Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. NPs Synthesis
2.2. ZnO-NPs’ Surface Modification
2.3. ZnO-NPs Characterization
2.4. Plant Material and Management
2.5. Growth and Plant Organs Biomass Measurement
2.6. Statistical Data Analyses
3. Results and Discussion
3.1. ZnO-NPs Characterization
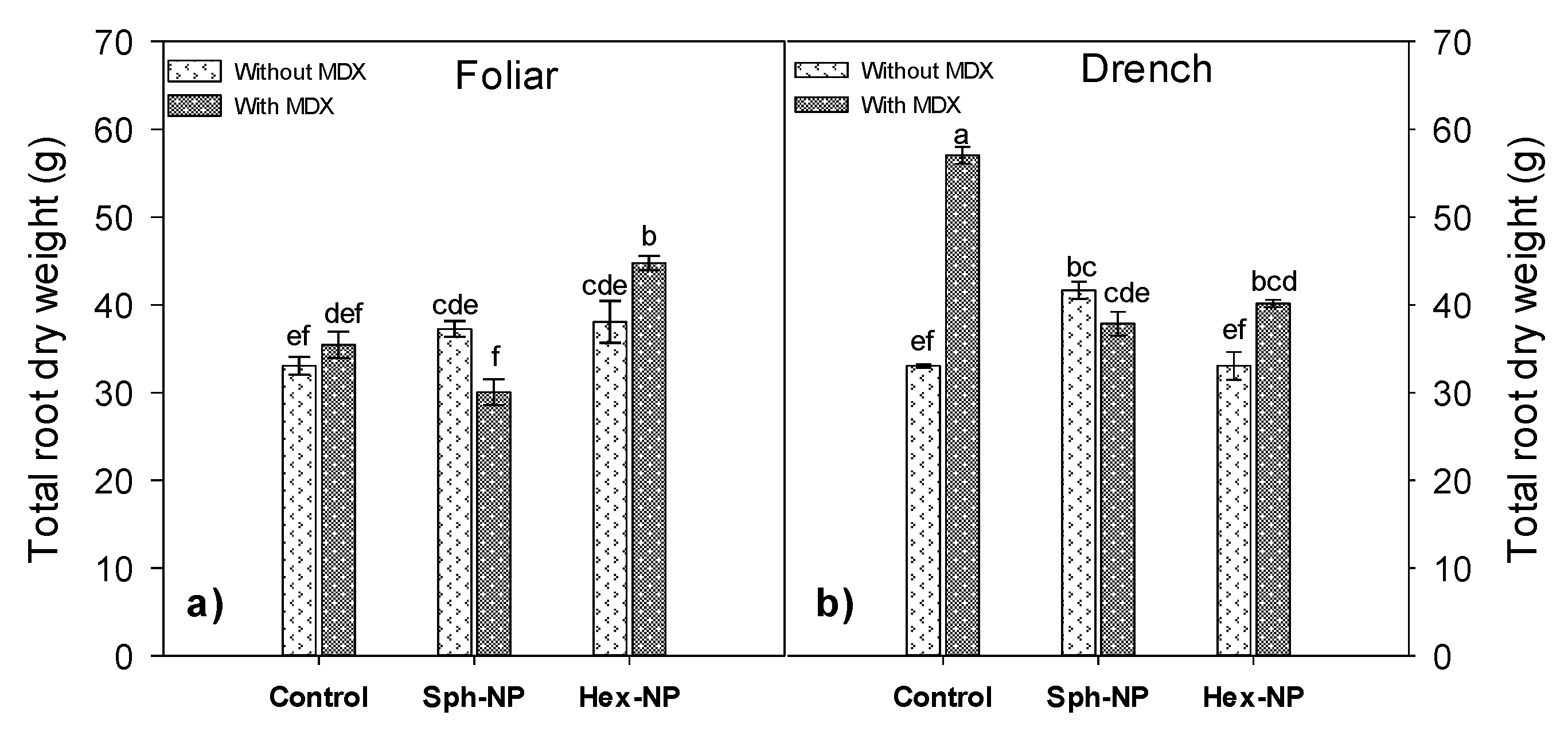
3.2. ZnO-NPs Effects on Plant Growth and Biomass
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano. 2016, 10, 3042–3068. [Google Scholar] [CrossRef]
- Chaudhri, N.; Soni, G.C.; Prajapati, S.K. Nanotechnology: An advance tool for nano-cosmetics preparation. Int. J. Pharma Res. Rev. 2015, 4, 28–40. [Google Scholar]
- Alam, F.; Naim, M.; Aziz, M.; Yadav, N. Unique roles of nanotechnology in medicine and cancer-II. Indian J. Cancer. 2015, 52, 1. [Google Scholar] [CrossRef]
- Korkin, A. Nanotechnology for Electronics, Photonics, and Renewable Energy; Krstić, P.S., Wells, J.C., Eds.; Springer: New York, NY, USA, 2010; Volume 78. [Google Scholar]
- Ozimek, L.; Pospiech, E.; Narine, S. Nanotechnologies in food and meat processing. ACTA Sci. Polonorum Technol. Aliment. 2010, 9, 401–412. [Google Scholar]
- Wu, J.; Yin, K.; Li, M.; Wu, Z.; Xiao, S.; Wang, H.; Duan, J.A.; He, J. Under-oil self-driven and directional transport of water on a femtosecond laser-processed superhydrophilic geometry-gradient structure. Nanoscale 2020, 12, 4077–4084. [Google Scholar] [CrossRef]
- Hullmann, A. The Economic Development of Nanotechnology—An Indicators Based Analysis. EU Report. 28 November 2006. Available online: http://nanotechnology.cz/storage/nanoarticle_.pdf (accessed on 29 October 2019).
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016. [Google Scholar] [CrossRef]
- Singh, M.D.; Chirag, G.; Prakash, P.O.; Mohan, M.H.; Prakasha, G. Nano-Fertilizers is a new way to increase nutrients use efficiency in crop production. Int. J. Agric. Sci. 2017, 9, 3831–3833. [Google Scholar]
- Grillo, R.; Abhilash, P.C.; Fraceto, L.F. Nanotechnology applied to bio-encapsulation of pesticides. J. Nanosci. Nanotechnol. 2016, 16, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A Review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Pandey, A.C.S.; Sanjay, S.S.; Yadav, R. Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 2010, 5, 488–497. [Google Scholar] [CrossRef]
- Rehman, H.U.; Aziz, T.; Farooq, M.; Wakeel, A. Zinc nutrition in rice production systems: A review. Plant Soil 2012, 361, 203–226. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Raliya, R.; Mahawar, H.; Rathore, I. Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agric. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- De la Rosa, G.; López-Moreno, M.L.; de Haro, D.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: Root development and X-ray absorption spectroscopy studies. Pure Appl. Chem. 2013, 85, 2161–2174. [Google Scholar] [CrossRef]
- Laware, S.L.; Raskar, S. Influence of zinc oxide nanoparticles on growth, flowering and seed productivity in onion. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 874–881. [Google Scholar]
- Tirani, M.M.; Haghjou, M.M.; Ismaili, A. Hydroponic grown tobacco plants respond to zinc oxide nanoparticles and bulk exposures by morphological, physiological and anatomical adjustments. Funct. Plant Biol. 2019, 46, 360–375. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica l.) under field conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Chen, G.; Zhao, M.; Watson, S.S.; Nguyen, T.; Chin, J.W.; Martin, J.W. Critical role of particle/polymer interface in photostability of nano-filled polymeric coatings. J. Coat. Technol. Res. 2012, 9, 251–267. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316. [Google Scholar] [CrossRef] [Green Version]
- Pompe, W.; Rödel, G.; Weiss, H.J.; Mertig, M. Bio-Nanomaterials: Designing Materials Inspired by Nature; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Hong, R.R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar] [CrossRef]
- Grasset, F.; Saito, N.; Li, D.; Park, D.; Sakaguchi, I.; Ohashi, N.; Haneda, H.; Roisnel, T.; Mornet, S.; Duguet, E. Surface modification of zinc oxide nanoparticles by aminopropyltriethoxysilane. J. Alloys Compd. 2003, 360, 298–311. [Google Scholar] [CrossRef]
- Hong, R.Y.; Li, J.H.; Chen, L.L.; Liu, D.Q.; Li, H.Z.; Zheng, Y.; Ding, J. Synthesis, surface modification and photocatalytic property of ZnO nanoparticles. Powder Technol. 2008, 189, 426–432. [Google Scholar] [CrossRef]
- Siddiquey, I.A.; Furusawa, T.; Sato, M.; Suzuki, N. Microwave-assisted silica coating and photocatalytic activities of ZnO nanoparticles. Mater. Res. Bull. 2008, 43, 3416–3424. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Li, D.; Ma, X.; Li, S.; Que, D. Controllable growth of ZnO microcrystals by a capping-molecule-assisted hydrothermal process. Cryst. Growth Design 2005, 5, 547–550. [Google Scholar] [CrossRef]
- Lumdubwong, N.; Seib, P.A. Low-and Medium-DE Maltodextrins From Waxy Wheat Starch: Preparation and Properties. Starch Stärke 2001, 53, 605–615. [Google Scholar] [CrossRef]
- Fountain, M.; Medd, N.C. Integrating pesticides and predatory mites in soft fruit crops. Phytoparasitica 2015, 43, 657–667. [Google Scholar] [CrossRef]
- Garanin, D.A.; Kachkachi, H. Surface contribution to the anisotropy of magnetic nanoparticles. Phys. Rev. Lett. 2003, 90, 065504. [Google Scholar] [CrossRef] [Green Version]
- Shyichuk, A.V.; Turton, T.J.; White, J.R.; Syrotynska, I.D. Different degradability of two similar polypropylenes as revealed by macromolecule scission and crosslinking rates. Polym. Degrad. Stab. 2004, 86, 377–383. [Google Scholar] [CrossRef]
- Fernández, R.V.; Sánchez, M.M.; Cámara, M.; Torija, M.E.; Chaya, C.; Galiana, B.L.; Roselló, S.; Nuez, F. Internal quality characterization of fresh tomato fruits. HortScience 2004, 39, 339–345. [Google Scholar] [CrossRef]
- Hsieh, C.H. Spherical zinc oxide nano particles from zinc acetate in the precipitation method. J. Chin. Chem. Soc. 2007, 54, 31–34. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Dutta, K.; Pramanik, A. Morphology control of ZnO with citrate: A time and concentration dependent mechanistic insight. Cryst. Eng. Comm. 2013, 15, 6349–6358. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Zinc oxide nanostructures: Synthesis and properties. J. Nanosci. Nanotechnol. 2005, 5, 1561–1573. [Google Scholar] [CrossRef] [Green Version]
- Baldoquin Hernandez, M.; Alonso Garcia, M.; Gomez Masjuan, Y.; Bertot Arosa, I.J. Agronomical response of lettuce (Lactuca sativa L.), variety” Black Seed Simpson”, to Enerplant biostimulant aplication. Centro Agricola 2015, 42, 55–59. [Google Scholar]
- Syu, Y.Y.; Hung, J.H.; Chen, J.C.; Chuang, H.W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.; Venkateswarlu, P.; Rao, V.R.; Rao, G.N. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 2013, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Faraz, A.; Hayat, S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant Biochem. Biotechnol. 2019, 1–5. [Google Scholar] [CrossRef]
- Wang, R.; Billone, P.S.; Mullett, W.M. Nanomedicine in action: An overview of cancer nanomedicine on the market and in clinical trials. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds ZnO-NP are not available. |










| Stem Diameter (mm) | Plant Height (cm) | Leaf Dry Weight (g) | Root Dry Weight (g) | Stem Dry Weight (g) | |
|---|---|---|---|---|---|
| Morphology | |||||
| Control | 21.8 b | 239 b | 223.9 c | 39.7 a | 84.2 a |
| Spherical | 21.9 b | 230 b | 253.2 b | 36.7 b | 85.4 a |
| Hexagonal | 24.5 a | 251 a | 278.5 a | 39.0 a | 90.5 a |
| ANOVA | p ≤ <.0001 | p ≤ <.0001 | p ≤ <.0001 | p ≤ 0.0045 | p ≤ 0.1396 |
| MDX | |||||
| Non-Modified | 22.4 a | 234 b | 245.3 b | 36.2 b | 82.8 b |
| Modified | 23.1 a | 246 a | 258.4 a | 40.9 a | 90.5 a |
| ANOVA | p ≤ 0.0898 | p ≤ 0.0006 | p ≤ <.0001 | p ≤ <.0001 | p ≤ 0.006 |
| Application method | |||||
| Foliar | 23.0 a | 239 a | 252.3 a | 36.4 b | 82.9 b |
| Drench | 22.5 a | 241.1 a | 251.4 a | 40.5 a | 90.4 a |
| ANOVA | p ≤ 0.2243 | p ≤ 0.5336 | p ≤ 0.0035 | p ≤ <.0001 | p ≤ 0.0073 |
| Interactions | |||||
| M*MDX | p ≤ 0.0003 | p ≤ <.0001 | p ≤ <.0001 | p ≤ <.0001 | p ≤ <.0001 |
| M*A | p ≤ 0.0003 | p ≤ 0.015 | p ≤ 0.0006 | p ≤ <.0001 | p ≤ 0.0024 |
| MDX*A | p ≤ 0.5866 | p ≤ 0.3323 | p ≤ 0.7354 | p ≤ <.0001 | p ≤ 0.0301 |
| M*MD*A | p ≤ 0.2135 | p ≤ 0.0005 | p ≤ 0.6123 | p ≤ <.0001 | p ≤ 0.6338 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Velasco, E.A.; Betancourt Galindo, R.; Valdez Aguilar, L.A.; González Fuentes, J.A.; Puente Urbina, B.A.; Lozano Morales, S.A.; Sánchez Valdés, S. Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants. Molecules 2020, 25, 1282. https://doi.org/10.3390/molecules25061282
Pérez Velasco EA, Betancourt Galindo R, Valdez Aguilar LA, González Fuentes JA, Puente Urbina BA, Lozano Morales SA, Sánchez Valdés S. Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants. Molecules. 2020; 25(6):1282. https://doi.org/10.3390/molecules25061282
Chicago/Turabian StylePérez Velasco, Eneida A., Rebeca Betancourt Galindo, Luis A. Valdez Aguilar, José A. González Fuentes, Bertha A. Puente Urbina, Samuel A. Lozano Morales, and Saúl Sánchez Valdés. 2020. "Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants" Molecules 25, no. 6: 1282. https://doi.org/10.3390/molecules25061282
APA StylePérez Velasco, E. A., Betancourt Galindo, R., Valdez Aguilar, L. A., González Fuentes, J. A., Puente Urbina, B. A., Lozano Morales, S. A., & Sánchez Valdés, S. (2020). Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants. Molecules, 25(6), 1282. https://doi.org/10.3390/molecules25061282







