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Abbasbeigi, S.; Adibi, H.; Moradi, S.; Ghadami, S.A.; Khodarahmi, R. Detection/quantification of amyloid aggregation in solution using the novel fluorescent benzofuranone-derivative compounds as amyloid fluorescent probes: Synthesis and in vitro characterization. J. Iran. Chem. Soc. 2019, 16, 1225–1237. [Google Scholar] [CrossRef]
- Crystal, A.S.; Giasson, B.I.; Crowe, A.; Kung, M.-P.; Zhuang, Z.-P.; Trojanowski, J.Q.; Lee, V.M.-Y. A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J. Neurochem. 2003, 86, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.L.; Schuck, T.; Sheridan, S.; Kung, M.P.; Kung, H.; Zhuang, Z.P.; Bergeron, C.; Lamarche, J.S.; Skovronsky, D.; Giasson, B.I.; et al. The fluorescent Congo red derivative, (trans, trans)-1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (BSB), labels diverse beta-pleated sheet structures in postmortem human neurodegenerative disease brains. Am. J. Pathol. 2001, 159, 937–943. [Google Scholar] [CrossRef]
- Styren, S.D.; Hamilton, R.L.; Styren, G.C.; Klunk, W.E. X-34, A Fluorescent Derivative of Congo Red: A Novel Histochemical Stain for Alzheimer’s Disease Pathology. J. Histochem. Cytochem. 2000, 48, 1223–1232. [Google Scholar] [CrossRef] [Green Version]
- He, X.-P.; Deng, Q.; Cai, L.; Wang, C.-Z.; Zang, Y.; Li, J.; Chen, G.-R.; Tian, H. Fluorogenic Resveratrol-Confined Graphene Oxide for Economic and Rapid Detection of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2014, 6, 5379–5382. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Balanda, A.O.; Losytskyy, M.Y.; Golub, A.G.; Vermeij, R.J.; Subramaniam, V.; Tolmachev, O.I.; Yarmoluk, S.M. Specific fluorescent detection of fibrillar α-synuclein using mono- and trimethine cyanine dyes. Bioorganic Med. Chem. 2008, 16, 1452–1459. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Balanda, A.O.; Vermeij, R.J.; Subramaniam, V.; Slominskii, Y.L.; Yarmoluk, S.M. Cyanine dye–protein interactions: Looking for fluorescent probes for amyloid structures. J. Biochem. Biophys. Methods 2007, 70, 727–733. [Google Scholar] [CrossRef]
- Luna-Muñoz, J.; Peralta-Ramirez, J.; Chávez-Macías, L.; Harrington, C.R.; Wischik, C.M.; Mena, R. Thiazin red as a neuropathological tool for the rapid diagnosis of Alzheimer’s disease in tissue imprints. Acta Neuropathol. 2008, 116, 507–515. [Google Scholar] [CrossRef]
- Sulatskaya, A.I.; Sulatsky, M.I.; Antifeeva, I.A.; Kuznetsova, I.M.; Turoverov, K.K. Structural Analogue of Thioflavin T, DMASEBT, as a Tool for Amyloid Fibrils Study. Anal. Chem. 2019, 91, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid nomenclature 2018: Recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2018, 25, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, A.J.; Parker, K.D.; Atkins, E.D.T.; Beighton, E. “Cross-β” conformation in proteins. J. Mol. Biol. 1968, 32, 343–358. [Google Scholar] [CrossRef]
- Sunde, M.; Blake, C. The Structure of Amyloid Fibrils by Electron Microscopy and X-Ray Diffraction. Adv. Protein Chem. 1997, 50, 123–159. [Google Scholar]
- Kodali, R.; Wetzel, R. Polymorphism in the intermediates and products of amyloid assembly. Curr. Opin. Struct. Biol. 2007, 17, 48–57. [Google Scholar] [CrossRef]
- Eanes, E.D.; Glennner, G.G. X-ray Diffraction Studies on Amyloid Filaments. J. Histochem. Cytochem. 1968, 16, 673–677. [Google Scholar] [CrossRef]
- Bonar, L.; Cohen, A.S.; Skinner, M.M. Characterization of the Amyloid Fibril as a Cross- Protein. Exp. Biol. Med. 1969, 131, 1373–1375. [Google Scholar] [CrossRef]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.; Eisenberg, D. Recent atomic models of amyloid fibril structure. Curr. Opin. Struct. Biol. 2006, 16, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.I.; Thompson, M.J.; Eisenberg, D. A systematic screen of β2-microglobulin and insulin for amyloid-like segments. Proc. Natl. Acad. Sci. USA 2006, 103, 4079–4082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, D.S.; Sawaya, M.R. Structural Studies of Amyloid Proteins at the Molecular Level. Annu. Rev. Biochem. 2017, 86, 69–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambashivan, S.; Liu, Y.; Sawaya, M.R.; Gingery, M.; Eisenberg, D. Amyloid-like fibrils of ribonuclease A with three-dimensional domain-swapped and native-like structure. Nature 2005, 437, 266–269. [Google Scholar] [CrossRef]
- Fändrich, M.; Meinhardt, J.; Grigorieff, N. Structural polymorphism of Alzheimer Aβ and other amyloid fibrils. Prion 2009, 3, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Meinhardt, J.; Sachse, C.; Hortschansky, P.; Grigorieff, N.; Fändrich, M. Aβ(1-40) Fibril Polymorphism Implies Diverse Interaction Patterns in Amyloid Fibrils. J. Mol. Biol. 2009, 386, 869–877. [Google Scholar] [CrossRef]
- Zapadka, K.L.; Becher, F.J.; Gomes dos Santos, A.L.; Jackson, S.E. Factors affecting the physical stability (aggregation) of peptide therapeutics. Interface Focus 2017, 7, 20170030. [Google Scholar] [CrossRef] [Green Version]
- Conchillo-Solé, O.; de Groot, N.S.; Avilés, F.X.; Vendrell, J.; Daura, X.; Ventura, S. AGGRESCAN: A server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinform. 2007, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- de Groot, N.S.; Castillo, V.; Graña-Montes, R.; Ventura, S. AGGRESCAN: Method, Application, and Perspectives for Drug Design. In Methods in Molecular Biology (Clifton, N.J.); Springer: New York, NY, USA, 2012; Volume 819, pp. 199–220. [Google Scholar]
- Zambrano, R.; Jamroz, M.; Szczasiuk, A.; Pujols, J.; Kmiecik, S.; Ventura, S. AGGRESCAN3D (A3D): Server for prediction of aggregation properties of protein structures. Nucleic Acids Res. 2015, 43, W306–W313. [Google Scholar] [CrossRef]
- Pujols, J.; Peña-Díaz, S.; Ventura, S. AGGRESCAN3D: Toward the Prediction of the Aggregation Propensities of Protein Structures. In Methods in Molecular Biology (Clifton, N.J.); Springer: New York, NY, USA, 2018; Volume 1762, pp. 427–443. [Google Scholar]
- Wozniak, P.P.; Kotulska, M. AmyLoad: Website dedicated to amyloidogenic protein fragments. Bioinformatics 2015, 31, 3395–3397. [Google Scholar] [CrossRef] [Green Version]
- Burdukiewicz, M.; Sobczyk, P.; Rödiger, S.; Duda-Madej, A.; Mackiewicz, P.; Kotulska, M. Amyloidogenic motifs revealed by n-gram analysis. Sci. Rep. 2017, 7, 12961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, C.W.; Waldispühl, J.; Lis, M.; Halfmann, R.; Devadas, S.; Lindquist, S.; Berger, B. A method for probing the mutational landscape of amyloid structure. Bioinformatics 2011, 27, i34–i42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frousios, K.K.; Iconomidou, V.A.; Karletidi, C.-M.; Hamodrakas, S.J. Amyloidogenic determinants are usually not buried. BMC Struct. Biol. 2009, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsolis, A.C.; Papandreou, N.C.; Iconomidou, V.A.; Hamodrakas, S.J. A Consensus Method for the Prediction of ‘Aggregation-Prone’ Peptides in Globular Proteins. PLoS ONE 2013, 8, e54175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Família, C.; Dennison, S.R.; Quintas, A.; Phoenix, D.A. Prediction of Peptide and Protein Propensity for Amyloid Formation. PLoS ONE 2015, 10, e0134679. [Google Scholar] [CrossRef] [Green Version]
- Bryan, A.W.; Menke, M.; Cowen, L.J.; Lindquist, S.L.; Berger, B. BETASCAN: Probable β-amyloids Identified by Pairwise Probabilistic Analysis. PLoS Comput. Biol. 2009, 5, e1000333. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Hoover, J.; et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016, 44, D862–D868. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei Ghomi, H.; Topp, E.M.; Lill, M.A. Fibpredictor: A computational method for rapid prediction of amyloid fibril structures. J. Mol. Modeling 2016, 22, 206. [Google Scholar] [CrossRef]
- Gasior, P.; Kotulska, M. FISH Amyloid—A new method for finding amyloidogenic segments in proteins based on site specific co-occurence of aminoacids. BMC Bioinform. 2014, 15, 54. [Google Scholar] [CrossRef] [Green Version]
- Garbuzynskiy, S.O.; Lobanov, M.Y.; Galzitskaya, O.V. FoldAmyloid: A method of prediction of amyloidogenic regions from protein sequence. Bioinformatics 2010, 26, 326–332. [Google Scholar] [CrossRef]
- Thangakani, A.M.; Kumar, S.; Nagarajan, R.; Velmurugan, D.; Gromiha, M.M. GAP: Towards almost 100 percent prediction for β-strand-mediated aggregating peptides with distinct morphologies. Bioinformatics 2014, 30, 1983–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, T.Z.; Jernigan, R.L.; Garnier, J.; Kloczkowski, A. GOR V server for protein secondary structure prediction. Bioinformatics 2005, 21, 2787–2788. [Google Scholar] [CrossRef] [PubMed]
- Kouza, M.; Faraggi, E.; Kolinski, A.; Kloczkowski, A. The GOR Method of Protein Secondary Structure Prediction and Its Application as a Protein Aggregation Prediction Tool. In Methods in Molecular Biology (Clifton, N.J.); Humana Press: New York, NY, USA, 2017; Volume 1484, pp. 7–24. [Google Scholar]
- Emily, M.; Talvas, A.; Delamarche, C. MetAmyl: A METa-Predictor for AMYLoid Proteins. PLoS ONE 2013, 8, e79722. [Google Scholar] [CrossRef] [PubMed]
- Munir, F.; Gull, S.; Asif, A.; Minhas, F.u.A.A. MILAMP: Multiple Instance Prediction of Amyloid Proteins. IEEE/ACM Trans. Comput. Biol. Bioinform. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Choi, J.; Lee, S.J.; Welsh, W.J.; Yoon, S. NetCSSP: Web application for predicting chameleon sequences and amyloid fibril formation. Nucleic Acids Res. 2009, 37, W469–W473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovato, A.; Chiti, F.; Maritan, A.; Seno, F. Insight into the Structure of Amyloid Fibrils from the Analysis of Globular Proteins. PLoS Comput. Biol. 2006, 2, e170. [Google Scholar] [CrossRef] [PubMed]
- Trovato, A.; Seno, F.; Tosatto, S.C.E. The PASTA server for protein aggregation prediction. Protein Eng. Des. Sel. 2007, 20, 521–523. [Google Scholar] [CrossRef] [Green Version]
- Walsh, I.; Seno, F.; Tosatto, S.C.E.; Trovato, A. PASTA 2.0: An improved server for protein aggregation prediction. Nucleic Acids Res. 2014, 42, W301–W307. [Google Scholar] [CrossRef]
- Niu, M.; Li, Y.; Wang, C.; Han, K. RFAmyloid: A Web Server for Predicting Amyloid Proteins. Int. J. Mol. Sci. 2018, 19, 2071. [Google Scholar] [CrossRef] [Green Version]
- De Baets, G.; Van Durme, J.; Reumers, J.; Maurer-Stroh, S.; Vanhee, P.; Dopazo, J.; Schymkowitz, J.; Rousseau, F. SNPeffect 4.0: On-line prediction of molecular and structural effects of protein-coding variants. Nucleic Acids Res. 2012, 40, D935–D939. [Google Scholar] [CrossRef]
- Bryan, A.W.; O’Donnell, C.W.; Menke, M.; Cowen, L.J.; Lindquist, S.; Berger, B. STITCHER: Dynamic assembly of likely amyloid and prion β-structures from secondary structure predictions. Proteins Struct. Funct. Bioinform. 2012, 80, 410–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Escamilla, A.-M.; Rousseau, F.; Schymkowitz, J.; Serrano, L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 2004, 22, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Stroh, S.; Debulpaep, M.; Kuemmerer, N.; de la Paz, M.L.; Martins, I.C.; Reumers, J.; Morris, K.L.; Copland, A.; Serpell, L.; Serrano, L.; et al. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nat. Methods 2010, 7, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Beerten, J.; Van Durme, J.; Gallardo, R.; Capriotti, E.; Serpell, L.; Rousseau, F.; Schymkowitz, J. WALTZ-DB: A benchmark database of amyloidogenic hexapeptides. Bioinformatics 2015, 31, 1698–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louros, N.; Konstantoulea, K.; De Vleeschouwer, M.; Ramakers, M.; Schymkowitz, J.; Rousseau, F. WALTZ-DB 2.0: An updated database containing structural information of experimentally determined amyloid-forming peptides. Nucleic Acids Res. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Sievers, S.A.; Karanicolas, J.; Ivanova, M.I.; Baker, D.; Eisenberg, D. The 3D profile method for identifying fibril-forming segments of proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 4074–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartaglia, G.G.; Vendruscolo, M. The Zyggregator method for predicting protein aggregation propensities. Chem. Soc. Rev. 2008, 37, 1395–1401. [Google Scholar] [CrossRef]
- GroB, M. Proteins that Convert from a Helix to b Sheet Implications for Folding and Disease. Curr. Protein Pept. Sci. 2000, 1, 339–347. [Google Scholar] [CrossRef]
- Hauser, C.A.E.; Deng, R.; Mishra, A.; Loo, Y.; Khoe, U.; Zhuang, F.; Cheong, D.W.; Accardo, A.; Sullivan, M.B.; Riekel, C.; et al. Natural tri- to hexapeptides self-assemble in water to amyloid beta-type fiber aggregates by unexpected alpha-helical intermediate structures. Proc. Natl. Acad. Sci. USA 2011, 108, 1361–1366. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Singh, P.K.; Sahay, S.; Jha, N.N.; Jacob, R.S.; Sen, S.; Kumar, A.; Riek, R.; Maji, S.K. Structure based aggregation studies reveal the presence of helix-rich intermediate during α-Synuclein aggregation. Sci. Rep. 2015, 5, 9228. [Google Scholar] [CrossRef] [Green Version]
- Cieślik-Boczula, K. Alpha-helix to beta-sheet transition in long-chain poly-l-lysine: Formation of alpha-helical fibrils by poly-l-lysine. Biochimie 2017, 137, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhuo, S.; Iliescu, C.; So, P.T.C.; Mehta, J.S.; Yu, H.; Hauser, C.A.E. Self-assembling amyloid-like peptides as exogenous second harmonic probes for bioimaging applications. J. Biophotonics 2019, 12, e201900065. [Google Scholar] [CrossRef] [PubMed]
- Tayeb-Fligelman, E.; Tabachnikov, O.; Moshe, A.; Goldshmidt-Tran, O.; Sawaya, M.R.; Coquelle, N.; Colletier, J.-P.; Landau, M. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science 2017, 355, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Bousset, L.; Thomson, N.H.; Radford, S.E.; Melki, R. The yeast prion Ure2p retains its native alpha-helical conformation upon assembly into protein fibrils in vitro. EMBO J. 2002, 21, 2903–2911. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K.S.; Lou, M.Z.; Chin, T.M.; Yang, N.C.; Garavito, R.M. A novel, multilayer structure of a helical peptide. Protein Sci. A Publ. Protein Soc. 1996, 5, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Privé, G.G.; Anderson, D.H.; Wesson, L.; Cascio, D.; Eisenberg, D. Packed protein bilayers in the 0.90 å resolution structure of a designed alpha helical bundle. Protein Sci. 1999, 8, 1400–1409. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Adler-Abramovich, L.; Lampel, A.; Bram, Y.; Lipstman, S.; Gazit, E. Formation of functional super-helical assemblies by constrained single heptad repeat. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Brunette, T.; Parmeggiani, F.; Huang, P.-S.; Bhabha, G.; Ekiert, D.C.; Tsutakawa, S.E.; Hura, G.L.; Tainer, J.A.; Baker, D. Exploring the repeat protein universe through computational protein design. Nature 2015, 528, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Pham, C.L.; Shanmugam, N.; Strange, M.; O’Carroll, A.; Brown, J.W.; Sierecki, E.; Gambin, Y.; Steain, M.; Sunde, M. Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep. 2019, 20. [Google Scholar] [CrossRef]
- Mompeán, M.; Li, W.; Li, J.; Laage, S.; Siemer, A.B.; Bozkurt, G.; Wu, H.; McDermott, A.E. The Structure of the Necrosome RIPK1-RIPK3 Core, a Human Hetero-Amyloid Signaling Complex. Cell 2018, 173, 1244–1253.e10. [Google Scholar] [CrossRef] [Green Version]
- O’Nuallain, B.; Williams, A.D.; Westermark, P.; Wetzel, R. Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem. 2004, 279, 17490–17499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oskarsson, M.E.; Paulsson, J.F.; Schultz, S.W.; Ingelsson, M.; Westermark, P.; Westermark, G.T. In Vivo Seeding and Cross-Seeding of Localized Amyloidosis: A Molecular Link between Type 2 Diabetes and Alzheimer Disease. Am. J. Pathol. 2015, 185, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.; Estrada, L.D.; Diaz-Espinoza, R.; Morales-Scheihing, D.; Jara, M.C.; Castilla, J.; Soto, C. Molecular cross talk between misfolded proteins in animal models of Alzheimer’s and prion diseases. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 4528–4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köppen, J.; Schulze, A.; Machner, L.; Wermann, M.; Eichentopf, R.; Guthardt, M.; Hähnel, A.; Klehm, J.; Kriegeskorte, M.-C.; Hartlage-Rübsamen, M.; et al. Amyloid-Beta Peptides Trigger Aggregation of Alpha-Synuclein In Vitro. Molecules 2020, 25, 580. [Google Scholar] [CrossRef] [Green Version]
- Gotz, J.; Chen, F.; van Dorpe, J.; Nitsch, R.M. Formation of Neurofibrillary Tangles in P301L Tau Transgenic Mice Induced by Abeta 42 Fibrils. Science 2001, 293, 1491–1495. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Guerrero-Muñoz, M.J.; Jackson, G.R.; Kayed, R. Preparation and Characterization of Neurotoxic Tau Oligomers. Biochemistry 2010, 49, 10039–10041. [Google Scholar] [CrossRef]
- Bhasne, K.; Sebastian, S.; Jain, N.; Mukhopadhyay, S. Synergistic Amyloid Switch Triggered by Early Heterotypic Oligomerization of Intrinsically Disordered α-Synuclein and Tau. J. Mol. Biol. 2018, 430, 2508–2520. [Google Scholar] [CrossRef]
- Lundmark, K.; Westermark, G.T.; Olsén, A.; Westermark, P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proc. Natl. Acad. Sci. USA 2005, 102, 6098–6102. [Google Scholar] [CrossRef] [Green Version]
- Derkatch, I.L.; Uptain, S.M.; Outeiro, T.F.; Krishnan, R.; Lindquist, S.L.; Liebman, S.W. Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 12934–12939. [Google Scholar] [CrossRef] [Green Version]
- Hammer, N.D.; Schmidt, J.C.; Chapman, M.R. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc. Natl. Acad. Sci. USA 2007, 104, 12494–12499. [Google Scholar] [CrossRef] [Green Version]
- Morris, K.L.; Serpell, L.C. X-Ray Fibre Diffraction Studies of Amyloid Fibrils. In Amyloid Proteins; Humana Press: Totowa, NJ, USA, 2012; pp. 121–135. [Google Scholar]
- Tycko, R. Solid-State NMR Studies of Amyloid Fibril Structure. Annu. Rev. Phys. Chem. 2011, 62, 279–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serpell, L.C.; Smith, J.M. Direct visualisation of the β-sheet structure of synthetic Alzheimer’s amyloid. J. Mol. Biol. 2000, 299, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.W.; Kelly, J.E.; Kelz, J.I. Advances in instrumentation and methodology for solid-state NMR of biological assemblies. J. Struct. Biol. 2019, 206, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.H.; Riek, R.; Böckmann, A. Emerging Structural Understanding of Amyloid Fibrils by Solid-State NMR. Trends Biochem. Sci. 2017, 42, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; McMullan, G.; Scheres, S.H. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 2015, 40, 49–57. [Google Scholar] [CrossRef]
- COHEN, A.S.; CALKINS, E. Electron Microscopic Observations on a Fibrous Component in Amyloid of Diverse Origins. Nature 1959, 183, 1202–1203. [Google Scholar] [CrossRef]
- Kühlbrandt, W. The Resolution Revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef]
- Milanesi, L.; Sheynis, T.; Xue, W.-F.; Orlova, E.V.; Hellewell, A.L.; Jelinek, R.; Hewitt, E.W.; Radford, S.E.; Saibil, H.R. Direct three-dimensional visualization of membrane disruption by amyloid fibrils. Proc. Natl. Acad. Sci. USA 2012, 109, 20455–20460. [Google Scholar] [CrossRef] [Green Version]
- Tipping, K.W.; van Oosten-Hawle, P.; Hewitt, E.W.; Radford, S.E. Amyloid fibres: Inert end-stage aggregates or key players in disease? Trends Biochem. Sci. 2015, 40, 719–727. [Google Scholar] [CrossRef]
- Verma, M.; Vats, A.; Taneja, V. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015, 18, 138–145. [Google Scholar]
- Evangelisti, E.; Cascella, R.; Becatti, M.; Marrazza, G.; Dobson, C.M.; Chiti, F.; Stefani, M.; Cecchi, C. Binding affinity of amyloid oligomers to cellular membranes is a generic indicator of cellular dysfunction in protein misfolding diseases. Sci. Rep. 2016, 6, 32721. [Google Scholar] [CrossRef] [PubMed]
- Vivoli Vega, M.; Cascella, R.; Chen, S.W.; Fusco, G.; De Simone, A.; Dobson, C.M.; Cecchi, C.; Chiti, F. The Toxicity of Misfolded Protein Oligomers Is Independent of Their Secondary Structure. ACS Chem. Biol. 2019, 14, 1593–1600. [Google Scholar] [CrossRef]
- Olzscha, H.; Schermann, S.M.; Woerner, A.C.; Pinkert, S.; Hecht, M.H.; Tartaglia, G.G.; Vendruscolo, M.; Hayer-Hartl, M.; Hartl, F.U.; Vabulas, R.M. Amyloid-like Aggregates Sequester Numerous Metastable Proteins with Essential Cellular Functions. Cell 2011, 144, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannini, B.; Mulvihill, E.; Sgromo, C.; Cascella, R.; Khodarahmi, R.; Ramazzotti, M.; Dobson, C.M.; Cecchi, C.; Chiti, F. Toxicity of Protein Oligomers Is Rationalized by a Function Combining Size and Surface Hydrophobicity. ACS Chem. Biol. 2014, 9, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Mannini, B.; Cascella, R.; Zampagni, M.; van Waarde-Verhagen, M.; Meehan, S.; Roodveldt, C.; Campioni, S.; Boninsegna, M.; Penco, A.; Relini, A.; et al. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc. Natl. Acad. Sci. USA 2012, 109, 12479–12484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucciantini, M.; Giannoni, E.; Chiti, F.; Baroni, F.; Formigli, L.; Zurdo, J.; Taddei, N.; Ramponi, G.; Dobson, C.M.; Stefani, M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416, 507–511. [Google Scholar] [CrossRef]
- McLaurin, J.; Chakrabartty, A. Membrane Disruption by Alzheimer β-Amyloid Peptides Mediated through Specific Binding to Either Phospholipids or Gangliosides. J. Biol. Chem. 1996, 271, 26482–26489. [Google Scholar] [CrossRef] [Green Version]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Goodchild, S.C.; Sheynis, T.; Thompson, R.; Tipping, K.W.; Xue, W.-F.; Ranson, N.A.; Beales, P.A.; Hewitt, E.W.; Radford, S.E. β2-Microglobulin Amyloid Fibril-Induced Membrane Disruption Is Enhanced by Endosomal Lipids and Acidic pH. PLoS ONE 2014, 9, e104492. [Google Scholar] [CrossRef] [Green Version]
- Winklhofer, K.F.; Haass, C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 29–44. [Google Scholar] [CrossRef]
- Roberts, H.; Brown, D. Seeking a Mechanism for the Toxicity of Oligomeric α-Synuclein. Biomolecules 2015, 5, 282–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaels, T.C.T.; Šarić, A.; Habchi, J.; Chia, S.; Meisl, G.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Chemical Kinetics for Bridging Molecular Mechanisms and Macroscopic Measurements of Amyloid Fibril Formation. Ann. Rev. Phys. Chem. 2018, 69, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Frieden, C. Protein aggregation processes: In search of the mechanism. Protein Sci. 2007, 16, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Buell, A.K.; Dobson, C.M.; Knowles, T.P.J. The physical chemistry of the amyloid phenomenon: Thermodynamics and kinetics of filamentous protein aggregation. Essays Biochem. 2014, 56, 11–39. [Google Scholar]
- Jarrett, J.T.; Lansbury, P.T. Amyloid fibril formation requires a chemically discriminating nucleation event: Studies of an amyloidogenic sequence from the bacterial protein OsmB. Biochemistry 1992, 31, 12345–12352. [Google Scholar] [CrossRef]
- Gosal, W.S.; Morten, I.J.; Hewitt, E.W.; Smith, D.A.; Thomson, N.H.; Radford, S.E. Competing Pathways Determine Fibril Morphology in the Self-assembly of β2-Microglobulin into Amyloid. J. Mol. Biol. 2005, 351, 850–864. [Google Scholar] [CrossRef]
- Soldi, G.; Bemporad, F.; Torrassa, S.; Relini, A.; Ramazzotti, M.; Taddei, N.; Chiti, F. Amyloid Formation of a Protein in the Absence of Initial Unfolding and Destabilization of the Native State. Biophys. J. 2005, 89, 4234–4244. [Google Scholar] [CrossRef] [Green Version]
- Plakoutsi, G.; Bemporad, F.; Calamai, M.; Taddei, N.; Dobson, C.M.; Chiti, F. Evidence for a Mechanism of Amyloid Formation Involving Molecular Reorganisation within Native-like Precursor Aggregates. J. Mol. Biol. 2005, 351, 910–922. [Google Scholar] [CrossRef]
- Bemporad, F.; Vannocci, T.; Varela, L.; Azuaga, A.I.; Chiti, F. A model for the aggregation of the acylphosphatase from Sulfolobus solfataricus in its native-like state. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2008, 1784, 1986–1996. [Google Scholar] [CrossRef]
- Garcia-Pardo, J.; Graña-Montes, R.; Fernandez-Mendez, M.; Ruyra, A.; Roher, N.; Aviles, F.X.; Lorenzo, J.; Ventura, S. Amyloid formation by human carboxypeptidase D transthyretin-like domain under physiological conditions. J. Biol. Chem. 2014, 289, 33783–33796. [Google Scholar] [CrossRef] [Green Version]
- Raso, S.W.; Abel, J.; Barnes, J.M.; Maloney, K.M.; Pipes, G.; Treuheit, M.J.; King, J.; Brems, D.N. Aggregation of granulocyte-colony stimulating factor in vitro involves a conformationally altered monomeric state. Protein Sci. 2005, 14, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, B.S.; Carpenter, J.F.; Cleland, J.L.; Randolph, T.W. A transient expansion of the native state precedes aggregation of recombinant human interferon-gamma. Proc. Natl. Acad. Sci. USA 1998, 95, 14142–14146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintas, A.; Saraiva, M.J.M.; Brito, R.M.M. The Tetrameric Protein Transthyretin Dissociates to a Non-native Monomer in Solution a novel model for amyloidogenesis. J. Biol. Chem. 1999, 274, 32943–32949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintas, A.; Vaz, D.C.; Cardoso, I.; Saraiva, M.J.; Brito, R.M. Tetramer dissociation and monomer partial unfolding precedes protofibril formation in amyloidogenic transthyretin variants. J. Biol. Chem. 2001, 276, 27207–27213. [Google Scholar] [CrossRef] [Green Version]
- Booth, D.R.; Sunde, M.; Bellotti, V.; Robinson, C.V.; Hutchinson, W.L.; Fraser, P.E.; Hawkins, P.N.; Dobson, C.M.; Radford, S.E.; Blake, C.C.F.; et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 1997, 385, 787–793. [Google Scholar] [CrossRef]
- Guijarro, J.I.; Sunde, M.; Jones, J.A.; Campbell, I.D.; Dobson, C.M. Amyloid fibril formation by an SH3 domain. Proc. Natl. Acad. Sci. USA 1998, 95, 4224–4228. [Google Scholar] [CrossRef] [Green Version]
- Truscott, R.J.W.; Augusteyn, R.C. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim. Et Biophys. Acta (BBA)-Protein Struct. 1977, 492, 43–52. [Google Scholar] [CrossRef]
- Guptasarma, P.; Balasubramanian, D.; Matsugo, S.; Saito, I. Hydroxyl radical mediated damage to proteins, with special reference to the crystallins. Biochemistry 1992, 31, 4296–4303. [Google Scholar] [CrossRef]
- Hermeling, S.; Schellekens, H.; Maas, C.; Gebbink, M.F.B.G.; Crommelin, D.J.A.; Jiskoot, W. Antibody Response to Aggregated Human Interferon Alpha2b in Wild-type and Transgenic Immune Tolerant Mice Depends on Type and Level of Aggregation. J. Pharm. Sci. 2006, 95, 1084–1096. [Google Scholar] [CrossRef]
- Mirzaei, H.; Regnier, F. Protein:protein aggregation induced by protein oxidation. J. Chromatogr. B 2008, 873, 8–14. [Google Scholar] [CrossRef]
- Landles, C.; Sathasivam, K.; Weiss, A.; Woodman, B.; Moffitt, H.; Finkbeiner, S.; Sun, B.; Gafni, J.; Ellerby, L.M.; Trottier, Y.; et al. Proteolysis of mutant huntingtin produces an exon 1 fragment that accumulates as an aggregated protein in neuronal nuclei in Huntington disease. J. Biol. Chem. 2010, 285, 8808–8823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, G.K.L.; Witkowski, A.; Gantz, D.L.; Zhang, T.O.; Zanni, M.T.; Jayaraman, S.; Cavigiolio, G. Myeloperoxidase-mediated Methionine Oxidation Promotes an Amyloidogenic Outcome for Apolipoprotein A-I. J. Biol. Chem. 2015, 290, 10958–10971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeld, M.A.; Leonova, V.B.; Konstantinova, M.L.; Razumovskii, S.D. Self-assembly of fibrin monomers and fibrinogen aggregation during ozone oxidation. Biochem. (Mosc.) 2009, 74, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Roostaee, A.; Côté, S.; Roucou, X. Aggregation and amyloid fibril formation induced by chemical dimerization of recombinant prion protein in physiological-like conditions. J. Biol. Chem. 2009, 284, 30907–30916. [Google Scholar] [CrossRef] [Green Version]
- Takata, T.; Oxford, J.T.; Demeler, B.; Lampi, K.J. Deamidation destabilizes and triggers aggregation of a lens protein, betaA3-crystallin. Protein Sci. A Publ. Protein Soc. 2008, 17, 1565–1575. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, L.; Chen, J.; Ge, L.; He, R.Q. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Redecke, L.; Binder, S.; Elmallah, M.I.Y.; Broadbent, R.; Tilkorn, C.; Schulz, B.; May, P.; Goos, A.; Eich, A.; Rübhausen, M.; et al. UV-light-induced conversion and aggregation of prion proteins. Free Radic. Biol. Med. 2009, 46, 1353–1361. [Google Scholar] [CrossRef]
- Roy, S.; Mason, B.D.; Schöneich, C.S.; Carpenter, J.F.; Boone, T.C.; Kerwin, B.A. Light-induced aggregation of type I soluble tumor necrosis factor receptor. J. Pharm. Sci. 2009, 98, 3182–3199. [Google Scholar] [CrossRef]
- Li, S.; Leblanc, R.M. Aggregation of Insulin at the Interface. J. Phys. Chem. B 2014, 118, 1181–1188. [Google Scholar] [CrossRef]
- Campioni, S.; Carret, G.; Jordens, S.; Nicoud, L.; Mezzenga, R.; Riek, R. The Presence of an Air–Water Interface Affects Formation and Elongation of α-Synuclein Fibrils. J. Am. Chem. Soc. 2014, 136, 2866–2875. [Google Scholar] [CrossRef]
- Trigg, B.J.; Lee, C.F.; Vaux, D.J.; Jean, L. The air–water interface determines the outcome of seeding during amyloidogenesis. Biochem. J. 2013, 456, 67–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, L.; Lee, C.F.; Vaux, D.J. Enrichment of Amyloidogenesis at an Air-Water Interface. Biophys. J. 2012, 102, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, L.; Lee, C.F.; Lee, C.; Shaw, M.; Vaux, D.J. Competing discrete interfacial effects are critical for amyloidogenesis. FASEB J. 2010, 24, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.; Cheng, C.-Y.; Kinnebrew, M.; Lew, J.; Dahlquist, F.W.; Han, S. Protein structural and surface water rearrangement constitute major events in the earliest aggregation stages of tau. Proc. Natl. Acad. Sci. USA 2016, 113, E127–E136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frachon, T.; Bruckert, F.; Le Masne, Q.; Monnin, E.; Weidenhaupt, M. Insulin Aggregation at a Dynamic Solid–Liquid–Air Triple Interface. Langmuir 2016, 32, 13009–13019. [Google Scholar] [CrossRef] [PubMed]
- Sluzky, V.; Tamada, J.A.; Klibanov, A.M.; Langer, R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. USA 1991, 88, 9377–9381. [Google Scholar] [CrossRef] [Green Version]
- Duerkop, M.; Berger, E.; Dürauer, A.; Jungbauer, A. Impact of Cavitation, High Shear Stress and Air/Liquid Interfaces on Protein Aggregation. Biotechnol. J. 2018, 13, 1800062. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, M.; Buck, P.M.; Alphonse Ignatius, A.; King, K.R.; Wang, W. Agitation-induced aggregation and subvisible particulate formation in model proteins. Eur. J. Pharm. Biopharm. 2014, 87, 299–309. [Google Scholar] [CrossRef]
- Kiese, S.; Papppenberger, A.; Friess, W.; Mahler, H.-C. Shaken, Not Stirred: Mechanical Stress Testing of an IgG1 Antibody. J. Pharm. Sci. 2008, 97, 4347–4366. [Google Scholar] [CrossRef]
- Fesinmeyer, R.M.; Hogan, S.; Saluja, A.; Brych, S.R.; Kras, E.; Narhi, L.O.; Brems, D.N.; Gokarn, Y.R. Effect of Ions on Agitation- and Temperature-Induced Aggregation Reactions of Antibodies. Pharm. Res. 2009, 26, 903–913. [Google Scholar] [CrossRef]
- Zhang, J.; Topp, E.M. Protein G, Protein A and Protein A-Derived Peptides Inhibit the Agitation Induced Aggregation of IgG. Mol. Pharm. 2012, 9, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Thirumangalathu, R.; Krishnan, S.; Ricci, M.S.; Brems, D.N.; Randolph, T.W.; Carpenter, J.F. Silicone oil- and agitation-induced aggregation of a monoclonal antibody in aqueous solution. J. Pharm. Sci. 2009, 98, 3167–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krielgaard, L.; Jones, L.S.; Randolph, T.W.; Frokjaer, S.; Flink, J.M.; Manning, M.C.; Carpenter, J.F. Effect of tween 20 on freeze-thawing- and agitation-induced aggregation of recombinant human factor XIII. J. Pharm. Sci. 1998, 87, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Abdolvahabi, A.; Shi, Y.; Rasouli, S.; Croom, C.M.; Chuprin, A.; Shaw, B.F. How Do Gyrating Beads Accelerate Amyloid Fibrillization? Biophys. J. 2017, 112, 250–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kueltzo, L.A.; Wang, W.e.i.; Randolph, T.W.; Carpenter, J.F. Effects of Solution Conditions, Processing Parameters, and Container Materials on Aggregation of a Monoclonal Antibody during Freeze-Thawing. J. Pharm. Sci. 2008, 97, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Perevozchikova, T.; Nanda, H.; Nesta, D.P.; Roberts, C.J. Protein adsorption, desorption, and aggregation mediated by solid-liquid interfaces. J. Pharm. Sci. 2015, 104, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, A.; Mcgraw, N.R.; Schwartz, D.K.; Bee, J.S.; Carpenter, J.F.; Randolph, T.W. Protein Aggregation and Particle Formation in Prefilled Glass Syringes. J. Pharm. Sci. 2014, 103, 1601–1612. [Google Scholar] [CrossRef]
- Basu, P.; Thirumangalathu, R.; Randolph, T.W.; Carpenter, J.F. IgG1 aggregation and particle formation induced by silicone–water interfaces on siliconized borosilicate glass beads: A model for siliconized primary containers. J. Pharm. Sci. 2013, 102, 852–865. [Google Scholar] [CrossRef]
- Bekard, I.B.; Asimakis, P.; Bertolini, J.; Dunstan, D.E. The effects of shear flow on protein structure and function. Biopolymers 2011, 95, 733–745. [Google Scholar] [CrossRef]
- Cao, E.; Chen, Y.; Cui, Z.; Foster, P.R. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef]
- Miller, Y.; Ma, B.; Nussinov, R. Zinc ions promote Alzheimer Aβ aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. USA 2010, 107, 9490–9495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagel, K.; Seri, T.; von Berlepsch, H.; Griebel, J.; Kirmse, R.; Böttcher, C.; Koksch, B. How metal ions affect amyloid formation: Cu2+-and Zn2+-sensitive peptides. ChemBioChem 2008, 9, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hoernke, M.; Koksch, B.; Brezesinski, G. Amyloidogenic peptides at hydrophobic–hydrophilic interfaces: Coordination affinities and the chelate effect dictate the competitive binding of Cu2+ and Zn2+. ChemPhysChem 2011, 12, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Hoernke, M.; Koksch, B.; Brezesinski, G. Influence of the hydrophobic interface and transition metal ions on the conformation of amyloidogenic model peptides. Biophys. Chem. 2010, 150, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Hoernke, M.; Falenski, J.A.; Schwieger, C.; Koksch, B.; Brezesinski, G. Triggers for β-sheet formation at the hydrophobic–hydrophilic interface: High concentration, in-plane orientational order, and metal ion complexation. Langmuir 2011, 27, 14218–14231. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.Y. Effect of protein–protein interactions on protein aggregation kinetics. J. Chem. Phys. 2003, 119, 10972–10976. [Google Scholar] [CrossRef]
- Galvagnion, C.; Buell, A.K.; Meisl, G.; Michaels, T.C.T.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015, 11, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Gorbenko, G.P.; Ioffe, V.M.; Kinnunen, P.K.J. Binding of Lysozyme to Phospholipid Bilayers: Evidence for Protein Aggregation upon Membrane Association. Biophys. J. 2007, 93, 140–153. [Google Scholar] [CrossRef] [Green Version]
- Terzi, E.; Hölzemann, G.; Seelig, J. Self-association of β-Amyloid Peptide (1–40) in Solution and Binding to Lipid Membranes. J. Mol. Biol. 1995, 252, 633–642. [Google Scholar] [CrossRef]
- Zhao, H.; Tuominen, E.K.J.; Kinnunen, P.K.J. Formation of Amyloid Fibers Triggered by Phosphatidylserine-Containing Membranes. Biochemistry 2004, 43, 10302–10307. [Google Scholar] [CrossRef]
- Sparr, E.; Engel, M.F.M.; Sakharov, D.V.; Sprong, M.; Jacobs, J.; de Kruijff, B.; Höppener, J.W.M.; Antoinette Killian, J. Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Lett. 2004, 577, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Z.; Hashemi, M.; Banerjee, S.; Zagorski, K.; Rochet, J.-C.; Lyubchenko, Y.L. Phospholipid membranes promote the early stage assembly of α-synuclein aggregates. bioRxiv 2018, 295782. [Google Scholar]
- Chauhan, A.; Ray, I.; Chauhan, V.P. Interaction of amyloid beta-protein with anionic phospholipids: Possible involvement of Lys28 and C-terminus aliphatic amino acids. Neurochem. Res. 2000, 25, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ege, C.; Lee, K.Y.C. Insertion of Alzheimer’s Aβ40 peptide into lipid monolayers. Biophys. J. 2004, 87, 1732–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrone, F. [17] Analysis of protein aggregation kinetics. Methods Enzymol. 1999, 309, 256–274. [Google Scholar]
- Morris, A.M.; Watzky, M.A.; Finke, R.G. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 375–397. [Google Scholar] [CrossRef]
- Arosio, P.; Knowles, T.P.J.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.D.; Lansbury, P.T. Models of Amyloid Seeding in Alzheimer’s Disease and Scrapie: Mechanistic Truths and Physiological Consequences of the Time-Dependent Solubility of Amyloid Proteins. Annu. Rev. Biochem. 1997, 66, 385–407. [Google Scholar] [CrossRef]
- O’Nuallain, B.; Shivaprasad, S.; Kheterpal, I.; Wetzel, R. Thermodynamics of Aβ(1−40) Amyloid Fibril Elongation. Biochemistry 2005, 44, 12709–12718. [Google Scholar] [CrossRef]
- Watzky, M.A.; Morris, A.M.; Ross, E.D.; Finke, R.G. Fitting Yeast and Mammalian Prion Aggregation Kinetic Data with the Finke−Watzky Two-Step Model of Nucleation and Autocatalytic Growth †. Biochemistry 2008, 47, 10790–10800. [Google Scholar] [CrossRef]
- Morris, A.M.; Watzky, M.A.; Agar, J.N.; Finke, R.G. Fitting Neurological Protein Aggregation Kinetic Data via a 2-Step, Minimal/“Ockham’s Razor” Model: The Finke−Watzky Mechanism of Nucleation Followed by Autocatalytic Surface Growth †. Biochemistry 2008, 47, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Watzky, M.A.; Finke, R.G. Transition Metal Nanocluster Formation Kinetic and Mechanistic Studies. A New Mechanism When Hydrogen Is the Reductant: Slow, Continuous Nucleation and Fast Autocatalytic Surface Growth. J. Am. Chem. Soc. 1997, 119, 10382–10400. [Google Scholar] [CrossRef]
- Morel, B.; Carrasco, M.P.; Jurado, S.; Marco, C.; Conejero-Lara, F. Dynamic micellar oligomers of amyloid beta peptides play a crucial role in their aggregation mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 20597–20614. [Google Scholar] [CrossRef] [PubMed]
- Schmit, J.D.; Ghosh, K.; Dill, K. What Drives Amyloid Molecules to Assemble into Oligomers and Fibrils? Biophys. J. 2011, 100, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Hasecke, F.; Miti, T.; Perez, C.; Barton, J.; Schölzel, D.; Gremer, L.; Grüning, C.S.R.; Matthews, G.; Meisl, G.; Knowles, T.P.J.; et al. Origin of metastable oligomers and their effects on amyloid fibril self-assembly. Chem. Sci. 2018, 9, 5937–5948. [Google Scholar] [CrossRef] [Green Version]
- Powers, E.T.; Powers, D.L. The Kinetics of Nucleated Polymerizations at High Concentrations: Amyloid Fibril Formation Near and Above the “Supercritical Concentration”. Biophys. J. 2006, 91, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Serio, T.R.; Cashikar, A.G.; Kowal, A.S.; Sawicki, G.J.; Moslehi, J.J.; Serpell, L.; Arnsdorf, M.F.; Lindquist, S.L. Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science 2000, 289, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Aucoin, D.; Davis, J.; Van Nostrand, W.E.; Smith, S.O. Mechanism of Nucleated Conformational Conversion of Aβ42. Biochemistry 2015, 54, 4197–4207. [Google Scholar] [CrossRef]
- Lee, J.; Culyba, E.K.; Powers, E.T.; Kelly, J.W. Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 2011, 7, 602–609. [Google Scholar] [CrossRef]
- Chimon, S.; Shaibat, M.A.; Jones, C.R.; Calero, D.C.; Aizezi, B.; Ishii, Y. Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer’s β-amyloid. Nat. Struct. Mol. Biol. 2007, 14, 1157–1164. [Google Scholar] [CrossRef]
- Ruzafa, D.; Morel, B.; Varela, L.; Azuaga, A.I.; Conejero-Lara, F. Characterization of Oligomers of Heterogeneous Size as Precursors of Amyloid Fibril Nucleation of an SH3 Domain: An Experimental Kinetics Study. PLoS ONE 2012, 7, e49690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzafa, D.; Conejero-Lara, F.; Morel, B. Modulation of the stability of amyloidogenic precursors by anion binding strongly influences the rate of amyloid nucleation. Phys. Chem. Chem. Phys. 2013, 15, 15508–15517. [Google Scholar] [CrossRef] [PubMed]
- Morel, B.; Ruzafa, D.; Conejero-Lara, F. SH3 Domains as Suitable Models to Study Amyloid Aggregation. In SH Domains; Springer International Publishing: Cham, Switzerland; New York, NY, USA, 2015; pp. 1–15. [Google Scholar]
- Fay, N.; Inoue, Y.; Bousset, L.; Taguchi, H.; Melki, R. Assembly of the yeast prion Ure2p into protein fibrils. Thermodynamic and kinetic characterization. J. Biol. Chem. 2003, 278, 30199–30205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, A.M.; Thakur, A.K.; Wetzel, R. polyglutamine aggregation nucleation: Thermodynamics of a highly unfavorable protein folding reaction. Proc. Natl. Acad. Sci. USA 2005, 102, 15400–15405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, A.S.; Gottschall, P.E.; Muschol, M. Sub-micron lysozyme clusters distort kinetics of crystal nucleation in supersaturated lysozyme solutions. Biophys. Chem 2007, 129, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.E.; Robinson, J.; Matthews, G.; Muschol, M. Amyloid Protofibrils of Lysozyme Nucleate and Grow Via Oligomer Fusion. Biophys. J. 2009, 96, 3781–3790. [Google Scholar] [CrossRef] [Green Version]
- Jarrett, J.T.; Lansbury, P.T. Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 1993, 73, 1055–1058. [Google Scholar] [CrossRef]
- Cohen, S.I.; Linse, S.; Luheshi, L.M.; Hellstrand, E.; White, D.A.; Rajah, L.; Otzen, D.E.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 9758–9763. [Google Scholar] [CrossRef] [Green Version]
- Meisl, G.; Yang, X.; Dobson, C.M.; Linse, S.; Knowles, T.P.J. Modulation of electrostatic interactions to reveal a reaction network unifying the aggregation behaviour of the Aβ42 peptide and its variants. Chem. Sci. 2017, 8, 4352–4362. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.I.A.; Vendruscolo, M.; Welland, M.E.; Dobson, C.M.; Terentjev, E.M.; Knowles, T.P.J. Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments. J. Chem. Phys. 2011, 135, 065105. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.I.A.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Nucleated polymerization with secondary pathways. II. Determination of self-consistent solutions to growth processes described by non-linear master equations. J. Chem. Phys. 2011, 135, 065106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, T.P.J.; Waudby, C.A.; Devlin, G.L.; Cohen, S.I.A.; Aguzzi, A.; Vendruscolo, M.; Terentjev, E.M.; Welland, M.E.; Dobson, C.M. An analytical solution to the kinetics of breakable filament assembly. Science (New York N.Y.) 2009, 326, 1533–1537. [Google Scholar] [CrossRef] [Green Version]
- Linse, S. Monomer-dependent secondary nucleation in amyloid formation. Biophys. Rev. 2017, 9, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Törnquist, M.; Michaels, T.C.T.; Sanagavarapu, K.; Yang, X.; Meisl, G.; Cohen, S.I.A.; Knowles, T.P.J.; Linse, S. Secondary nucleation in amyloid formation. Chem. Commun. 2018, 54, 8667–8684. [Google Scholar] [CrossRef] [Green Version]
- Meisl, G.; Yang, X.; Hellstrand, E.; Frohm, B.; Kirkegaard, J.B.; Cohen, S.I.A.; Dobson, C.M.; Linse, S.; Knowles, T.P.J. Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proc. Natl. Acad. Sci. USA 2014, 111, 9384–9389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, G.; Udgaonkar, J.B. Evidence for the existence of a secondary pathway for fibril growth during the aggregation of tau. J. Mol. Biol. 2012, 421, 296–314. [Google Scholar] [CrossRef]
- Buell, A.K.; Galvagnion, C.; Gaspar, R.; Sparr, E.; Vendruscolo, M.; Knowles, T.P.; Linse, S.; Dobson, C.M. Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2014, 111, 7671–7676. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, R.; Meisl, G.; Buell, A.K.; Young, L.; Kaminski, C.F.; Knowles, T.P.J.; Sparr, E.; Linse, S. Secondary nucleation of monomers on fibril surface dominates α -synuclein aggregation and provides autocatalytic amyloid amplification. Q. Rev. Biophys. 2017, 50, e6. [Google Scholar] [CrossRef] [Green Version]
- Padrick, S.B.; Miranker, A.D. Islet Amyloid: Phase Partitioning and Secondary Nucleation Are Central to the Mechanism of Fibrillogenesis. Biochemistry 2002, 41, 4694–4703. [Google Scholar] [CrossRef]
- Foderà, V.; Librizzi, F.; Groenning, M.; van de Weert, M.; Leone, M. Secondary Nucleation and Accessible Surface in Insulin Amyloid Fibril Formation. J. Phys. Chem. B 2008, 112, 3853–3858. [Google Scholar] [CrossRef]
- Garg, D.K.; Kundu, B. Clues for divergent, polymorphic amyloidogenesis through dissection of amyloid forming steps of bovine carbonic anhydrase and its critical amyloid forming stretch. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2016, 1864, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.I.A.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Nucleated Polymerisation in the Presence of Pre-Formed Seed Filaments. Int. J. Mol. Sci. 2011, 12, 5844–5852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, R.; Moreno-Gonzalez, I.; Soto, C. Cross-Seeding of Misfolded Proteins: Implications for Etiology and Pathogenesis of Protein Misfolding Diseases. PLoS Pathog. 2013, 9, e1003537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.C.; Diamond, M.I.; Duff, K.E.; Hyman, B.T. Mechanisms of Protein Seeding in Neurodegenerative Diseases. JAMA Neurol. 2013, 70, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Come, J.H.; Fraser, P.E.; Lansbury, P.T. A kinetic model for amyloid formation in the prion diseases: Importance of seeding. Proc. Natl. Acad. Sci. USA 1993, 90, 5959–5963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundin, P.; Melki, R.; Kopito, R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2010, 11, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.C.; Jucker, M. Neurodegenerative Diseases: Expanding the Prion Concept. Annu. Rev. Neurosci. 2015, 38, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540. [Google Scholar] [CrossRef]
- Ono, K.; Takahashi, R.; Ikeda, T.; Yamada, M. Cross-seeding effects of amyloid β-protein and α-synuclein. J. Neurochem. 2012, 122, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Oosawa, F.; Kasai, M. A theory of linear and helical aggregations of macromolecules. J. Mol. Biol. 1962, 4, 10–21. [Google Scholar] [CrossRef]
- Oosawa, F.; Asakura, S. Thermodynamics of the Polymerization of Protein; Academic Press: London, UK, 1975. [Google Scholar]
- Ruzafa, D.; Varela, L.; Azuaga, A.I.; Conejero-Lara, F.; Morel, B. Mapping the structure of amyloid nucleation precursors by protein engineering kinetic analysis. Phys. Chem. Chem. Phys. 2014, 16, 2989–3000. [Google Scholar] [CrossRef]
- Hori, Y.; Hashimoto, T.; Wakutani, Y.; Urakami, K.; Nakashima, K.; Condron, M.M.; Tsubuki, S.; Saido, T.C.; Teplow, D.B.; Iwatsubo, T. The Tottori (D7N) and English (H6R) familial Alzheimer disease mutations accelerate Abeta fibril formation without increasing protofibril formation. J. Biol. Chem. 2007, 282, 4916–4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Udgaonkar, J.B. Conformational Conversion May Precede or Follow Aggregate Elongation on Alternative Pathways of Amyloid Protofibril Formation. J. Mol. Biol. 2009, 385, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Hurshman, A.R.; White, J.T.; Powers, E.T.; Kelly, J.W. Transthyretin Aggregation under Partially Denaturing Conditions Is a Downhill Polymerization. Biochemistry 2004, 43, 7365–7381. [Google Scholar] [CrossRef] [PubMed]
- Faria, T.Q.; Almeida, Z.L.; Cruz, P.F.; Jesus, C.S.H.; Castanheira, P.; Brito, R.M.M. A look into amyloid formation by transthyretin: Aggregation pathway and a novel kinetic model. Phys. Chem. Chem. Phys. 2015, 17, 7255–7263. [Google Scholar] [CrossRef] [PubMed]
- O’Nuallain, B.; Freir, D.B.; Nicoll, A.J.; Risse, E.; Ferguson, N.; Herron, C.E.; Collinge, J.; Walsh, D.M. Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 14411–14419. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, V.; Moore, B.D.; Reed, D.K.; Sonoda, L.K.; Bridges, A.W.; Conboy, E.; Hartigan, D.; Rosenberry, T.L. Amyloid-β (1− 42) rapidly forms protofibrils and oligomers by distinct pathways in low concentrations of sodium dodecylsulfate. Biochemistry 2007, 46, 12451–12462. [Google Scholar] [CrossRef]
- Ramachandran, G.; Udgaonkar, J.B. Understanding the kinetic roles of the inducer heparin and of rod-like protofibrils during amyloid fibril formation by Tau protein. J. Biol. Chem. 2011, 286, 38948–38959. [Google Scholar] [CrossRef] [Green Version]
- Juárez, J.; Taboada, P.; Mosquera, V. Existence of Different Structural Intermediates on the Fibrillation Pathway of Human Serum Albumin. Biophys. J. 2009, 96, 2353–2370. [Google Scholar] [CrossRef] [Green Version]
- Holm, N.K.; Jespersen, S.K.; Thomassen, L.V.; Wolff, T.Y.; Sehgal, P.; Thomsen, L.A.; Christiansen, G.; Andersen, C.B.; Knudsen, A.D.; Otzen, D.E. Aggregation and fibrillation of bovine serum albumin. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2007, 1774, 1128–1138. [Google Scholar] [CrossRef]
- Campioni, S.; Mossuto, M.F.; Torrassa, S.; Calloni, G.; de Laureto, P.P.; Relini, A.; Fontana, A.; Chiti, F. Conformational properties of the aggregation precursor state of HypF-N. J. Mol. Biol. 2008, 379, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, P.; Navarro, S.; Baño-Polo, M.; Morel, B.; Graña-Montes, R.; Sabe, A.; Canals, F.; Fernandez, M.R.; Conejero-Lara, F.; Ventura, S. Global Protein Stabilization Does Not Suffice to Prevent Amyloid Fibril Formation. ACS Chem. Biol. 2018, 13, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Calamai, M.; Taddei, N.; Stefani, M.; Ramponi, G.; Chiti, F. Relative Influence of Hydrophobicity and Net Charge in the Aggregation of Two Homologous Proteins. Biochemistry 2003, 42, 15078–15083. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Griffin, M.D.W.; Binger, K.J.; Schuck, P.; Howlett, G.J. An Equilibrium Model for Linear and Closed-Loop Amyloid Fibril Formation. J. Mol. Biol. 2012, 421, 364–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, M.D.W.; Mok, M.L.Y.; Wilson, L.M.; Pham, C.L.L.; Waddington, L.J.; Perugini, M.A.; Howlett, G.J. Phospholipid Interaction Induces Molecular-level Polymorphism in Apolipoprotein C-II Amyloid Fibrils via Alternative Assembly Pathways. J. Mol. Biol. 2008, 375, 240–256. [Google Scholar] [CrossRef]
- Morel, B.; Varela, L.; Azuaga, A.I.; Conejero-Lara, F. Environmental Conditions Affect the Kinetics of Nucleation of Amyloid Fibrils and Determine Their Morphology. Biophys. J. 2010, 99, 3801–3810. [Google Scholar] [CrossRef] [Green Version]
- Zurdo, J.; Guijarro, J.; Jiménez, J.L.; Saibil, H.R.; Dobson, C.M. Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. J. Mol. Biol. 2001, 311, 325–340. [Google Scholar] [CrossRef]
- Varela, L.; Morel, B.; Azuaga, A.I.; Conejero-Lara, F. A single mutation in an SH3 domain increases amyloid aggregation by accelerating nucleation, but not by destabilizing thermodynamically the native state. FEBS Lett. 2009, 583, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Watters, A.L.; Deka, P.; Corrent, C.; Callender, D.; Varani, G.; Sosnick, T.; Baker, D. The highly cooperative folding of small naturally occurring proteins is likely the result of natural selection. Cell 2007, 128, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Vendruscolo, M.; Paci, E.; Karplus, M.; Dobson, C. Structures and relative free energies of partially folded states of proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 14817–14821. [Google Scholar] [CrossRef] [Green Version]
- Brockwell, D.J.; Radford, S.E. Intermediates: Ubiquitous species on folding energy landscapes? Curr. Opin. Struct. Biol. 2007, 17, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Jahn, T.R.; Radford, S.E. Folding versus aggregation: Polypeptide conformations on competing pathways. Arch. Biochem. Biophys. 2008, 469, 100–117. [Google Scholar] [CrossRef] [Green Version]
- Turoverov, K.K.; Kuznetsova, I.M.; Uversky, V.N. The protein kingdom extended: Ordered and intrinsically disordered proteins, their folding, supramolecular complex formation, and aggregation. Prog. Biophys. Mol. Biol. 2010, 102, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Sekijima, Y.; Wiseman, R.L.; Matteson, J.; Hammarström, P.; Miller, S.R.; Sawkar, A.R.; Balch, W.E.; Kelly, J.W. The Biological and Chemical Basis for Tissue-Selective Amyloid Disease. Cell 2005, 121, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.I.A.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. From Macroscopic Measurements to Microscopic Mechanisms of Protein Aggregation. J. Mol. Biol. 2012, 421, 160–171. [Google Scholar] [CrossRef]
- Routledge, K.E.; Tartaglia, G.G.; Platt, G.W.; Vendruscolo, M.; Radford, S.E. Competition between Intramolecular and Intermolecular Interactions in an Amyloid-Forming Protein. J. Mol. Biol. 2009, 389, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Modler, A.; Gast, K.; Lutsch, G.; Damaschun, G. Assembly of Amyloid Protofibrils via Critical Oligomers—A Novel Pathway of Amyloid Formation. J. Mol. Biol. 2003, 325, 135–148. [Google Scholar] [CrossRef]
- Thirumalai, D.; Reddy, G. Protein thermodynamics: Are native proteins metastable? Nat. Chem. 2011, 3, 910. [Google Scholar] [CrossRef]
- Chiti, F.; Taddei, N.; Baroni, F.; Capanni, C.; Stefani, M.; Ramponi, G.; Dobson, C.M. Kinetic partitioning of protein folding and aggregation. Nat. Struct. Biol. 2002, 9, 137–143. [Google Scholar] [CrossRef]
- Jiménez, J.L.; Nettleton, E.J.; Bouchard, M.; Robinson, C.V.; Dobson, C.M.; Saibil, H.R. The protofilament structure of insulin amyloid fibrils. Proc. Natl. Acad. Sci. USA 2002, 99, 9196–9201. [Google Scholar] [CrossRef] [Green Version]
- Eichner, T.; Radford, S.E. A Diversity of Assembly Mechanisms of a Generic Amyloid Fold. Mol. Cell 2011, 43, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.J.; Knowles, T.P.J.; Tartaglia, G.G.; Fitzpatrick, A.W.; Devlin, G.L.; Shammas, S.L.; Waudby, C.A.; Mossuto, M.F.; Meehan, S.; Gras, S.L.; et al. Metastability of Native Proteins and the Phenomenon of Amyloid Formation. J. Am. Chem. Soc. 2011, 133, 14160–14163. [Google Scholar] [CrossRef]
- Chong, S.-H.; Ham, S. Distinct Role of Hydration Water in Protein Misfolding and Aggregation Revealed by Fluctuating Thermodynamics Analysis. Acc. Chem. Res. 2015, 48, 956–965. [Google Scholar] [CrossRef]
- Sunde, M.; Blake, C.C.F. From the globular to the fibrous state: Protein structure and structural conversion in amyloid formation. Q. Rev. Biophys. 1998, 31, 1–39. [Google Scholar] [CrossRef]
- Dorta-Estremera, S.M.; Li, J.; Cao, W. Rapid Generation of Amyloid from Native Proteins In vitro. J. Vis. Exp. 2013, e50869. [Google Scholar]
- Goldschmidt, L.; Teng, P.K.; Riek, R.; Eisenberg, D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 2010, 107, 3487–3492. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, R. Kinetics and Thermodynamics of Amyloid Fibril Assembly. Acc. Chem. Res. 2006, 39, 671–679. [Google Scholar] [CrossRef]







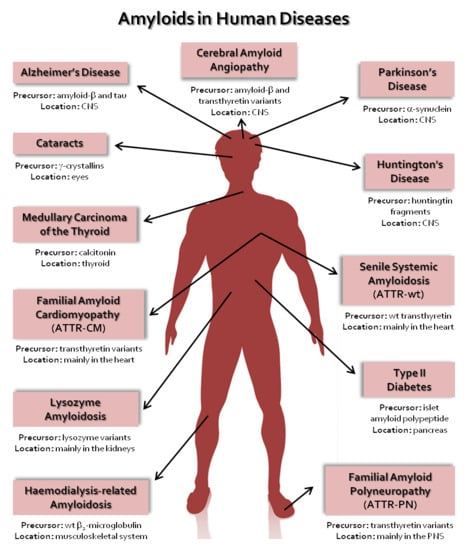
| Disease | Precursor Protein | Polypeptide Length (n° of Residues) | Structural Organization of Precursor |
|---|---|---|---|
| Neurodegenerative Diseases | |||
| Alzheimer’s disease | Amyloid-β variants | 37–44 | IDP |
| Spongiform encephalopathies | Prion protein or its fragments | 208 | IDP and α-helical |
| Parkinson’s disease | α-synuclein | 140 | IDP |
| Frontotemporal dementia with Parkinsonism | Tau | 352–441 | IDP |
| Amyotrophic lateral sclerosis | Superoxide dismutase 1 | 153 | β-sheet |
| Huntington’s disease | Huntingtin with polyQ expansion | 3144 | Mostly IDP |
| Neuroferritinopathy | Ferritin | 175 or 183 | α-helical |
| Familial British dementia | ABri | 34 | IDP |
| Familial Danish dementia | ADan | 34 | IDP |
| Familial amyloid polyneuropathy | Transthyretin variants | 127 | β-sheet |
| Non-Neuropathic Systemic Amyloidosis | |||
| Amyloid light chain amyloidosis | Immunoglobulin light chains or its fragments | ~90 | β-sheet |
| Amyloid heavy chain amyloidosis | Immunoglobulin heavy chains or its fragments | ~220 | β-sheet |
| Amyloid A amyloidosis | Serum amyloid A protein fragments | 45–104 | α-helical and unknown fold |
| Familial Mediterranean fever | Serum amyloid A protein fragments | 45–104 | α-helical and unknown fold |
| Apolipoprotein A1 amyloidosis | Apo A-1 fragments | 80–93 | IDP |
| Senile systemic amyloidosis | Wild-type transthyretin | 127 | β-sheet |
| Familial amyloid cardiomyopathy | Transthyretin variants | 127 | β-sheet |
| Haemodialysis-related amyloidosis | β2-microglobulin | 99 | β-sheet |
| Lysozyme amyloidosis | Lysozyme variants | 130 | α-helical and β-sheet |
| Finnish hereditary amyloidosis | Fragments of gelsolin variants | 53 or 71 | IDP |
| Non-Neuropathic Localized Amyloidosis | |||
| Type II diabetes | Islet amyloid polypeptide | 37 | IDP |
| Injection-localized amyloidosis | Insulin | 21 and 30 | α-helical |
| Gelatinous drop-like corneal dystrophy | Lactoferrin | 691 | α-helical and β-sheet |
| Medullary carcinoma of the thyroid | Calcitonin | 32 | IDP |
| Localized cutaneous amyloidosis | Galectin 7 | 136 | β-sheet |
| Atrial amyloidosis | Atrial natriuretic factor | 28 | IDP |
| Cataracts | γ-crystallins | variable | β-sheet |
| Technique | Advances | Advantages | Disadvantages | References |
|---|---|---|---|---|
| X-ray and electron diffraction | 1. Discovery of short protein segments that can themselves form amyloid fibrils and closely related crystals; 2. Development of synchrotron X-ray microbeams sufficiently focused and intense to determine a structure from a single crystal. | 1. May yield atomic resolution; 2. Is not limited by the molecular weight of the specimen. | 1. Well-ordered microcrystals needed; 2. The fibrils formed by some segments may represent the spines of polymorphs of full fibrils, but others may not; 3. The crystallized segment is only a few residues in length, thus nothing is revealed about the fibril structure outside the spine; 4. The steric zippers structures only show homo-steric zippers. | [23,60,62,126] |
| ssNMR | 1. Innovations in high-field magnets, pulse sequences, high-resolution multi-channel magic-angle spinning (MAS) probes, ultrafast MAS, isotopic labeling schemes, use of quadrupolar nuclei as spectroscopic probes and solid-state dynamic nuclear polarization (DNP). | 1. No need for crystals; 2. Structural information obtained on: identity of residues, recognition of parallel versus antiparallel β-sheets, register of strands within a sheet, and inter-residue contacts of amino acid side chains; 3. ssNMR-determined models show the overall conformation of the well-ordered portion of the chain around the protofilament spine; 4. Can be used to determine dihedral angles and inter-atom distances in the fibril subunits. | 1. Amyloid-forming proteins are expressed recombinantly from media containing isotopically labeled amino acids; 2. Reliability of molecular models is highly dependent on the number of experimental constraints that have been collected; 3. The relative positions of atoms are not as accurately determined as in an atomic-resolution crystal structure; 4. The sensitivity of the experiments and spectral resolution decrease with the increase in molecular weight. | [127,129,130] |
| cryo-EM | 1. Introduction of high-field microscopes; 2. New generation of direct detectors record the incident electrons in a thin, sensitive layer so that the signal is not scattered into surrounding pixels resulting in an improvement in image processing. | 1. Near atomic-resolution structures of large molecular complexes without the need for crystals; 2. May yield the overall fibril structure: the number of protofilaments; the degree of twist; and, depending on the number of well-ordered specimens, information on the atomic structure of the fibril. | 1. Due to a lack of contrast, images often have a very low signal-to-noise ratio, requiring highly advanced detection hardware and image processing; 2. Sample preparation can be difficult, not only to optimize thickness, but also to optimize particle distribution; 3. The most advanced cryo-EM equipment is very expensive. | [131,132,133] |
| Nature of Monomeric Species | Description | References |
| Native monomer | Normally folded proteins may retain a substantial tendency to aggregate through direct assembly of monomers in their native state when the native state exposes complementary surfaces. | [153,154,155,156] |
| Conformationally altered monomer | The native monomer has very low propensity to associate. Partial unfolding or conformational changes of the native monomer are required, resulting in a non-native species prone to aggregate. | [157,158,159,160,161,162] |
| Chemically modified monomer | Chemical modifications (deamidation, isomerization, hydrolysis, oxidation, photolysis, etc.) may cause conformational changes in native monomers, leading to species with high propensity to aggregate. | [163,164,165,166,167,168,169,170,171,172,173,174] |
| Nature of Aggregation Interfaces | Description | References |
| Gas-liquid interface | Hydrophobic–hydrophilic interfaces may induce aggregation reactions. | [175,176,177,178,179,180,181] |
| Mechanical stress (agitation, stirring, pumping, or shaking) has been associated with cavitation which generates air bubbles and, consequently, the formation of an air-water interface which facilitates protein denaturation and aggregation. | [176,182,183,184,185,186,187,188,189] | |
| The use of beads during agitation accelerates the aggregation process by enhancing cavitation. | [190] | |
| Solid-liquid interface | Solid-liquid interfaces may facilitate monomer encounters and initial monomer to monomer association and later further aggregation. | |
| In vitro, interaction with glass, silicone, graphite, polypropylene, Teflon, mica, gold, etc. might lead to protein partial unfolding and aggregation. | [181,191,192,193,194] | |
| In vitro and in vivo, flow through tubes and vessels produce shear forces that may lead to protein partial unfolding and aggregation. | [195] | |
| Freeze-thaw cycles create new ice-water interfaces which may induce protein partial unfolding and aggregation. | [189,191,196] | |
| Presence of metal ions, in particular, Cu2+ and Zn2+, may promote aggregation of protein monomers bearing metal-ion binding sites or binding residues (e.g., histidines). | [197,198,199,200,201] | |
| Monomer association at the surface of biomembranes or biomolecules may also enhance aggregation. | [202,203,204,205,206,207,208,209,210] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, Z.L.; Brito, R.M.M. Structure and Aggregation Mechanisms in Amyloids. Molecules 2020, 25, 1195. https://doi.org/10.3390/molecules25051195
Almeida ZL, Brito RMM. Structure and Aggregation Mechanisms in Amyloids. Molecules. 2020; 25(5):1195. https://doi.org/10.3390/molecules25051195
Chicago/Turabian StyleAlmeida, Zaida L., and Rui M. M. Brito. 2020. "Structure and Aggregation Mechanisms in Amyloids" Molecules 25, no. 5: 1195. https://doi.org/10.3390/molecules25051195
APA StyleAlmeida, Z. L., & Brito, R. M. M. (2020). Structure and Aggregation Mechanisms in Amyloids. Molecules, 25(5), 1195. https://doi.org/10.3390/molecules25051195