Incorporation of Lutein on Layered Double Hydroxide for Improving the Environmental Stability
Abstract
:1. Introduction
2. Results and Discussion
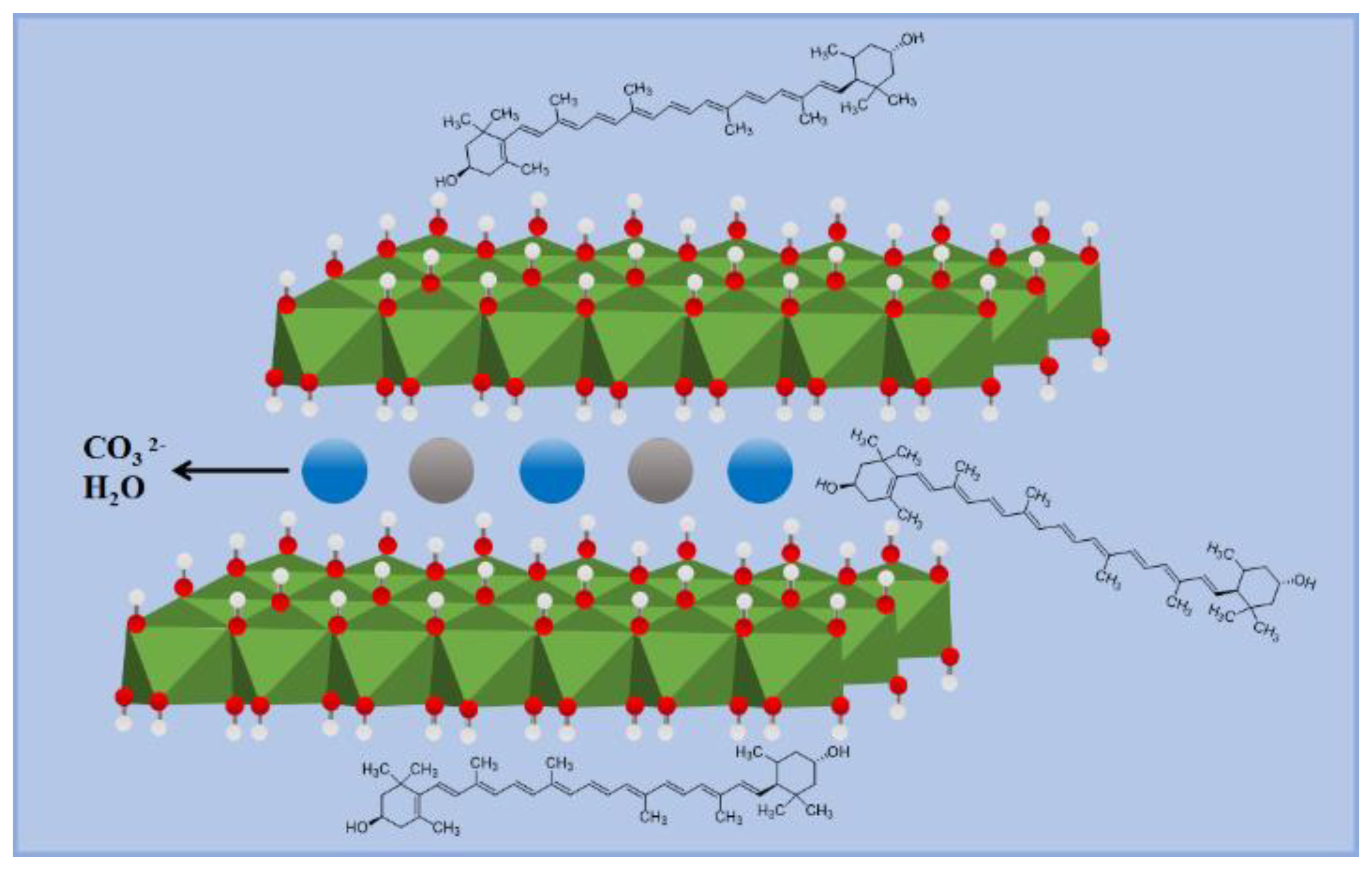
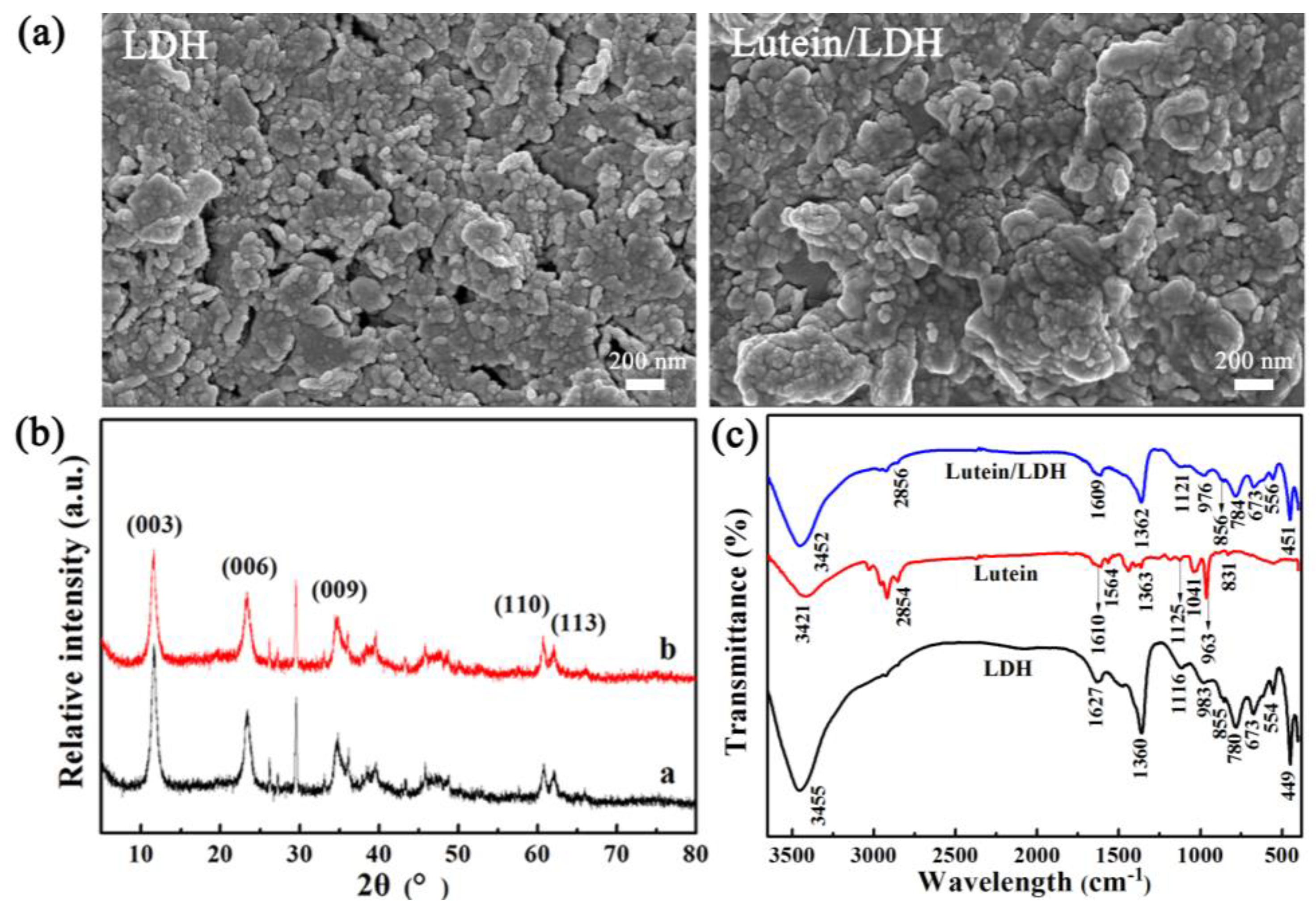
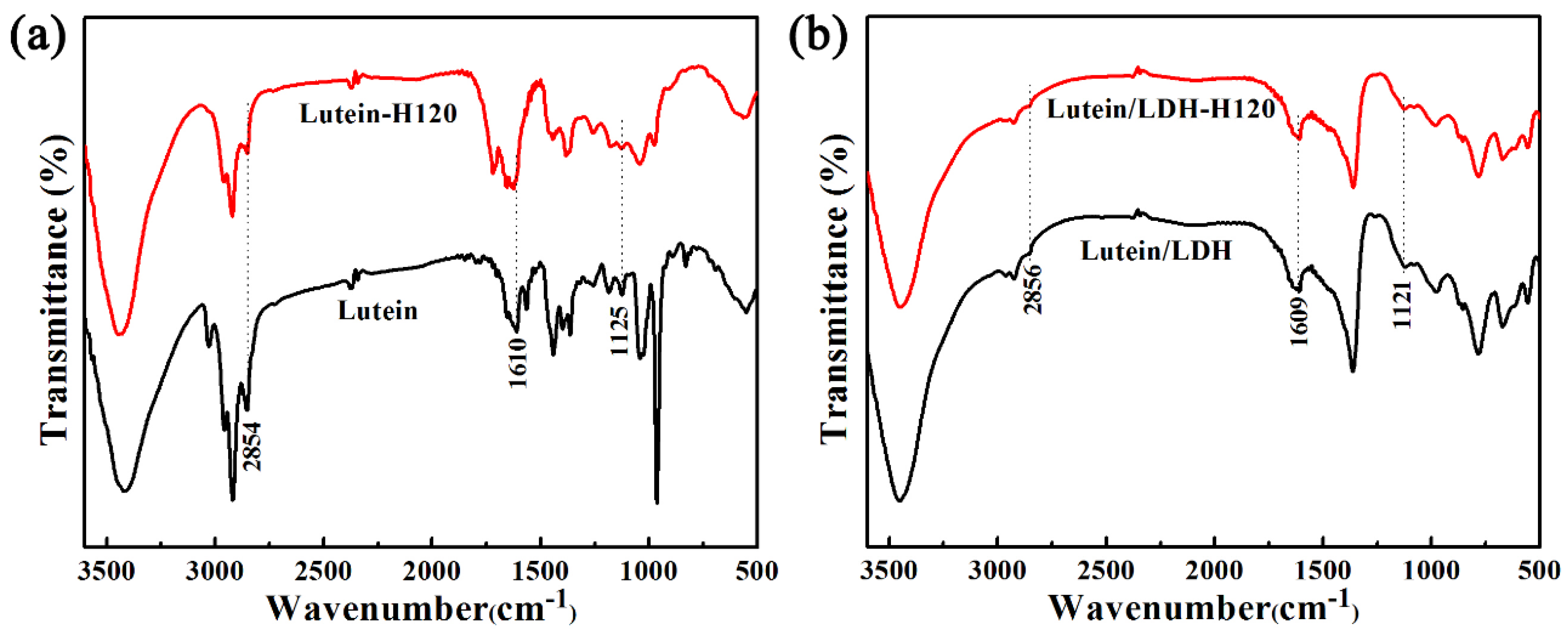
2.1. Characterization of Lutein/LDH Composites
2.2. Environmental Stability of Lutein/LDH Composites
2.2.1. Chemical Stability
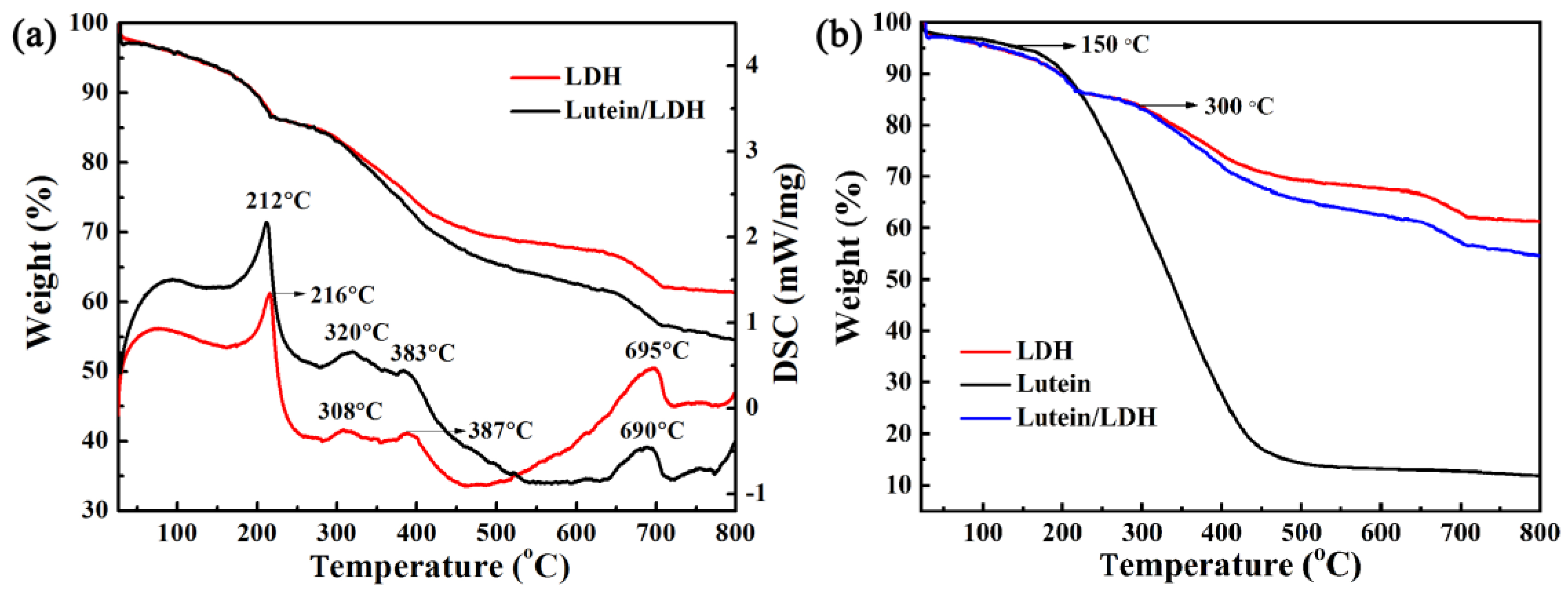
2.2.2. Thermal Stability
2.2.3. Photostability and Storage Stability
3. Materials and Methods
3.1. Materials
3.2. Preparation of Lutein/LDH Composites
3.3. Chemical stabilityEvaluation of Lutein/LDH Composites
3.4. Thermal Stability Evaluation of Lutein/LDH Composites
3.5. Photostability and Storage Stability of Lutein/LDH Composites
3.6. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jia, K.P.; Baz, L.; Al-Babil, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczynski, P.; Oziewicz, S.; Jurgaand, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Teo, A.; Lee, S.J.; Goh, K.K.T.; Wolber, F.M. Kinetic stability and cellular uptake of lutein in WPI-stabilised nanoemulsions and emulsions prepared by emulsification and solvent evaporation method. Food Chem. 2017, 221, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.N.; Gao, S.S.; Zhou, J.L.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.W.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef] [Green Version]
- Mehariya, S.; Iovine, A.; Sanzo, G.D.; Larocca, V.; Martino, M.; Leone, G.P.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical fluid extraction of lutein from scenedesmusalmeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Liu, F.G.; Duan, X.; Xiao, C.X.; Liu, X.B. Physicochemical properties of lutein-loaded microcapsules and their uptake via caco-2 monolayers. Molecules 2018, 23, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesterberg, K.; Schanzer, S.; Patzelt, A.; Sterry, W.; Fluhr, J.W.; Meinke, M.C.; Lademann, J.; Darvin, M.E. Raman spectroscopic analysis of the carotenoid concentration in egg yolks depending on the feeding and housing conditions of the laying hens. J. Biophotonics 2012, 5, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Prashanth, K.V.H.; Baskaran, V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: Characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013, 141, 327–337. [Google Scholar] [CrossRef]
- Li, S.N.; Wang, C.; Fu, X.; Li, C.; He, X.W.; Zhang, B.; Huang, Q. Encapsulation of lutein into swelled cornstarch granules: Structure, stability and in vitro digestion. Food Chem. 2018, 268, 362–368. [Google Scholar] [CrossRef]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef]
- Jia, Y.P.; Sun, L.; Yu, H.S.; Liang, L.P.; Li, W.; Ding, H.; Song, X.B.; Zhang, L.J. The pharmacological effects of lutein and zeaxanthinon visual disorders and cognition diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Madaan, T.; Choudhary, A.N.; Gyenwalee, S.; Thomas, S.; Mishra, H.; Tariq, M.; Vohora, D.; Talegaonkar, S. Lutein, a versatile phyto-nutraceutical: An insight on pharmacology, therapeutic indications, challenges and recent advances in drug delivery. Pharma. Nutr. 2017, 5, 64–75. [Google Scholar] [CrossRef]
- Qv, X.Y.; Zeng, Z.P.; Jiang, J.G. Preparation of lutein microencapsulation by complex coacervation method and its physicochemical properties and stability. Food Hydrocoll. 2011, 25, 1596–1603. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Lou, X.W.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.M.; Xia, S.Q. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Zhao, C.H.; Shen, X.; Guo, M.R. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLoS ONE 2018, 13, e0192511. [Google Scholar] [CrossRef]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Apanasenko, I.E.; Selyutina, O.Y.; Polyakov, N.E.; Suntsova, L.P.; Meteleva, E.S.; Dushkin, A.V.; Vachali, P.; Bernstein, P.S. Solubilization and stabilization of macular carotenoids by water soluble oligosaccharides and polysaccharides. Arch. Biochem. Biophys. 2015, 572, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Polyakov, N.E.; Magyar, A.; Kispert, L.D. Photochemical and optical properties of water-soluble Xanthophyll antioxidants: Aggregation vs complexation. J. Phys. Chem. B 2013, 117, 10173–10182. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular carotenoid complexes of enhanced solubility and stability-The way of bioavailability improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Gao, Y.; Zhou, Z.; Strappe, P.; Blanchard, C. Fabrication and characterization of ferritin-chitosan-lutein shell-core nanocomposites and lutein stability and release evaluation in vitro. RSC Adv. 2016, 6, 35267–35279. [Google Scholar] [CrossRef]
- Wang, Q.; O’ Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Cheng, H.M.; Gao, X.W.; Zhou, W.; Li, S.J.; Cao, X.L.; Yan, D.P. Intercalation assembly of kojic acid into Zn-Ti layered double hydroxide with antibacterial and whitening performances. Chin. Chem. Lett. 2019, 30, 919–923. [Google Scholar] [CrossRef]
- Wang, X.R.; Li, Y.; Tang, L.P.; Gan, W.; Zhou, W.; Zhao, Y.F.; Bai, D.F. Fabrication of Zn-Ti layered double hydroxide by varying cationic ratio of Ti4+ and its application as UV absorbent. Chin. Chem. Lett. 2017, 28, 394–399. [Google Scholar] [CrossRef]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical layered double hydroxide nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martos, A.M.; Herrero, M.; Várez, A.; Levenfeld, B. Synthesis and characterization of new membranes based on sulfonated polysulfone/Zn,Al-heptamolibdate LDH. Mater. Lett. 2015, 152, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.F.; Liu, P.; Zhao, K.C.; Li, L. Photostability enhancement of azoic dyes adsorbed and intercalated into Mg-Al-layered double hydroxide. Opt. Laser Technol. 2015, 74, 23–28. [Google Scholar] [CrossRef]
- Li, L.; Liu, P.F.; Zhang, L.; Chen, D.Z. Photophysical properties of donor-∏-acceptor azoic chromophores adsorbed and intercalated into Mg-Al-LDH. J. Solid State Chem. 2013, 198, 218–223. [Google Scholar] [CrossRef]
- Zhu, H.F.; Wang, L.R.; Tang, P.G.; Feng, Y.J.; Li, D.Q. Improving thermal stability and light fastness of Acid Red 114 by incorporating its anions in a ZnAl-layered double hydroxides matrix. Particuology 2012, 10, 503–508. [Google Scholar] [CrossRef]
- Kohno, Y.; Asai, S.; Shibata, M.; Fukuhara, C.; Maeda, Y.; Tomita, Y.; Kobayashi, K. Improved photostability of hydrophobic natural dye incorporated in organo-modified hydrotalcite. J. Phys. Chem. Sol. 2014, 75, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Kohno, Y.; Totsuka, K.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Photostability enhancement of anionic natural dye by intercalation into hydrotalcite. J. Colloid Interface Sci. 2009, 337, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring hybrid layered double hydroxides for the development of innovative applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Maruyama, S.A.; Tavares, S.R.; Leitão, A.A.; Wypych, F. Intercalation of indigo carmine anions into zinc hydroxide salt: A novel alternative blue pigment. Dyes Pigment. 2016, 128, 158–164. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inorg. Chin. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Ay, A.N.; Zümreoglu-Karan, B.; Mafra, L. A simple mechanochemical route to layered double hydroxides: Synthesis of hydrotalcite-like Mg-Al-NO3-LDH by manual grinding in a mortar. Z. Anorg. Allg. Chem. 2009, 635, 1470–1475. [Google Scholar] [CrossRef]
- BhojarajArulraj, J.; Kolinjavadi, M.R.; Rajamathi, M. Solvent-mediated and mechanochemical methods for anion exchange of carbonate from layered double hydroxides using ammonium salts. ACS Omega 2019, 4, 20072–20079. [Google Scholar] [CrossRef] [Green Version]
- You, W.Y.; Vance, G.E.; Zhao, H.T. Selenium adsorption on Mg-Al and Zn-Al layered double hydroxides. Appl. Clay Sci. 2001, 20, 13–25. [Google Scholar] [CrossRef]
- Bontchev, R.P.; Liu, S.; Krumhansl, J.L.; Voigt, J.; Nenoff, T.M. Synthesis, characterization, and ion exchange properties of hydrotalcite Mg6Al2(OH)16(A)x(A′)2−x·4H2O (A, A′ = Cl−, Br−, I−, and NO3−,2 ≥ x ≥ 0) derivatives. Chem. Mater. 2003, 15, 3669–3675. [Google Scholar] [CrossRef]
- Pourfaraj, R.; Fatemi, S.J.; Kazemi, S.J.; Biparva, P. Synthesis of hexagonal mesoporous MgAl LDH nanoplatelets adsorbent for the effective adsorption of Brilliant Yellow. J. Colloid Interf. Sci. 2017, 508, 65–74. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rogowski, J.; Maniukiewicz, W.; Zaborski, M. Insight into the formation mechanism of azo dye-based hybrid colorant: Physico-chemical properties and potential applications. Dyes Pigment. 2019, 167, 236–244. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Zaborski, M. Characterization and structure–property relationships of organic–inorganic hybrid composites based on aluminum–magnesium hydroxycarbonate and azo chromophore. Molecules 2019, 24, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyi, N.; Matsumoto, T.; Kaneko, Y.; Kitamura, K. Deintercalation of carbonate ions from a hydrotalcite-like compound: Enhanced decarbonation using acid-salt mixed solution. Chem. Mater. 2004, 16, 2926–2932. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Kozanecki, M.; Moszyński, D.; Zaborski, M. Characterization and properties of new color-tunable hybrid pigments based on layered double hydroxides (LDH) and 1,2-dihydroxyanthraquinone dye. J. Ind. Eng. Chem. 2019, 70, 427–438. [Google Scholar] [CrossRef]
- Silva, J.T.D.; Geiss, J.M.T.; Oliveira, S.M.; Brum, E.D.S.; Sagae, S.C.; Becker, D.; Leimann, F.V.; Ineu, R.P.; Guerra, G.P.; Goncalves, O.H. Nanoencapsulation of lutein and its effect on mice’s declarative memory. Mater. Sci. Eng. C 2017, 76, 1005–1011. [Google Scholar] [CrossRef]
- Zhao, C.D.; Cheng, H.; Jiang, P.F.; Yao, Y.J.; Han, J. Preparation of lutein-loaded particles for improving solubility and stability by polyvinylpyrrolidone (PVP) as an emulsion-stabilizer. Food Chem. 2014, 156, 123–128. [Google Scholar] [CrossRef]
- Wang, L.D.; Zhang, K.Y.; Sun, W.; Wu, T.T.; He, H.R.; Liu, G.C. Hydrothermal synthesis of corrosion resistant hydrotalcite conversion coating on AZ91D alloy. Mater. Lett. 2013, 106, 111–114. [Google Scholar] [CrossRef]
- El Gaini, L.; Lakraimi, M.; Sebbar, E.; Meghea, A.; Bakasse, M. Removal of indigo carmine dye from water to Mg-Al-CO3-calcined layered double hydroxides. J. Hazard. Mater. 2009, 161, 627–632. [Google Scholar] [CrossRef]
- Laguna, H.; Loera, S.; Ibarra, I.A.; Lima, E.; Vera, M.A.; Lara, V. Azoic dyes hosted on hydrotalcite-like compounds: Non-toxic hybrid pigments. Microporous Mesoporous Mater. 2007, 98, 234–241. [Google Scholar] [CrossRef]
- Zeng, R.C.; Liu, Z.G.; Zhang, F.; Li, S.Q.; He, Q.K.; Cui, H.Z.; Han, E.H. Corrosion resistance of in-situ Mg-Al hydrotalcite conversion film on AZ31 magnesium alloy by one-step formation. Trans. Nonferr. Metal. Soc. 2015, 25, 1917–1925. [Google Scholar] [CrossRef]
- Dong, W.K.; Lu, Y.S.; Wang, W.B.; Zhang, M.M.; Jing, Y.M.; Wang, A.Q. A sustainable approach to fabricate new 1D and 2D nanomaterials from natural abundant palygorskite clay for antibacterial and adsorption. Chem. Eng. J. 2020, 382, 122984. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Watanabe, K.; Suzuki, H. Corrosion resistance of Mg-Al layered double hydroxide container-containing magnesium hydroxide films formed directly on magnesium alloy by chemical-free steam coating. J. Mater. Chem. A 2013, 1, 8968. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhu, X.P.; Meng, X.R. Preparation of a Mg/Al/Fe layered supramolecular compound and application for removal of Cr (VI) from laboratory wastewater. RSC Adv. 2017, 7, 34984–34993. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.T.D.; da Silva, A.C.; Geiss, J.M.T.; de Araujo, P.H.H.; Becker, D.; Bracht, L.; Leimann, F.V.; Bona, E.; Guerra, G.P.; Goncalves, O.H. Analytical validation of an ultraviolet-visible procedure for determining lutein concentration and application to lutein-loaded nanoparticles. Food Chem. 2017, 230, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Boonnoun, P.; Nerome, H.; Machmudah, S.; Goto, M.; Shotipruk, A. Supercritical anti-solvent micronization of chromatography purified marigold lutein using hexane and ethyl acetate solvent mixture. J. Supercrit. Fluid. 2013, 80, 15–22. [Google Scholar] [CrossRef]
- Wang, G.Y.; Chen, J.; Shi, Y.P. Preparation of microencapsulated xanthophyll for improving solubility and stability by nanoencapsulation. J. Food Eng. 2013, 117, 82–88. [Google Scholar] [CrossRef]
- Nalawade, P.; Gajjar, A. Preparation and characterization of spray dried complexes of lutein with cyclodextrins. J. Incl. Phenom. Macro. 2015, 83, 77–87. [Google Scholar] [CrossRef]
- Ranganathan, A.; Hindupur, R.; Vallikannan, B. Biocompatible lutein-polymer-lipid nanocapsules: Acute and subacute toxicity and bioavailability in mice. Mater. Sci. Eng. C 2016, 69, 1318–1327. [Google Scholar] [CrossRef]
- Tang, P.G.; Feng, Y.J.; Li, D.Q. Improved thermal and photostability of an anthraquinone dye by intercalation in a zinc–aluminum layered double hydroxides host. Dyes Pigment. 2011, 90, 253–258. [Google Scholar] [CrossRef]
- Chen, T.W.; Tang, P.G.; Feng, Y.G.; Li, D.Q. Facile color tuning, characterization, and application of Acid green 25 and Acid yellow 25 Co-intercalated layered double hydroxides. Ind. Eng. Chem. Res. 2017, 56, 5495–5504. [Google Scholar] [CrossRef]
- Bharali, D.; Devi, R.; Bharali, P.; Deka, R.C. Synthesis of high surface area mixed metal oxide from the NiMgAl LDH precursor for nitro-aldol condensation reaction. New J. Chem. 2015, 39, 172–178. [Google Scholar] [CrossRef]
- Jiang, D.B.; Jing, C.; Yuan, Y.S.; Feng, L.; Liu, X.Y.; Dong, F.; Dong, B.Q.; Zhang, Y.X. 2D-2D growth of NiFe LDH nanoflakes on montmorillonite for cationic and anionic dye adsorption performance. J. Colloid Interface Sci. 2019, 540, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Sobhana, S.S.L.; Mehedi, R.; Malmivirta, M.; Paturi, P.; Lastusaari, M.; Dîrtu, M.M.; Garcia, Y.; Fardim, P. Heteronuclear nanoparticles supported hydrotalcites containing Ni(II) and Fe(III) stable photocatalysts for Orange II degradation. Appl. Clay Sci. 2016, 132–133, 641–649. [Google Scholar]
- Bauer, J.; Behrens, P.; Speckbacher, M.; Langhals, H. Composites of perylenechromophores and layered double hydroxides: Direct synthesis, characterization, and photo-and chemical stability. Adv. Funct. Mater. 2003, 13, 241–248. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Muller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F. Process for Extraction and Purification of Lutein, Zeaxanthin and Rare Carotenoids from Marigold Flowers and Plants. Available online: https://patents.google.com/patent/US6262284B1/en (accessed on 17 April 2019).
- American Society of Testing Materials.ASTM F1980: 2002, Standard Guide for Accelerated Aging of Sterile Medical Device Package. Available online: https://www.astm.org/Standards/F1980.htm (accessed on 8 May 2019).
Sample Availability: Samples of the compounds are not available from the authors. |

| Samples | SBET (m2/g) | Vtotal (cm3/g) | Average Pore Width (nm) | Zeta Potentials (mV) |
|---|---|---|---|---|
| LDH | 81.70 | 0.20 | 9.83 | 3.75 |
| Lutein/LDH | 62.70 | 0.17 | 10.75 | −4.54 |
| Color Parameters | Pure Lutein | Raw Samples | 0.1 M NaOH | Acetone | Ethanol | Ethylacetate Acetate |
|---|---|---|---|---|---|---|
| L* | 44.25 | 55.5 | 51.36 | 50.86 | 50.43 | 52.73 |
| a* | 37.48 | 16.42 | 17.76 | 15.09 | 15.41 | 14.76 |
| b* | 43.84 | 44.15 | 42.79 | 30.88 | 29.23 | 31.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Mu, B.; Dong, W.; Liang, O.; Shao, S.; Wang, A. Incorporation of Lutein on Layered Double Hydroxide for Improving the Environmental Stability. Molecules 2020, 25, 1231. https://doi.org/10.3390/molecules25051231
Li S, Mu B, Dong W, Liang O, Shao S, Wang A. Incorporation of Lutein on Layered Double Hydroxide for Improving the Environmental Stability. Molecules. 2020; 25(5):1231. https://doi.org/10.3390/molecules25051231
Chicago/Turabian StyleLi, Shue, Bin Mu, Wenkai Dong, Oing Liang, Shijun Shao, and Aiqin Wang. 2020. "Incorporation of Lutein on Layered Double Hydroxide for Improving the Environmental Stability" Molecules 25, no. 5: 1231. https://doi.org/10.3390/molecules25051231
APA StyleLi, S., Mu, B., Dong, W., Liang, O., Shao, S., & Wang, A. (2020). Incorporation of Lutein on Layered Double Hydroxide for Improving the Environmental Stability. Molecules, 25(5), 1231. https://doi.org/10.3390/molecules25051231