Deciphering Pathways for Carotenogenesis in Haloarchaea
Abstract
:1. Introduction
2. Results
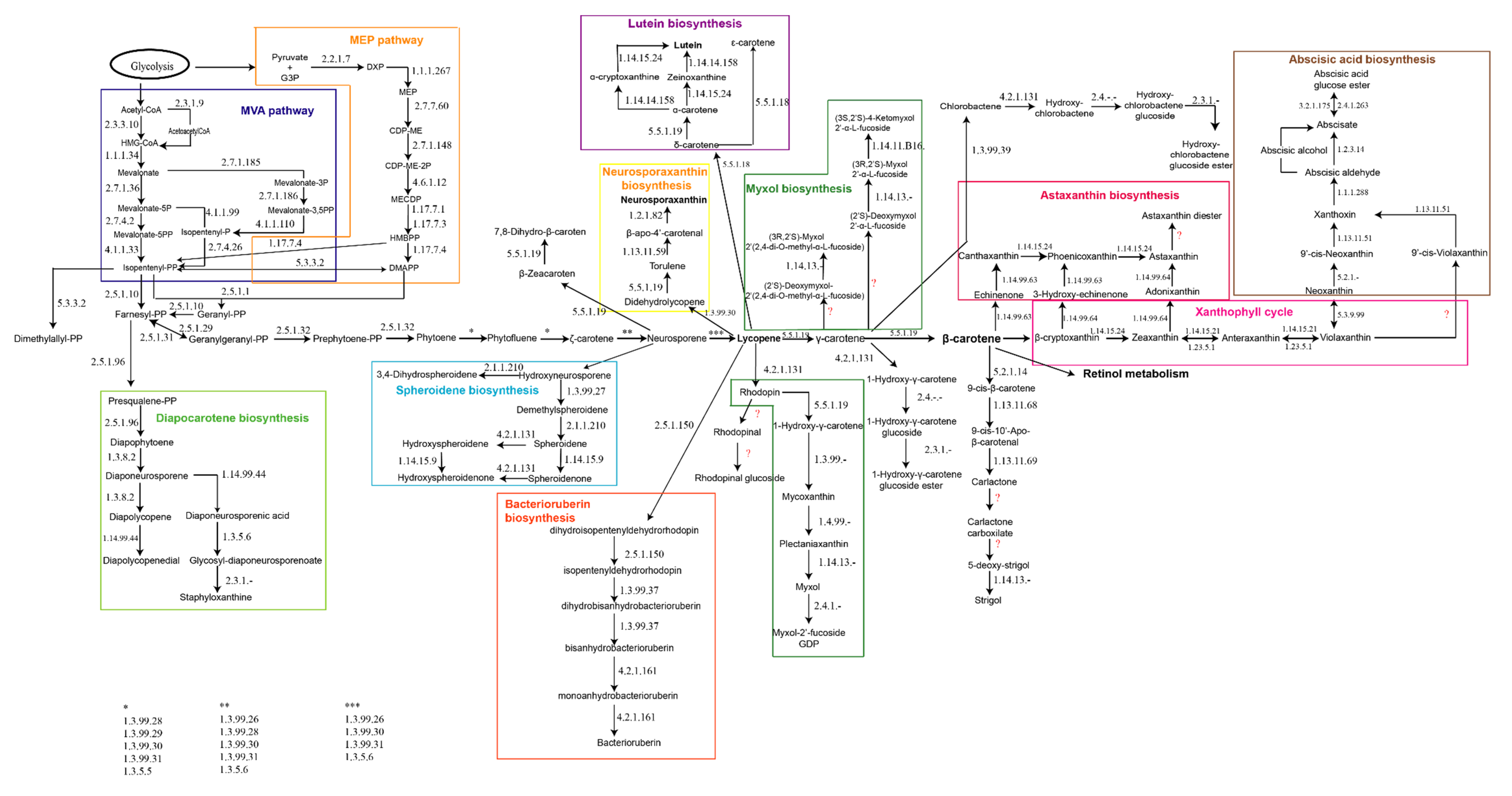
2.1. Reconstruction of a Metabolic Map for Global Carotenogenesis.
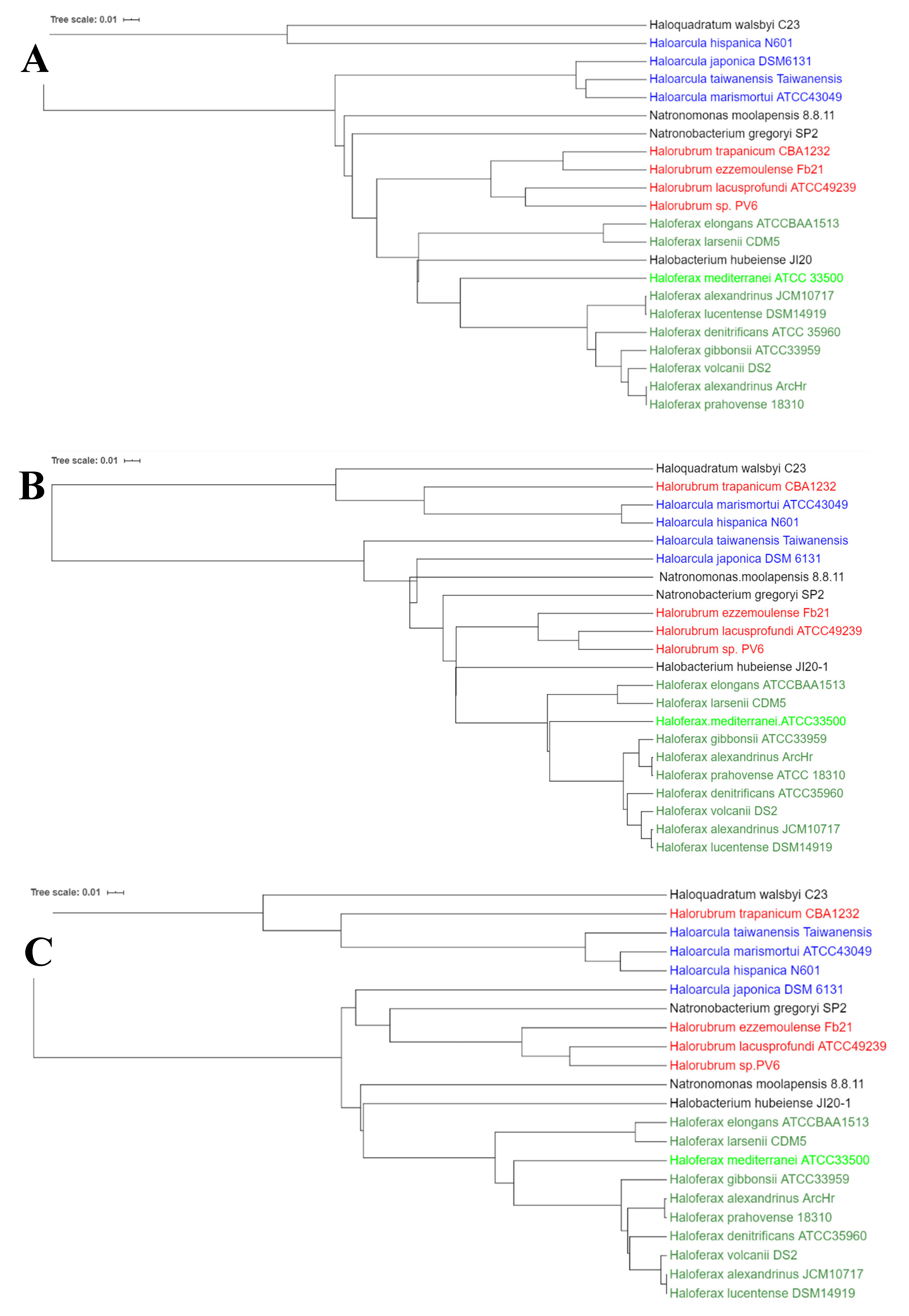
2.2. A Search of the Genes for Carotenogenesis in Haloarchaeal Genomes
- (i)
- Genes involved in the synthesis of bacterioruberin in Haloarcula japonica DSM 6131 were used as a query to look for their homologs in haloarchaeal available genomes at NCBI genome database [12]. Special interest has been paid on the following genomes because these are the haloarchaeal species better described from a biochemical point of view at the time of writing this work: Haloferax mediterranei ATCC3500, Haloferax volcanii DS2, Haloferax gibbonsii ARA6, Haloarcula marismortui ATCC43049, Haloarcula hispanica N601, Halorubrum trapanicum CBA1232, Halorubrum ezzemoulense Fb21, Natronobacterium gregoryi SP2, Natronomonas moolapensis 8.8.11 and Haloquadratum walsbyi C23.
- (ii)
- All these sequences identified in the previous step were used as a query in BlastN to look for their homologs in other haloarchaeal genomes available at NCBI, in a one-to-one comparison. The enzymes encoded by these genes were identified using BlastX and Uniprot. Data about genes homology and identity are summarized in tables displayed in Supplementary Materials (for more details, see Section 4.2) (Supplementary materials: Tables S2–S14).
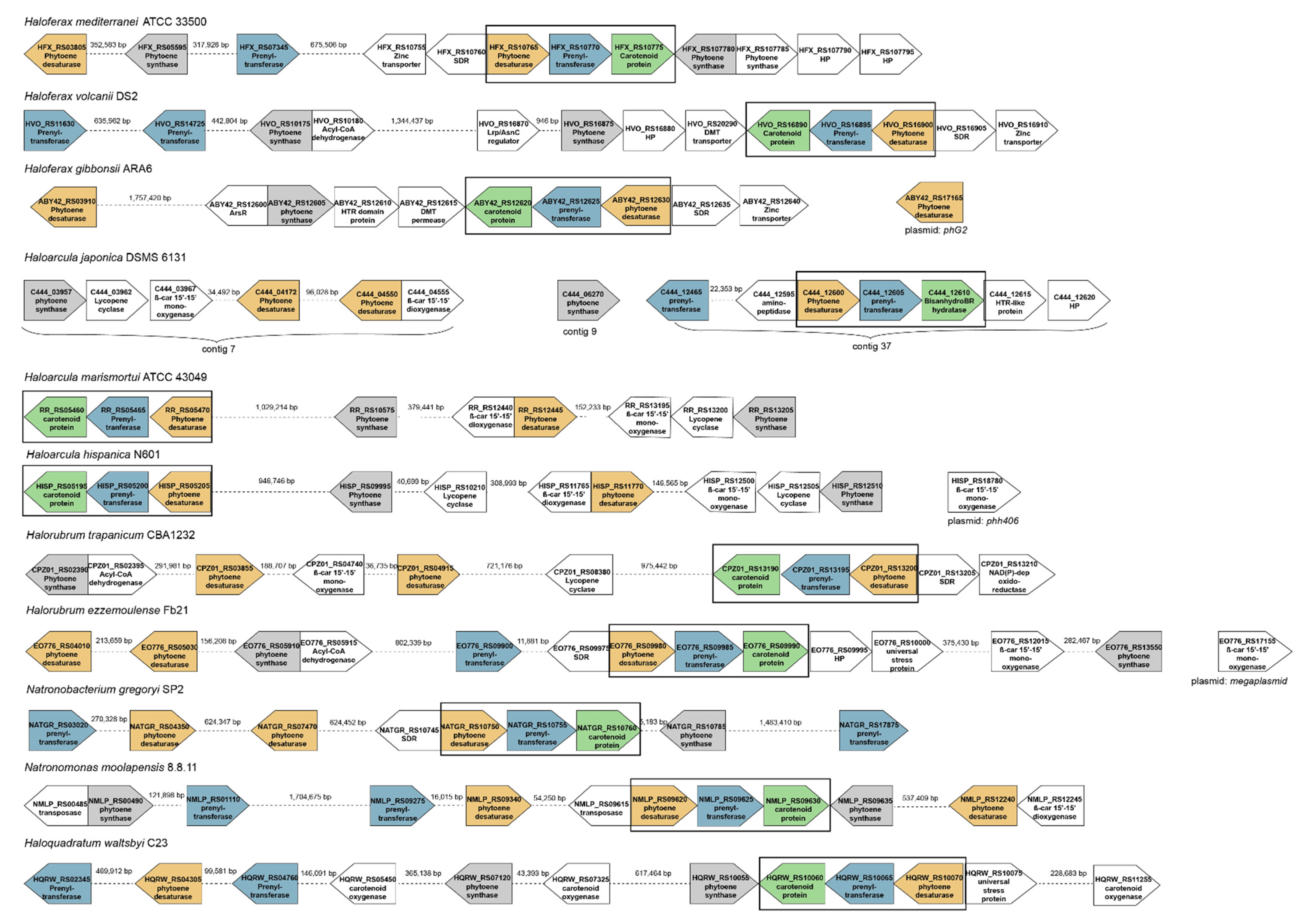
2.3. Organization of the Genomes around the Genes Coding for the Enzymes Involved in the Synthesis of Bacterioruberin
- (1)
- The analysis of the Hfx. mediterranei genome has revealed that this strain presents two genes encoding a phytoene desaturase: one inside the three-gene cluster and another one in another locus of the genome, both showing no resemblance between them. The one included in the three-gene cluster shows an identity between 68% and 87% with the correspondent gene of all the other species included in this study. On the other hand, when observing the gene located outside the cluster, it shares the highest homology (identity = 85.71%) with a phytoene desaturase-coding gene of Haloferax gibbonsii, also located outside the cluster; and shows also some identity with the correspondent gene of Natronobacterium gregoryi (67.13%) and with one of the two genes outside the cluster coding for this enzyme in Natronomonas moolapensis (65.67%), being both species haloalkalophiles.
- (2)
- Hfx. mediterranei genome also has two non-homolog genes coding prenyltransferase: one being part of the cluster and the other one positioned upstream in the genome. The one inside the cluster shows a homology between 69% and 82% with the correspondent gene of all the other studied species; while the one located out of the cluster is very similar to the out-of-cluster gene coding the same enzyme of Haloferax volcanii (84.22%) and also, to the one of Natronobacterium gregoryi (66.81%) and Natronomonas moolapensis (69.58%).
- (3)
- There is only one copy of the gene coding the unidentified ‘’carotenoid biosynthesis protein’’ in all the genomes studied. The one from Hfx. mediterranei genome shows a homology between 64% and 86% with the correspondent gene in the rest of the studied species, being the most similar Hfx. volcanii and Hfx. gibbonsii. However, the most remarkable hit is that this gene shows a 66.58% of homology with the gene coding the enzyme identified as bisanhydrobacterioruberin hydratase, also called C50 carotenoid 2′’,3′’-hydratase, in Haloarcula japonica [12].
- (4)
- Finally, as a significant feature of the Haloferax genus, it is worth mentioning the presence of a gene coding a zinc transporter next to the carotenogenesis three-gene cluster in all the species of this genus. The possible implication of this gene is still unknown.
3. Discussion
4. Materials and Methods
4.1. Data Sample
4.2. In Silico Analysis of the Haloarchaeal Genomes.
4.3. Bioinformatic Tools
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mata-Gómez, L.; Montañez, J.; Méndez-Zavala, A.; Aguilar, C. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelis, H.J.; De Leenheer, A.P. Microbial sources of carotenoid pigments used in foods and feeds. J. Appl. Bacteriol. 1991, 70, 181–191. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. (Eds.) Carotenoids; Birkhäuser Verlag: Basel, Switzerland, 1995; ISBN 978-3-7643-2908-2. [Google Scholar]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Heider, S.A.E.; Peters-Wendisch, P.; Wendisch, V.F.; Beekwilder, J.; Brautaset, T. Metabolic engineering for the microbial production of carotenoids and related products with a focus on the rare C50 carotenoids. Appl. Microbiol. Biotechnol. 2014, 98, 4355–4368. [Google Scholar] [CrossRef]
- Krubasik, P.; Kobayashi, M.; Sandmann, G. Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation: Decaprenoxanthin formation. Eur. J. Biochem. 2001, 268, 3702–3708. [Google Scholar] [CrossRef]
- Norgård, S.; Aasen, A.J.; Liaaen-Jensen, S.; Holme, D.; Lamvik, A.; Sunde, E.; Sørensen, N.A. Bacterial Carotenoids. XXXII. C50-Carotenoids 6. Carotenoids from Corynebacterium poinsettiae Including Four New C50-Diols. Acta Chem. Scand. 1970, 24, 2183–2197. [Google Scholar] [CrossRef] [Green Version]
- Netzer, R.; Stafsnes, M.H.; Andreassen, T.; Goksoyr, A.; Bruheim, P.; Brautaset, T. Biosynthetic Pathway for -Cyclic Sarcinaxanthin in Micrococcus luteus: Heterologous Expression and Evidence for Diverse and Multiple Catalytic Functions of C50 Carotenoid Cyclases. J. Bacteriol. 2010, 192, 5688–5699. [Google Scholar] [CrossRef] [Green Version]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yatsunami, R.; Ando, A.; Miyoko, N.; Fukui, T.; Takaichi, S.; Nakamura, S. Complete Biosynthetic Pathway of the C 50 Carotenoid Bacterioruberin from Lycopene in the Extremely Halophilic Archaeon Haloarcula japonica. J. Bacteriol. 2015, 197, 1614–1623. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Saavedra, C.; Stange, C. Biosynthesis of Carotenoids in Plants: Enzymes and Color. In Carotenoids in Nature; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 79, pp. 35–69. ISBN 978-3-319-39124-3. [Google Scholar]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment: Carotenoids: Pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Henríquez, V.; Escobar, C.; Galarza, J.; Gimpel, J. Carotenoids in Microalgae. In Carotenoids in Nature; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 79, pp. 219–237. ISBN 978-3-319-39124-3. [Google Scholar]
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.; Martínez-Espinosa, R. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Halophiles, coming stars for industrial biotechnology. Biotechnol. Adv. 2015, 33, 1433–1442. [Google Scholar] [CrossRef]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complementary Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Naushad, S.; Baker, S. Phylogenomic analyses and molecular signatures for the class Halobacteria and its two major clades: A proposal for division of the class Halobacteria into an emended order Halobacteriales and two new orders, Haloferacales ord. nov. and Natrialbales ord. nov., containing the novel families Haloferacaceae fam. nov. and Natrialbaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 1050–1069. [Google Scholar]
- Falb, M.; Müller, K.; Königsmaier, L.; Oberwinkler, T.; Horn, P.; von Gronau, S.; Gonzalez, O.; Pfeiffer, F.; Bornberg-Bauer, E.; Oesterhelt, D. Metabolism of halophilic archaea. Extremophiles 2008, 12, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Córdova, D.I.; Camacho-Ruíz, R.M.; Córdova-López, J.A.; Cervantes-Martínez, J. Estimation of bacterioruberin by Raman spectroscopy during the growth of halophilic archaeon Haloarcula marismortui. Appl. Opt. 2014, 53, 7470. [Google Scholar] [CrossRef]
- Fang, C.-J.; Ku, K.-L.; Lee, M.-H.; Su, N.-W. Influence of nutritive factors on C50 carotenoids production by Haloferax mediterranei ATCC 33500 with two-stage cultivation. Bioresour. Technol. 2010, 101, 6487–6493. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.M.; Oren, A. Bacterioruberin and salinixanthin carotenoids of extremely halophilic Archaea and Bacteria: A Raman spectroscopic study. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2013, 106, 99–103. [Google Scholar] [CrossRef]
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef]
- Naziri, D.; Hamidi, M.; Hassanzadeh, S.; Tarhriz, V.; Maleki Zanjani, B.; Nazemyieh, H.; Hejazi, M.A.; Hejazi, M.S. Analysis of Carotenoid Production by Halorubrum sp. TBZ126; an Extremely Halophilic Archeon from Urmia Lake. Adv. Pharm. Bull. 2014, 4, 61–67. [Google Scholar]
- Matsumi, R.; Atomi, H.; Driessen, A.J.M.; van der Oost, J. Isoprenoid biosynthesis in Archaea – Biochemical and evolutionary implications. Res. Microbiol. 2011, 162, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Sui, L.; Liu, L.; Deng, Y. Characterization of halophilic C50 carotenoid-producing archaea isolated from solar saltworks in Bohai Bay, China. Chin. J. Oceanol. Limnol. 2014, 32, 1280–1287. [Google Scholar] [CrossRef]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.-L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Othman, R.; Mohd Zaifuddin, F.A.; Hassan, N.M. Carotenoid Biosynthesis Regulatory Mechanisms in Plants. J. Oleo Sci. 2014, 63, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Arteni, A.-A.; LaFountain, A.M.; Alexandre, M.T.A.; Fradot, M.; Mendes-Pinto, M.M.; Sahel, J.-A.; Picaud, S.; Frank, H.A.; Robert, B.; Pascal, A.A. Carotenoid composition and conformation in retinal oil droplets of the domestic chicken. PLoS ONE 2019, 14, e0217418. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H.; Zeng, Y.; Xu, Q. Plastids and Carotenoid Accumulation. In Carotenoids in Nature; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 79, pp. 273–293. ISBN 978-3-319-39124-3. [Google Scholar]
- Hartz, P.; Milhim, M.; Trenkamp, S.; Bernhardt, R.; Hannemann, F. Characterization and engineering of a carotenoid biosynthesis operon from Bacillus megaterium. Metab. Eng. 2018, 49, 47–58. [Google Scholar] [CrossRef]
- Raisig, A.; Sandmann, G. Functional properties of diapophytoene and related desaturases of C30 and C40 carotenoid biosynthetic pathways. Biochim. Et Biophys. Acta (Bba) - Mol. Cell Biol. Lipids 2001, 1533, 164–170. [Google Scholar] [CrossRef]
- Takaichi, S.; Inoue, K.; Akaike, M.; Kobayashi, M.; Oh-oka, H.; Madigan, M.T. The major carotenoid in all known species of heliobacteria is the C30 carotenoid 4,4′-diaponeurosporene, not neurosporene. Arch. Microbiol. 1997, 168, 277–281. [Google Scholar] [CrossRef]
- Furubayashi, M.; Li, L.; Katabami, A.; Saito, K.; Umeno, D. Construction of carotenoid biosynthetic pathways using squalene synthase. Febs Lett. 2014, 588, 436–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiger, S.; Perez-Fons, L.; Cutting, S.M.; Fraser, P.D.; Sandmann, G. Annotation and functional assignment of the genes for the C30 carotenoid pathways from the genomes of two bacteria: Bacillus indicus and Bacillus firmus. Microbiology 2015, 161, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirsakova, V.; Reiss-Husson, F. A specific carotenoid is required for reconstitution of the Rubrivivax gelatinosus B875 light harvesting complex from its subunit form B820. FEBS Lett. 1994, 353, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Ashikhmin, A.A.; Makhneva, Z.K.; Bolshakov, M.A.; Shastik, E.S.; Moskalenko, A.A. Embedding carotenoids of spheroidene-branch biosynthesis into antenna complexes of sulfur photosynthetic bacteria. Dokl. Biochem. Biophys. 2016, 468, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Ashikhmin, A.; Makhneva, Z.; Bolshakov, M.; Moskalenko, A. Incorporation of spheroidene and spheroidenone into light-harvesting complexes from purple sulfur bacteria. J. Photochem. Photobiol. B Biol. 2017, 170, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Zhou, Z.; Jiang, Y. Microalgae as a Source of Lutein: Chemistry, Biosynthesis, and Carotenogenesis. In Microalgae Biotechnology; Posten, C., Feng Chen, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 153, pp. 37–58. ISBN 978-3-319-23807-4. [Google Scholar]
- Van den Berg, H. Effect of lutein on beta-carotene absorption and cleavage. Int. J. Vitam Nutr. Res. 1998, 68, 360–365. [Google Scholar]
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524. [Google Scholar] [CrossRef] [Green Version]
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodríguez-Ortiz, R.; Hornero-Méndez, D.; Limón, M. Carotenoid Biosynthesis in Fusarium. J. Fungi 2017, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Hornero-Méndez, D.; Limón, M.C.; Avalos, J. HPLC Analysis of Carotenoids in Neurosporaxanthin-Producing Fungi. In Microbial Carotenoids; Barreiro, C., Barredo, J.-L., Eds.; Springer: New York, NY, USA, 2018; Volume 1852, pp. 269–281. ISBN 978-1-4939-8741-2. [Google Scholar]
- Avalos, J.; Prado-Cabrero, A.; Estrada, A.F. Neurosporaxanthin Production by Neurospora and Fusarium. In Microbial Carotenoids from Fungi; Barredo, J.-L., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 898, pp. 263–274. ISBN 978-1-61779-917-4. [Google Scholar]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.; Reig García-Galbis, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [Green Version]
- Francis, G.W.; Liaaen-Jensen, S.; Silvennoinen, K.; Vaahtera, K.; Shimizu, A. Bacterial Carotenoids. XXXIII. Carotenoids of Thiorhodaceae. 9. The Structures of the Carotenoids of the Rhodopinal Series. Acta Chemica Scandinavica 1970, 24, 2705–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teramoto, M.; Onodera, K.; Moriyama, H.; Komatsu, A.; Akakabe, M.; Nishijima, M. Aurantiacicella marina gen. nov., sp. nov., a myxol-producing bacterium from surface seawater. Int. J. Syst. Evol. Microbiol. 2016, 66, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Bryant, D.A. The Biosynthetic Pathway for Myxol-2′ Fucoside (Myxoxanthophyll) in the Cyanobacterium Synechococcus sp. Strain PCC 7002. J. Bacteriol. 2009, 191, 3292–3300. [Google Scholar] [CrossRef] [Green Version]
- Mochimaru, M.; Masukawa, H.; Maoka, T.; Mohamed, H.E.; Vermaas, W.F.J.; Takaichi, S. Substrate Specificities and Availability of Fucosyltransferase and -Carotene Hydroxylase for Myxol 2′-Fucoside Synthesis in Anabaena sp. Strain PCC 7120 Compared with Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2008, 190, 6726–6733. [Google Scholar] [CrossRef] [Green Version]
- Takaichi, S.; Maoka, T.; Akimoto, N.; Carmona, M.L.; Yamaoka, Y. Carotenoids in a Corynebacterineae, Gordonia terrae AIST-1: Carotenoid Glucosyl Mycoloyl Esters. Biosci. Biotechnol. Biochem. 2008, 72, 2615–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarroya, F.; Giralt, M.; Iglesias, R. Retinoids and adipose tissues: Metabolism, cell differentiation and gene expression. Int. J. Obes. 1999, 23, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.; Hoekstra, M.; Hoeke, M.O.; Heegsma, J.; Faber, K.N. The interrelationship between bile acid and vitamin A homeostasis. BBA-Mol. Cell Biol. L. 2017, 1862, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.C. Vitamin A and Vision. In The Biochemistry of Retinoid Signaling II.; Asson-Batres, M.A., Rochette-Egly, C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2016; Volume 81, pp. 231–259. ISBN 978-94-024-0943-7. [Google Scholar]
- Goss, R.; Jakob, T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 2010, 106, 103–122. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P. Xanthophylls as modulators of membrane protein function. Arch. Biochem. Biophys. 2010, 504, 78–85. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Et Biophys. Acta (Bba)—Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwanenburg, B.; Blanco-Ania, D. Strigolactones: New plant hormones in the spotlight. J. Exp. Bot. 2018, 69, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Beltran, J.C.M.; Stange, C. Apocarotenoids: A New Carotenoid-Derived Pathway. In Carotenoids in Nature; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 79, pp. 239–272. ISBN 978-3-319-39124-3. [Google Scholar]
- Humphrey, A.; Beale, M. Strigol: Biogenesis and physiological activity. Phytochemistry 2006, 67, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Asker, D. Ohta Haloferax alexandrinus sp. nov., an extremely halophilic canthaxanthin-producing archaeon from a solar saltern in Alexandria (Egypt). Int. J. Syst. Evol. Microbiol. 2002, 52, 729–738. [Google Scholar]
- Asker, D.; Ohta, Y. Production of canthaxanthin by extremely halophilic bacteria. J. Biosci. Bioeng. 1999, 88, 617–621. [Google Scholar] [CrossRef]
- Calo, P.; Velazquez, J.B.; Sieiro, C.; Blanco, P.; Longo, E.; Villa, T.G. Analysis of astaxanthin and other carotenoids from several Phaffia rhodozyma mutants. J. Agric. Food Chem. 1995, 43, 1396–1399. [Google Scholar] [CrossRef]
- Müller, W.J.; Smit, M.S.; van Heerden, E.; Capes, M.D.; DasSarma, S. Complex Effects of Cytochrome P450 Monooxygenase on Purple Membrane and Bacterioruberin Production in an Extremely Halophilic Archaeon: Genetic, Phenotypic, and Transcriptomic Analyses. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; González-Torres, P.; Bautista, V.; Esclapez, J.M.; Pire, C.; Camacho, M.; Bonete, M.J.; Richardson, D.J.; Watmough, N.J.; Martínez-Espinosa, R.M. Analysis of multiple haloarchaeal genomes suggests that the quinone-dependent respiratory nitric oxide reductase is an important source of nitrous oxide in hypersaline environments: Analysis of multiple haloarchaeal genomes. Environ. Microbiol. Rep. 2017, 9, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.M. Carotenoid pigments of halophilic bacteria. Can. J. Microbiol. 1960, 6, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.L.; Brown, A.D. The membrane lipids of Halobacterium halobium. Biochem. J. 1968, 110, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Calegari-Santos, R.; Diogo, R.A.; Fontana, J.D.; Bonfim, T.M.B. Carotenoid Production by Halophilic Archaea Under Different Culture Conditions. Curr. Microbiol. 2016, 72, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Theochari, I.; Papadimitriou, V.; Xenakis, A.; Ammar, E. Encapsulation of carotenoids extracted from halophilic Archaea in oil-in-water (O/W) micro- and nano-emulsions. Colloids Surf. B Biointerfaces 2018, 161, 219–227. [Google Scholar] [CrossRef]
- Hou, J.; Cui, H.-L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the extreme halophilic archaeon Haloterrigena turkmenica: Identification and antioxidant activity. Extremophiles 2017, 21, 933–945. [Google Scholar] [CrossRef]
- Dellas, N.; Thomas, S.T.; Manning, G.; Noel, J.P. Discovery of a metabolic alternative to the classical mevalonate pathway. eLife 2013, 2. [Google Scholar] [CrossRef]
- Swift, I.E.; Milborrow, B.V. Stereochemistry of allene biosythesis and the formation of the acetylenic carotenoid diadinoxanthin and peridinin (C37) from neoxanthin. Biochem. J. 2005, 389, 919. [Google Scholar] [CrossRef] [Green Version]
- VanNice, J.C.; Skaff, D.A.; Keightley, A.; Addo, J.K.; Wyckoff, G.J.; Miziorko, H.M. Identification in Haloferax volcanii of Phosphomevalonate Decarboxylase and Isopentenyl Phosphate Kinase as Catalysts of the Terminal Enzyme Reactions in an Archaeal Alternate Mevalonate Pathway. J. Bacteriol. 2014, 196, 1055–1063. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Ramos-Merchante, A.; Fuentes, J.; Sayago, A.; Fernández-Recamales, Á.; Martínez-Espinosa, R.; Vega, J.; Vílchez, C.; Garbayo, I. Optimization of Growth and Carotenoid Production by Haloferax mediterranei Using Response Surface Methodology. Mar. Drugs 2018, 16, 372. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, S.C.; Kates, M.K. Effect of glycerol on carotenogenesis in the extreme halophile, Halobacterium Cutirubrum. Can. J. Microbiol. 1979, 25, 1288–1291. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Kates, M. Studies of the biosynthesis of C50 carotenoids in Halobacterium Cutirubrum. Can. J. Microbiol. 1979, 25, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Radwan, M.O.; Hamasaki, A.; Ejima, A.; Obata, E.; Koga, R.; Tateishi, H.; Okamoto, Y.; Fujita, M.; Nakao, M.; et al. A novel inhibitor of farnesyltransferase with a zinc site recognition moiety and a farnesyl group. Bioorganic Med. Chem. Lett. 2017, 27, 3862–3866. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.G.; Goulding, C.W. Enzyme-catalyzed methyl transfers to thiols: The role of zinc. Curr. Opin. Chem. Biol. 1997, 1, 332–339. [Google Scholar] [CrossRef]
- Hu, X.; Dong, Q.; Yang, J.; Zhang, Y. Recognizing metal and acid radical ion-binding sites by integrating ab initio modeling with template-based transferals. Bioinformatics 2016, 32, 3260–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarasov, V.Y.; Besir, H.; Schwaiger, R.; Klee, K.; Furtwängler, K.; Pfeiffer, F.; Oesterhelt, D. A small protein from the bop-brp intergenic region of Halobacterium salinarum contains a zinc finger motif and regulates bop and crtB1 transcription: Zinc finger regulator of bop and crtB1 expression. Mol. Microbiol. 2008, 67, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarasov, V.; Schwaiger, R.; Furtwängler, K.; Dyall-Smith, M.; Oesterhelt, D. A small basic protein from the brz-brb operon is involved in regulation of bop transcription in Halobacterium salinarum. BMC Mol. Biol. 2011, 12, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DasSarma, P.; Zamora, R.C.; Muller, J.A.; DasSarma, S. Genome-Wide Responses of the Model Archaeon Halobacterium sp. Strain NRC-1 to Oxygen Limitation. J. Bacteriol. 2012, 194, 5530–5537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, A.; Hallsworth, J.E. Microbial weeds in hypersaline habitats: The enigma of the weed-like Haloferax mediterranei. FEMS Microbiol. Lett. 2014, 359, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Z.Q.; Xue, Q.; Zhou, J.; Zhao, D.H.; Han, J.; Xiang, H. Engineering Haloferax mediterranei as an Efficient Platform for High Level Production of Lycopene. Front. Microbiol. 2018, 29, 2893. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, N.; Paggi, R.; Troetschel, C.; Ferrari, M.C.; Ramallo Guevara, C.; Albaum, S.; Poetsch, A.; De Castro, R. LonB Protease is a novel regulator of carotenogenesis controlling degradation of phytoene synthase in Haloferax volcanii. J. Proteome Res. 2018, 17, 1158–1171. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2014, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Soding, J. Protein homology detection by HMM-HMM comparison. Bioinformatics 2005, 21, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: This study is based on bioinformatics analysis. Consequently, details about sample availability are not required. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giani, M.; Miralles-Robledillo, J.M.; Peiró, G.; Pire, C.; Martínez-Espinosa, R.M. Deciphering Pathways for Carotenogenesis in Haloarchaea. Molecules 2020, 25, 1197. https://doi.org/10.3390/molecules25051197
Giani M, Miralles-Robledillo JM, Peiró G, Pire C, Martínez-Espinosa RM. Deciphering Pathways for Carotenogenesis in Haloarchaea. Molecules. 2020; 25(5):1197. https://doi.org/10.3390/molecules25051197
Chicago/Turabian StyleGiani, Micaela, Jose María Miralles-Robledillo, Gloria Peiró, Carmen Pire, and Rosa María Martínez-Espinosa. 2020. "Deciphering Pathways for Carotenogenesis in Haloarchaea" Molecules 25, no. 5: 1197. https://doi.org/10.3390/molecules25051197
APA StyleGiani, M., Miralles-Robledillo, J. M., Peiró, G., Pire, C., & Martínez-Espinosa, R. M. (2020). Deciphering Pathways for Carotenogenesis in Haloarchaea. Molecules, 25(5), 1197. https://doi.org/10.3390/molecules25051197






