Enhancement of Macarpine Production in Eschscholzia Californica Suspension Cultures under Salicylic Acid Elicitation and Precursor Supplementation
Abstract
1. Introduction
2. Results
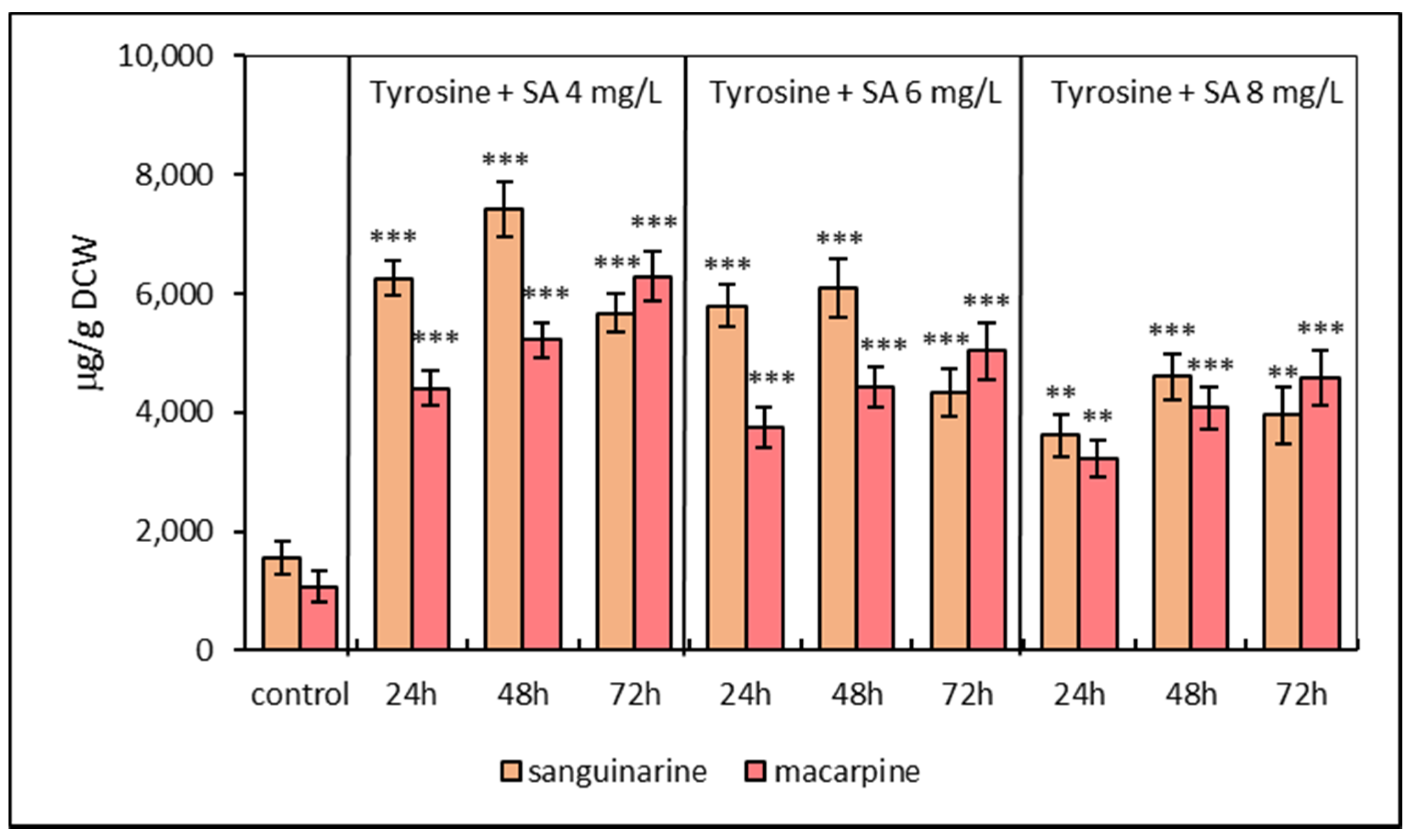
2.1. Alkaloid Production Pattern in Suspension Cultures under Elicitation
2.2. Gene Expression Assessment
3. Discussion
4. Materials and Methods
4.1. Preparation of Plant Material
4.2. Elicitor Preparation and Elicitation Models
4.3. Isolation and Purification of Macarpine
4.4. Isolation and Quantification of Alkaloids
4.5. Isolation of Total RNA and Quantitative RT-PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slaninová, I.; López-Sánchez, N.; Šebrlová, K.; Vymazal, O.; Frade, J.M.; Táborská, E. Introduction of macarpine as a novel cell-permeant DNA dye for live cell imaging and flow cytometry sorting. Biol. Cell 2016, 108, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Eschscholzia californica: A phytochemical and pharmacological—Review. Indo Am. J. Pharm. Sci. 2017, 4, 257–263. [Google Scholar] [CrossRef]
- Arakawa, H.; Clark, W.G.; Psenak, M.; Coscia, C.J. Purification and characterization of dihydrobenzophenanthridine oxidase from elicited Sanguinaria canadensis cell cultures. Arch. Biochem. Biophys. 1992, 299, 1–7. [Google Scholar] [CrossRef]
- Weiss, D.; Baumert, A.; Vogel, M.; Roos, W. Sanguinarine reductase, a key enzyme of benzophenanthridine detoxification. Plant Cell Environ. 2006, 29, 291–302. [Google Scholar] [CrossRef]
- De-Eknamkul, W.; Tanahashi, T.; Zenk, M.H. Enzymic 10-hydroxylation and 10-O-methylation of dihydrosanguinarine in dihydrochelirubine formation by Eschscholtzia. Phytochemistry 1992, 31, 2713–2717. [Google Scholar] [CrossRef]
- Kammerer, L.; de-Eknamkul, W.; Zenk, M.H. Enzymic 12-hydroxylation and 12-O-methylation of dihydrochelirubine in dihydromacarpine formation by Thalictrum bulgaricum. Phytochemistry 1994. [Google Scholar] [CrossRef]
- Purwanto, R.; Hori, K.; Yamada, Y.; Sato, F. Unraveling Additional O-Methylation Steps in Benzylisoquinoline Alkaloid Biosynthesis in California Poppy (Eschscholzia californica). Plant Cell Physiol. 2017, 58, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Yamada, Y.; Purwanto, R.; Minakuchi, Y.; Toyoda, A.; Hirakawa, H.; Sato, F. Mining of the Uncharacterized Cytochrome P450 Genes Involved in Alkaloid Biosynthesis in California Poppy Using a Draft Genome Sequence. Plant Cell Physiol. 2018, 59, 222–233. [Google Scholar] [CrossRef]
- Orhan, I.; Özçelik, B.; Karaoǧlu, T.; Şener, B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Z. Naturforsch. 2007, 62c, 19–26. [Google Scholar] [CrossRef]
- Slunská, Z.; Gelnarová, E.; Hammerová, J.; Táborská, E.; Slaninová, I. Effect of quaternary benzo[c]phenanthridine alkaloids sanguilutine and chelilutine on normal and cancer cells. Toxicol. Vitr. 2010, 24, 697–706. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, P.; Bao, H.; Liang, P.; Wang, W.; Xing, A.; Sun, J. Anti-inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp. Ther. Med. 2017, 13, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Slaninová, I.; Slunská, Z.; Šinkora, J.; Vlková, M.; Táborská, E. Screening of minor benzo(c)phenanthridine alkaloids for antiproliferative and apoptotic activities. Pharm. Biol. 2007, 45, 131–139. [Google Scholar] [CrossRef]
- Pica, F.; Balestrieri, E.; Serafino, A.; Sorrentino, R.; Gaziano, R.; Moroni, G.; Moroni, N.; Palmieri, G.; Mattei, M.; Garaci, E.; et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine in a rat syngeneic model of colorectal cancer. Anticancer. Drugs 2012, 23, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, R.; Moroni, G.; Buè, C.; Miele, M.T.; Sinibaldi-Vallebona, P.; Pica, F. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: Evidence and perspectives. World J. Gastrointest. Oncol. 2016, 8, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Saito, T.; Ishii, H. Synthesis of macarpine and its cytotoxicity: Toward a synthetic route for 12-alkoxybenzo[c]phenanthridine alkaloids through aromatic nitrosation under basic condition. Tetrahedron 1995, 51, 8447–8458. [Google Scholar] [CrossRef]
- Singh, S.; Samineni, R.; Pabbaraja, S.; Mehta, G. Nitromethane as a Carbanion Source for Domino Benzoannulation with Ynones: One-Pot Synthesis of Polyfunctional Naphthalenes and a Total Synthesis of Macarpine. Angew. Chemie Int. Ed. 2018, 57, 16847–16851. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, J.; Liu, Y.-Q. Inhibitory activity of dihydrosanguinarine and dihydrochelerythrine against phytopathogenic fungi. Nat. Prod. Res. 2011, 25, 1082–1089. [Google Scholar] [CrossRef]
- Yang, X.; Miao, F.; Yao, Y.; Cao, F.J.; Yang, R.; Ma, Y.N.; Qin, B.F.; Zhou, L. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules 2012, 17, 13026–13035. [Google Scholar] [CrossRef]
- Holková, I.; Bezáková, L.; Bilka, F.; Balažová, A.; Vanko, M.; Blanáriková, V. Involvement of lipoxygenase in elicitor-stimulated sanguinarine accumulation in Papaver somniferum suspension cultures. Plant Physiol. Biochem. 2010, 48, 887–892. [Google Scholar] [CrossRef]
- Bilka, F.; Balažová, A.; Bilková, A.; Holková, I. Effect of abiotic elicitation on the sanguinarine production and polyphenol oxidase activity in the suspension culture of Eschscholtzia californica CHAM. Ceska Slov. Farm. 2013, 62, 169–173. [Google Scholar]
- Balažová, A.; Urdová, J.; Bilka, F.; Holková, I.; Horváth, B.; Forman, V.; Mučaji, P. Evaluation of Manganese Chloride’s Effect on Biosynthetic Properties of In Vitro Cultures of Eschscholzia californica Cham. Molecules 2018, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Angelova, S.; Buchheim, M.; Frowitter, D.; Schierhorn, A.; Roos, W. Overproduction of alkaloid phytoalexins in california poppy cells is associated with the co-expression of biosynthetic and stress-protective enzymes. Mol. Plant 2010, 3, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, N.; Iwasa, K.; Sato, F. Molecular cloning and characterization of methylenedioxy bridge-forming enzymes involved in stylopine biosynthesis in Eschscholzia californica. FEBS J. 2007, 274, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Šebrlová, K.; Slaninová, I.; Táborská, E. Benzophenanthridine alkaloid macarpine—Biological effects and plant sources. Planta Med. 2014. [Google Scholar] [CrossRef]
- Kollárová, R.; Obložinsḱy, M.; Kováčiková, V.; Holková, I.; Balažová, A.; Pekárová, M.; Hoffman, P.; Bezáková, L. Lipoxygenase activity and sanguinarine production in cell suspension cultures of California poppy (Eschscholtzia californica CHAM.). Pharmazie 2014, 69, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Balažová, A.; Bilka, F.; Blanáriková, V.; Pšenák, M. Changes in sanguinarine content and polyphenoloxidase activity due to a fungal elicitor in suspension cultures of the opium plant Papaver somniferum L. Ceska Slov. Farm. 2002, 51, 182–185. [Google Scholar]
- Cho, H.Y.; Son, S.Y.; Rhee, H.S.; Yoon, S.Y.H.; Lee-Parsons, C.W.T.; Park, J.M. Synergistic effects of sequential treatment with methyl jasmonate, salicylic acid and yeast extract on benzophenanthridine alkaloid accumulation and protein expression in Eschscholtzia californica suspension cultures. J. Biotechnol. 2008, 135, 117–122. [Google Scholar] [CrossRef]
- Ju, Y.W.; Byun, S.Y. Precursor Feeding Experiments with Elicitation in Suspension Cultures of Eschscholtzia californica. Plant tissue Cult. Lett. 1994, 11, 112–115. [Google Scholar] [CrossRef]
- Verma, P.; Khan, S.A.; Mathur, A.K.; Ghosh, S.; Shanker, K.; Kalra, A. Improved sanguinarine production via biotic and abiotic elicitations and precursor feeding in cell suspensions of latex-less variety of Papaver somniferum with their gene expression studies and upscaling in bioreactor. Protoplasma 2014, 251, 1359–1371. [Google Scholar] [CrossRef]
- Hu, G.S.; Jia, J.M.; Kim, D.H. Effects of feeding tyrosine and phenylalanine on the accumulation of phenylethanoid glycosides to Cistanche deserticola cell suspension culture. Chin. J. Nat. Med. 2014, 12, 367–372. [Google Scholar] [CrossRef]
- Vogel, M.; Lawson, M.; Sippl, W.; Conrad, U.; Roos, W. Structure and Mechanism of Sanguinarine Reductase, an Enzyme of Alkaloid Detoxification. J. Biol. Chem. 2010, 285, 18397–18406. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Rhee, H.S.; Yoon, S.Y.H.; Park, J.M. Differential induction of protein expression and benzophenanthridine alkaloid accumulation in Eschscholtzia californica suspension cultures by methyl jasmonate and yeast extract. J. Microbiol. Biotechnol. 2008, 18, 255–262. [Google Scholar] [PubMed]
- Facchini, P.J.; Park, S.-U. Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy. Phytochemistry 2003, 64, 177–186. [Google Scholar] [CrossRef]
- Hanaoka, M.; Cho, W.J.; Yoshida, S.; Fueki, T.; Mukai, C. Chemical Transformation of Protoberberines. XVI. Regioselective Introduction of an Oxy Functionality at the C12 Position of the Benzo[c]phenanthridine Skeleton: A Convenient Synthesis of Macarpine from Oxychelirubine. Chem. Pharm. Bull. 1990, 38, 3335–3340. [Google Scholar] [CrossRef][Green Version]
- Winer, J.; Jung, C.K.S.; Shackel, I.; Williams, P.M. Development and Validation of Real-Time Quantitative Reverse Transcriptase–Polymerase Chain Reaction for Monitoring Gene Expression in Cardiac Myocytesin Vitro. Anal. Biochem. 1999, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of macarpine is available from the authors. |





| Primer Name | Oligonucleotide Sequences (5′- to 3′-) |
|---|---|
| 4′-OMT | forward CCTAGAAGAGGAATCAGAACATCCA reverse TCACTTCTCTCCCTTCCACCA |
| CYP719A | forward GTCGTAATTAATCACTTAACCGTGCTCG reverse GAAAGAAACAGAGCAAATCTTATCCTTTTACC |
| CYP719A3 | forward CCTCGTAACTAATATACCAGTGTGGTG reverse GACAACCAAGCAAACTCTTATTCTTGTAC |
| β-actin | forward GGTATTGTGCTGGATTCTGGTG reverse GTAGGATTGCGTGGGGTAGTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balažová, A.; Urdová, J.; Forman, V.; Mučaji, P. Enhancement of Macarpine Production in Eschscholzia Californica Suspension Cultures under Salicylic Acid Elicitation and Precursor Supplementation. Molecules 2020, 25, 1261. https://doi.org/10.3390/molecules25061261
Balažová A, Urdová J, Forman V, Mučaji P. Enhancement of Macarpine Production in Eschscholzia Californica Suspension Cultures under Salicylic Acid Elicitation and Precursor Supplementation. Molecules. 2020; 25(6):1261. https://doi.org/10.3390/molecules25061261
Chicago/Turabian StyleBalažová, Andrea, Júlia Urdová, Vladimír Forman, and Pavel Mučaji. 2020. "Enhancement of Macarpine Production in Eschscholzia Californica Suspension Cultures under Salicylic Acid Elicitation and Precursor Supplementation" Molecules 25, no. 6: 1261. https://doi.org/10.3390/molecules25061261
APA StyleBalažová, A., Urdová, J., Forman, V., & Mučaji, P. (2020). Enhancement of Macarpine Production in Eschscholzia Californica Suspension Cultures under Salicylic Acid Elicitation and Precursor Supplementation. Molecules, 25(6), 1261. https://doi.org/10.3390/molecules25061261






