Crosslinked Nanocomposite Sodium Alginate-Based Membranes with Titanium Dioxide for the Dehydration of Isopropanol by Pervaporation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Membrane Characterization
2.1.1. Fourier Transform Infra-red Spectroscopy Studies
2.1.2. WAXD Studies
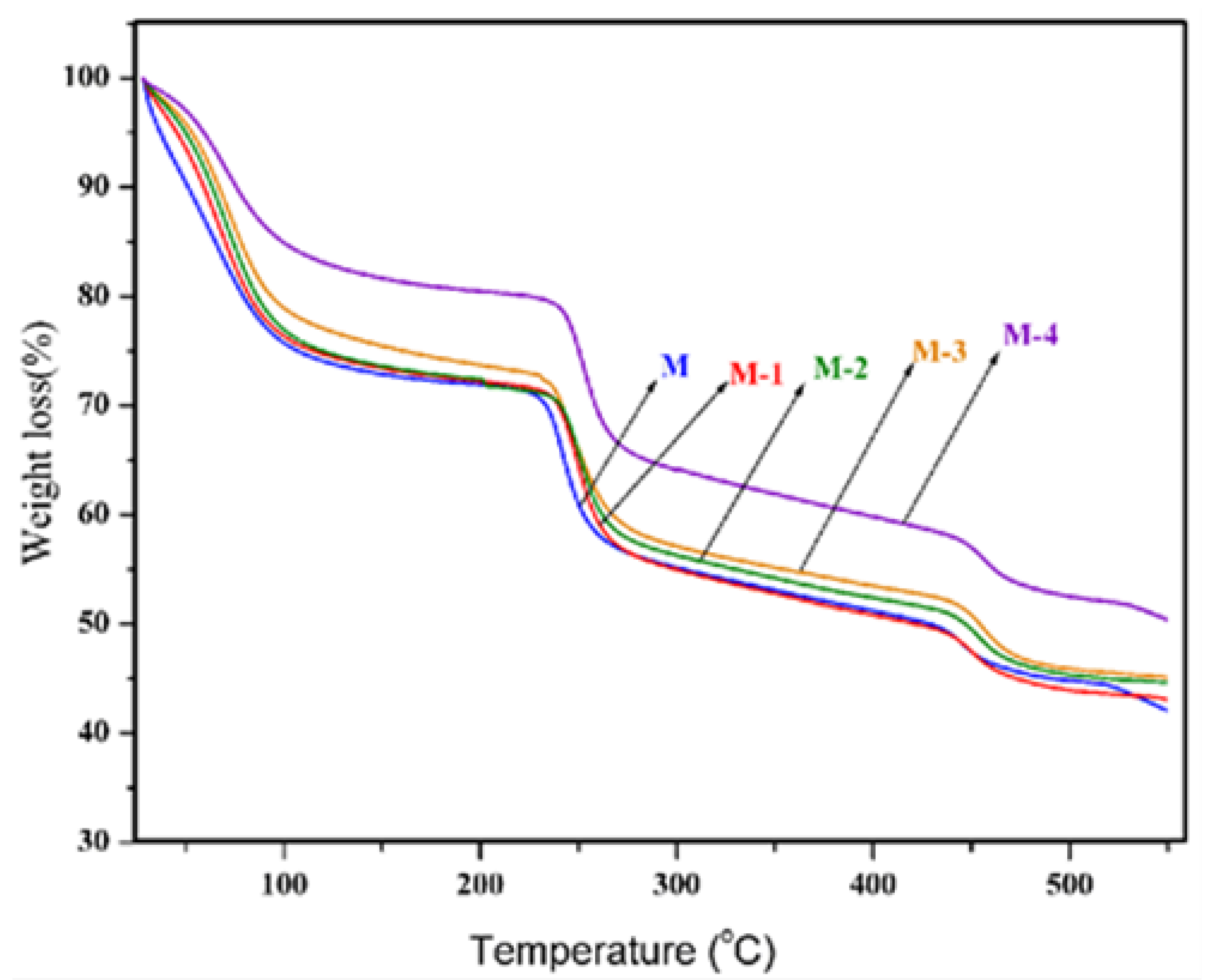
2.1.3. Thermal Studies
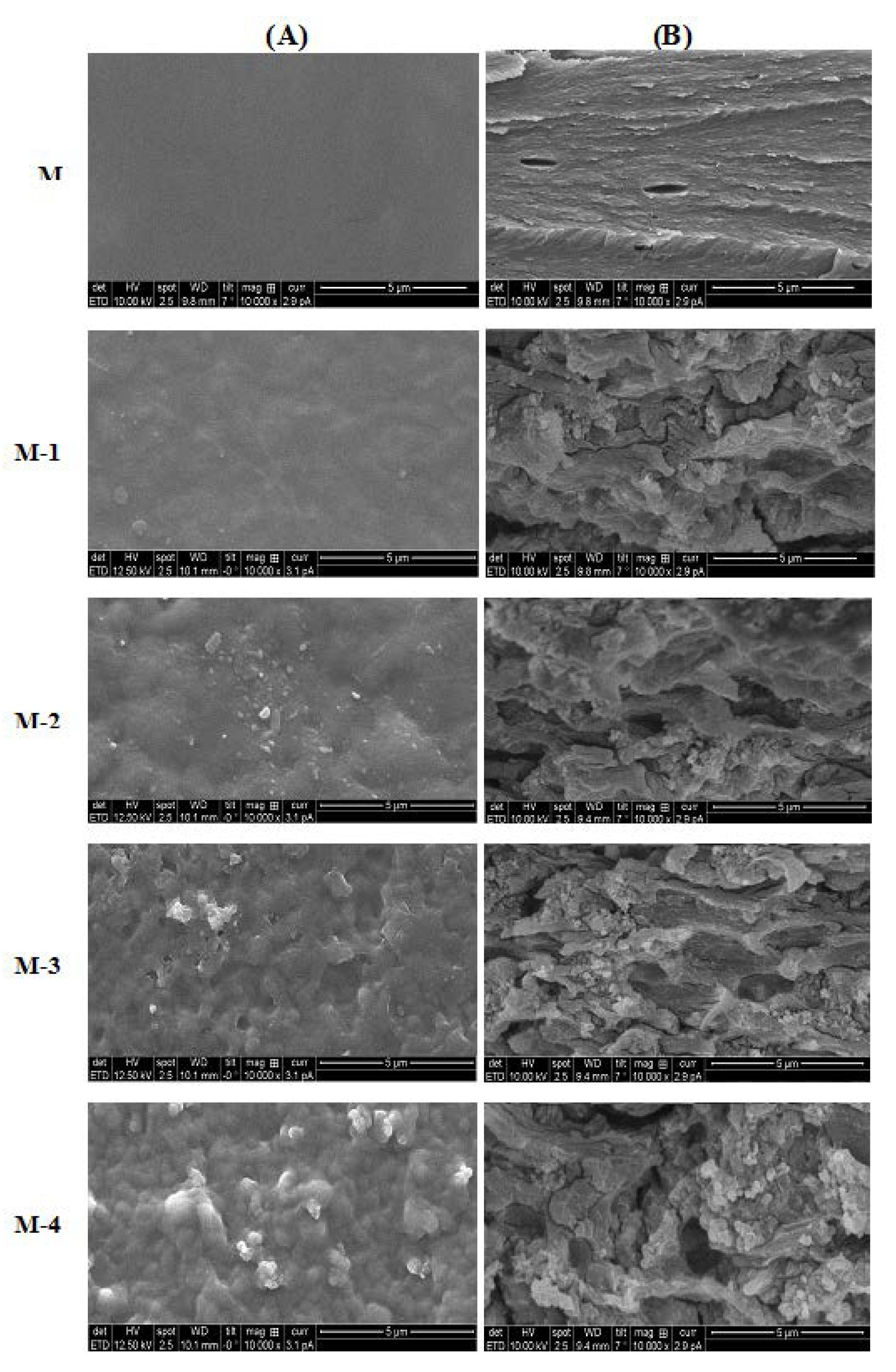
2.1.4. SEM Studies
2.2. Mechanical Properties
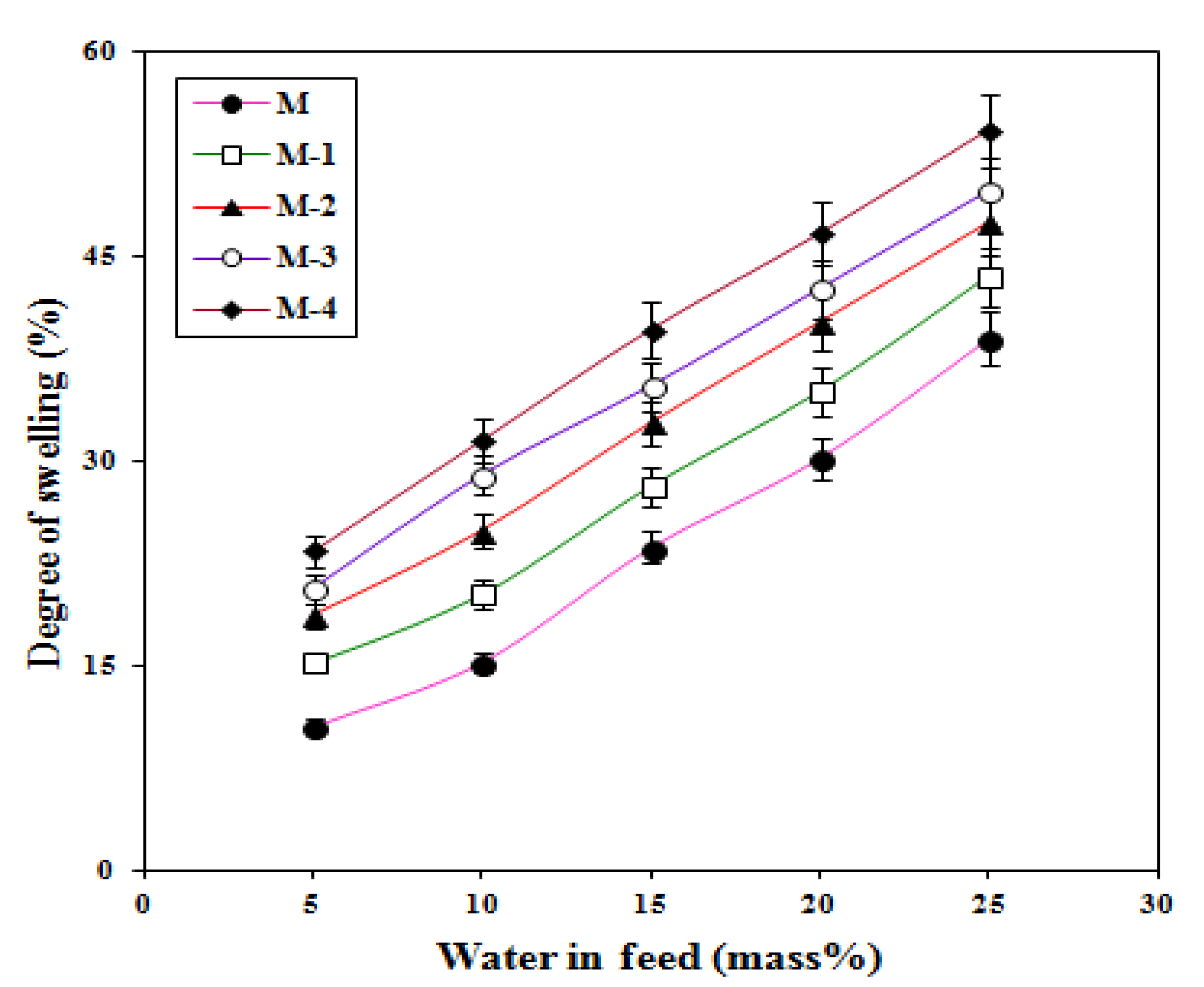
2.3. Effects of Feed Composition and Titanium Dioxide on Membrane Swelling
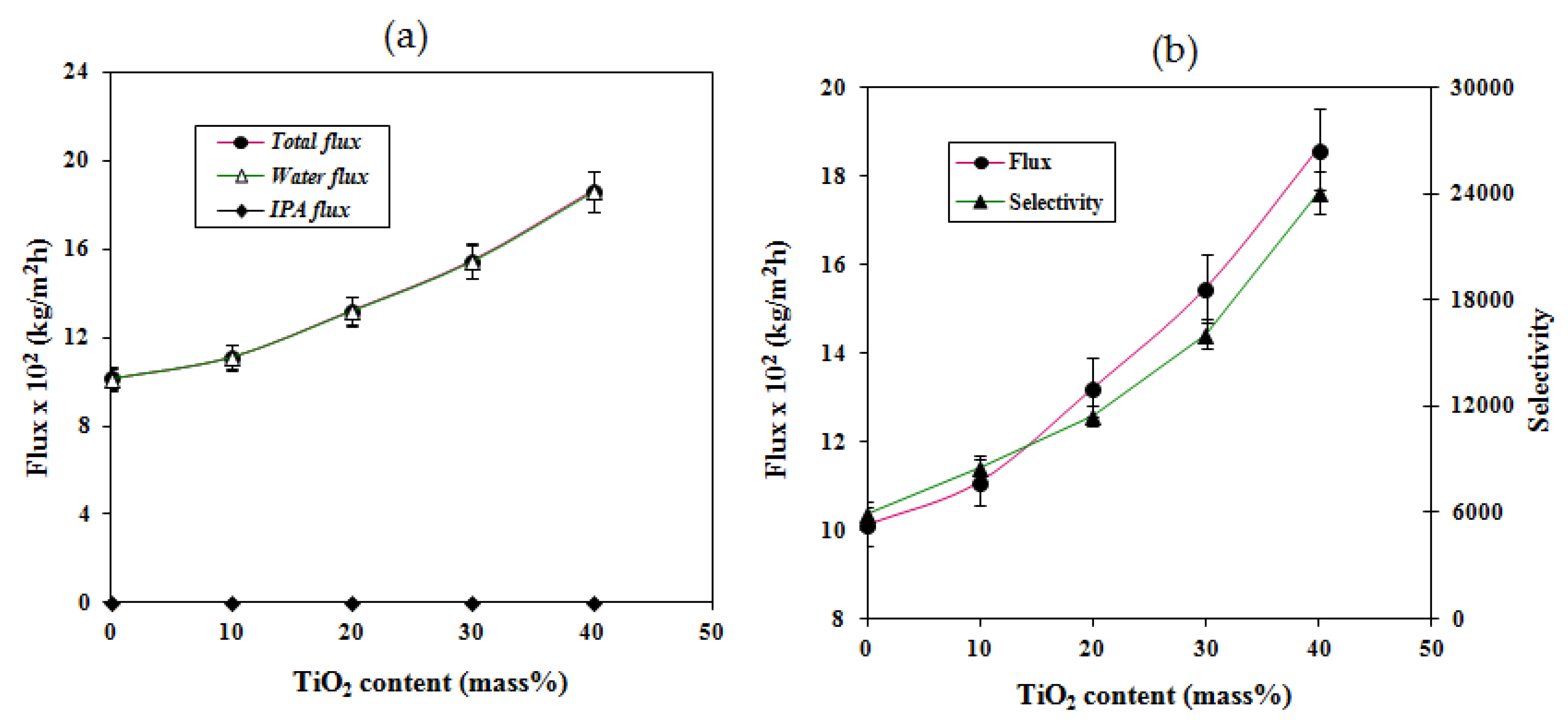
2.4. Effects of Feed Composition and Titanium Dioxide on Pervaporation
2.5. Comparison of Pervaporation Performance of Sodium Alginate-Based Membranes
2.6. Diffusion Coefficients
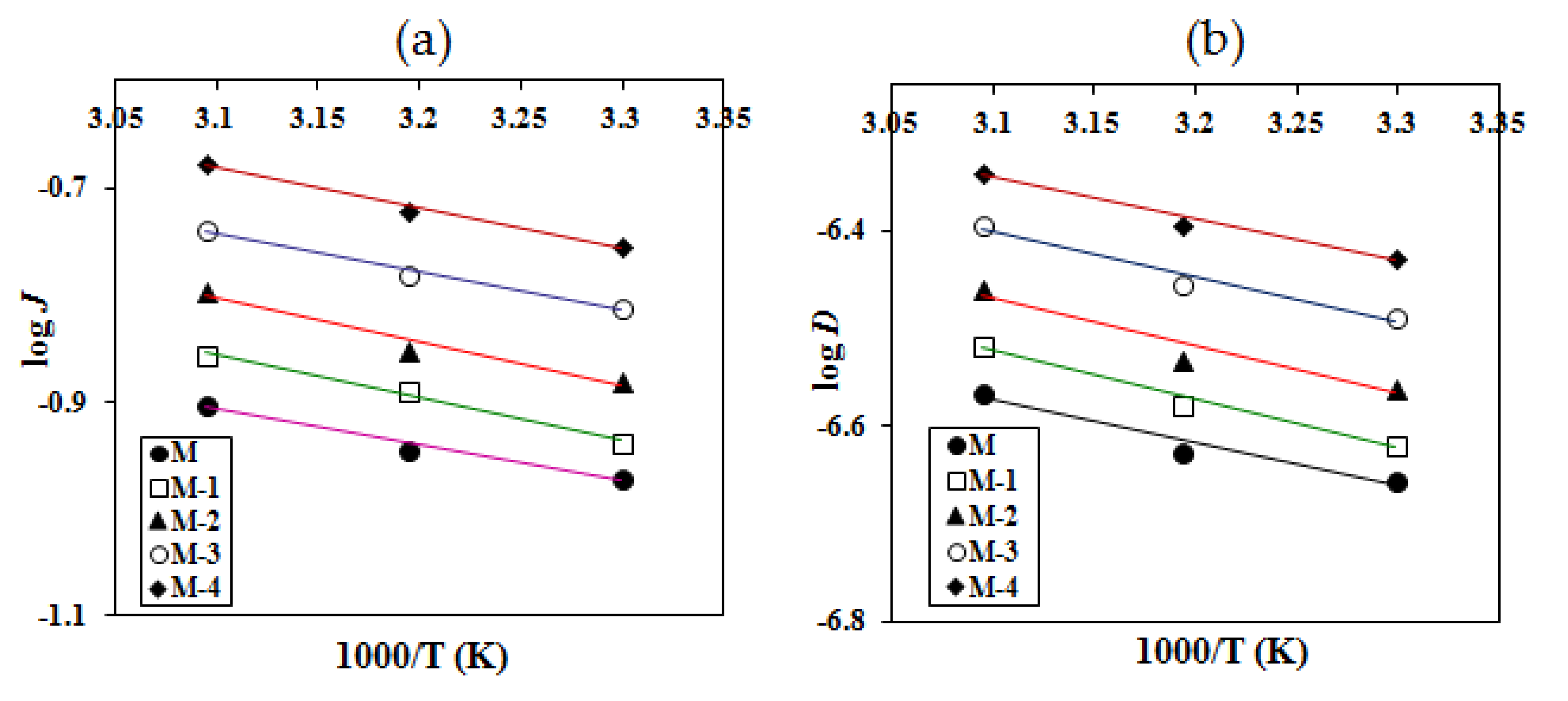
2.7. Effect of Temperature on the Pervaporation Performance
3. Materials and Methods
3.1. Materials
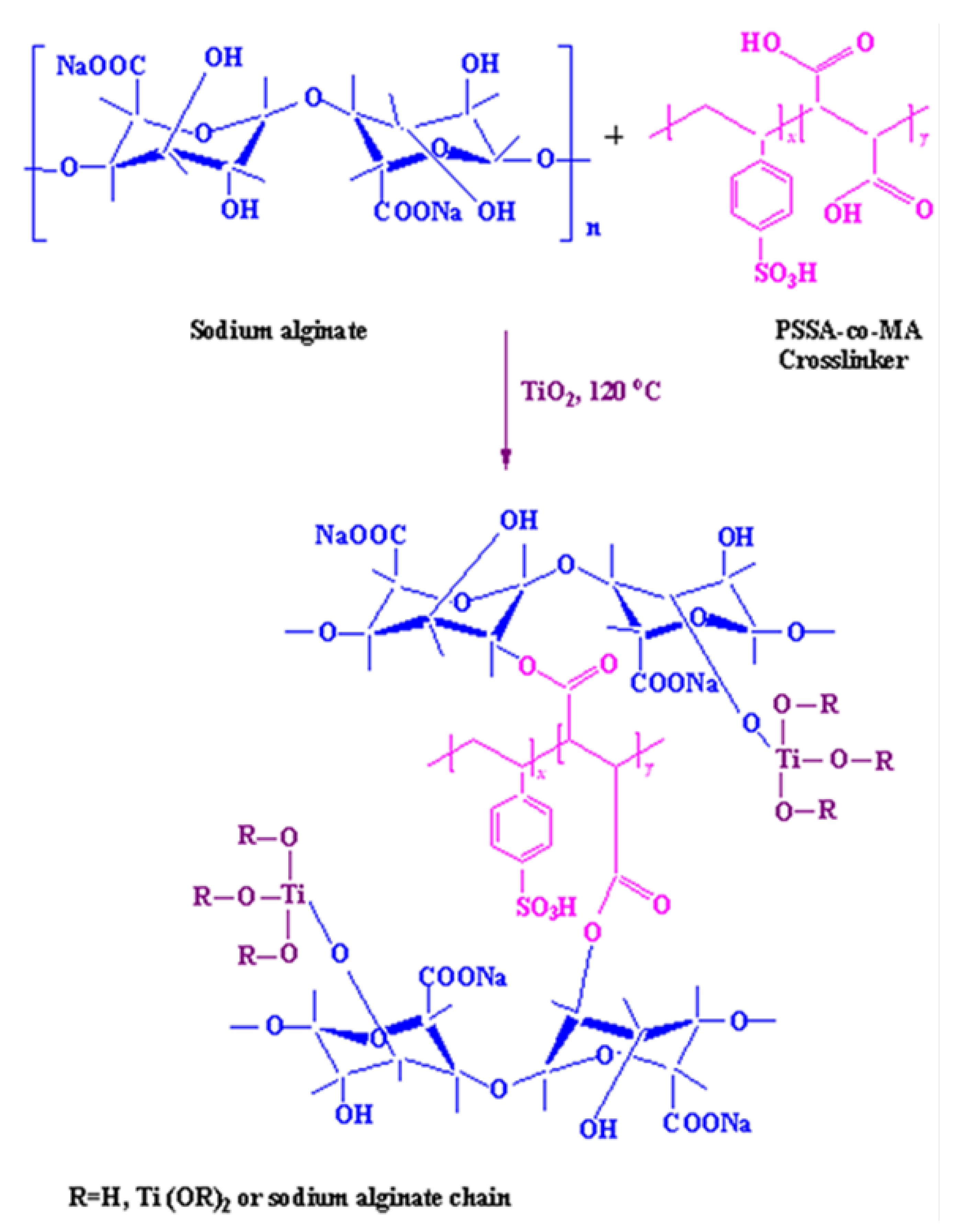
3.2. Membrane Preparation
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Wide-Angle X-Ray Diffraction (WAXD)
3.5. Thermal Analysis
3.6. Scanning Electron Microscopy (SEM)
3.7. Tensile Properties
3.8. Swelling Measurement
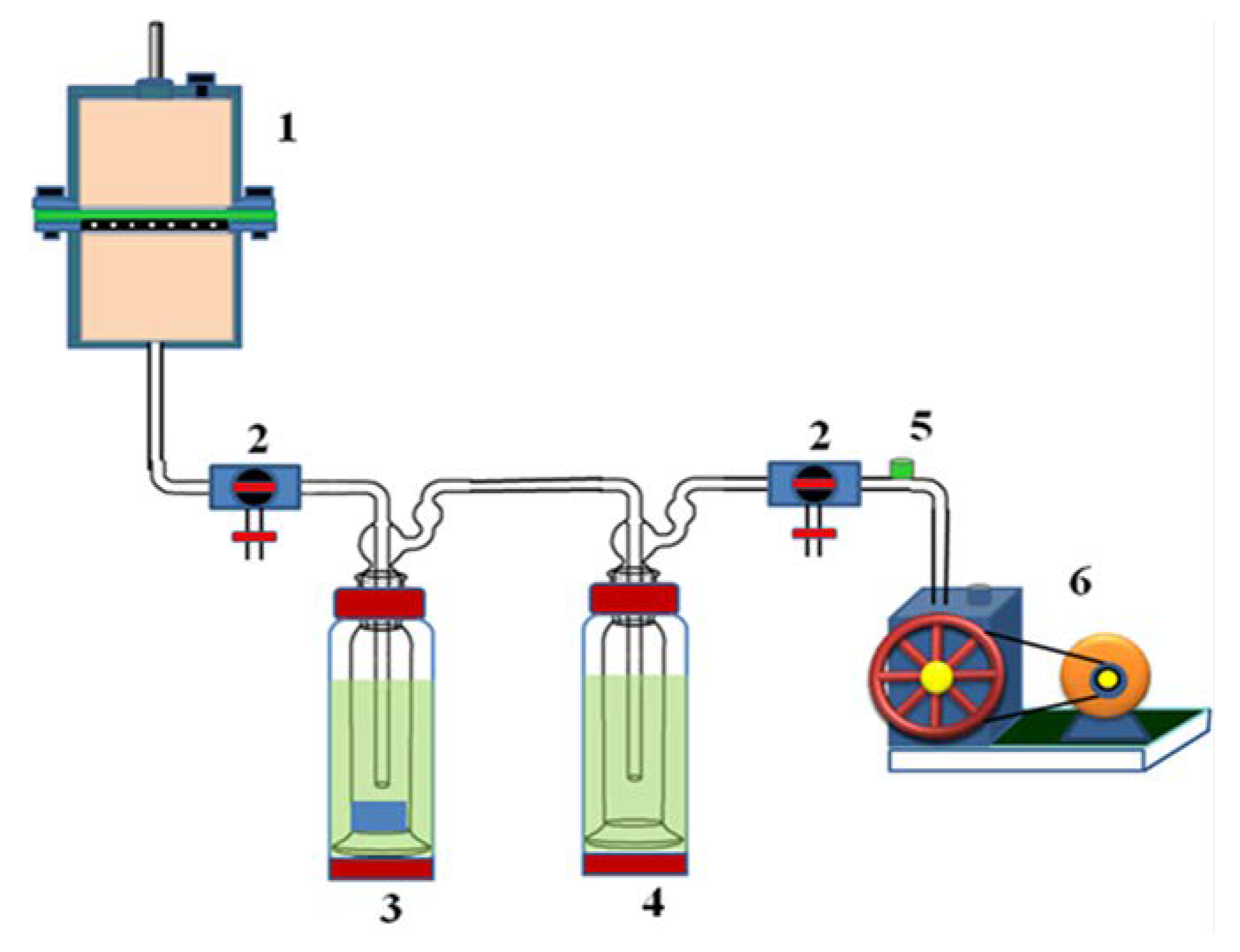
3.9. Pervaporation Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Carlo, B.V.; Habert, A.C. Plasma-treated polyethersulfone coated with crosslinked poly(vinyl alcohol): Composite membranes for pervaporation dehydration of ethanol. J. Mater. Sci. 2013, 48, 1457–1464. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Shi, F.F.; Xiong, Y. Structure and permeation of organic inorganic hybrid membranes composed of poly(vinyl alcohol) and polysilisesquioxane. J. Mater. Chem. 2008, 18, 4646–4653. [Google Scholar] [CrossRef] [Green Version]
- Shea, K.J.; Loy, D.A. Bridged polysilsesquioxanes molecular-engineered hybrid organic−inorganic materials. Chem. Mater. 2001, 13, 3306–3319. [Google Scholar] [CrossRef]
- Cot, L.; Ayral, A.; Durand, J.; Guizard, C.; Hovnanian, N.; Julbe, A.; Larbot, A. Inorganic membranes and solid state sciences. Solid State Sci. 2000, 2, 313–334. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- George, S.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Han, G.L.; Gong, Y.; Zhang, Q.G.; Zhu, A.M.; Ye, M.L.; Liu, Q.L. Facile preparation of homogeneous polyelectrolyte complex membranes for separation of methanol/methyl tert-butyl ether mixtures. J. Membr. Sci. 2013, 447, 246–252. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Chen, Y.; Chen, J.H. Dehydration of isopropanol by novel poly(vinyl alcohol)-silicone hybrid membranes. Ind. Eng. Chem. Res. 2007, 46, 913–920. [Google Scholar] [CrossRef]
- Kalla, S.; Upadhyaya, S.; Singh, K.; Dohare, R.K.; Agarwal, M. A case study on separation of IPA-water mixture by extractive distillation using aspen plus. Int. J. Adv. Techn. Eng. Explor. 2016, 3, 2394–5443. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Liang, L. Pervaporation of ethanol-water mixtures through polyvinyl alcohol-polyacrylamide interpenetrating polymer network membranes unsupported and supported on polyethersulfone ultrafiltration membranes: A comparison. J. Membr. Sci. 1996, 110, 99–107. [Google Scholar] [CrossRef]
- Kim, H.J.; Jo, W.H.; Kang, Y.S. Modified free-volume model for pervaporation of water/ethanol mixtures through membranes containing hydrophilic groups or ions. J. Appl. Polym. Sci. 1995, 57, 63–76. [Google Scholar] [CrossRef]
- Nam, S.Y.; Chun, H.J.; Lee, Y.M. Pervaporation separation of water-isopropanol mixture using carboxymethylated poly(vinyl alcohol) composite membranes. J. Appl. Polym. Sci. 1999, 72, 241–249. [Google Scholar] [CrossRef]
- Shah, D.; Kissick, K.; Ghorpade, A.; Hannah, R. Bhattacharyya, Pervaporation of alcohol-water and dimethylformamide-water mixtures using hydrophilic zeolite NaY membranes: Mechanisms and experimental results. J. Membr. Sci. 2000, 179, 185–205. [Google Scholar] [CrossRef]
- Premakshi, H.G.; Ramesh, K.; Kariduraganavar, M.Y. Modification of crosslinked chitosan membrane using NaY zeolite for pervaporation separation of water–isopropanol mixtures. Chem. Eng. Res. Des. 2015, 94, 32–43. [Google Scholar] [CrossRef]
- Premakshi, H.G.; Sajjan, A.M.; Kariduraganavar, M.Y. Development of pervaporation membranes using chitosan and titanium glycine-N,Ndimethylphosphonate for dehydration of isopropanol. J. Mater. Chem. A 2015, 3, 3952–3961. [Google Scholar] [CrossRef]
- Kittur, A.A.; Tambe, S.M.; Kulkarni, S.S.; Kariduraganavar, M.Y. Pervaporation separation of water–acetic acid mixtures through NaY Zeolite-incorporated sodium alginate membranes . J. Appl. Polym. Sci. 2004, 94, 2101–2109. [Google Scholar] [CrossRef]
- Swayampakula, K.; Biduru, S.; Sundergopal, S.; Abburi, K. Separation of ethanol–water mixtures by pervaporation using sodium alginate/poly(vinyl pyrrolidone) blend membrane crosslinked with phosphoric acid. Ind. Eng. Chem. Res. 2006, 45, 9088–9095. [Google Scholar]
- Yeom, C.K.; Lee, K.H. Characterization of permeation behaviors of ethanol–water mixtures through sodium alginate membrane with crosslinking membrane with crosslinking gradient during pervaporation separation. J. Appl. Polym. Sci. 1998, 69, 1607–1619. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, K.H. Characterization of sodium alginate membrane crosslinked with glutaraldehyde in pervaporation separation. J. Appl. Polym. Sci. 1998, 67, 209–219. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Pal, R.; Moon, G.Y. Characteristics of sodium alginate membranes for the pervaporation dehydration of ethanol-water and isopropanol-water mixtures. J. Membr. Sci. 1999, 160, 101–113. [Google Scholar] [CrossRef]
- Rachipudi, P.S.; Kittur, A.A.; Sajjan, A.M.; Kamble, R.R.; Kariduraganavar, M.Y. Solving the trade-off phenomenon in separation of water-dioxin mixtures by pervaporation through crosslinked sodium–alginate membranes with polystyrene sulfonic acid-co-maleic acid. Chem. Eng. Sci. 2013, 94, 84–92. [Google Scholar] [CrossRef]
- Premakshi, H.G.; Kariduraganavar, M.Y.; Mitchell, G.R. Development of composite anion-exchange membranes using poly(vinyl alcohol) and silica precursor for pervaporation separation of water–isopropanol mixtures. RSC Adv. 2016, 6, 11802–11814. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, L.; Wang, N.; Li, J.; Ji, S.; Guo, H.; Zhang, G. In situ ultraviolet-light-induced TiO2 nanohybrid superhydrophilic membrane for pervaporation dehydration. Sep. Puri. Techn. 2014, 122, 32–40. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Varghese, J.G.; Choudhari, S.K.; Olley, R.H. Organic inorganic hybrid membranes: Solving the trade-off phenomenon between permeation flux and selectivity in pervaporation. Ind. Eng. Chem. Res. 2009, 48, 4002–4013. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature (London) 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature (London) 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sirghi, L.; Aoki, T.; Hatanaka, Y. Hydrophilicity of thin films obtained by radio frequency magnetron sputtering deposition. Thin Solid Films 2002, 422, 55–61. [Google Scholar] [CrossRef]
- Gao, Y.; Masuda, Y.; Koumoto, K. Light excited super-hydrophilicity of amorphous TiO2 thin films deposited in an aqueous peroxotitanate solution. Langmuir 2004, 20, 3188–3194. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.Y. Role of moisture in adsorption, photocatalytic oxidation and reemission of elemental mercury on a SiO2-TiO2 nanocomposite. Environ. Sci. Technol. 2006, 40, 6444–6448. [Google Scholar] [CrossRef]
- Lokesh, B.G.; Krishna Rao, K.S.V.; Mallikarjuna Reddy, K.; Chowdoji Rao, K. Novel nanocomposite membranes of sodium alginate filled with polyaniline-coated titanium dioxide for dehydration of 1,4-dioxane/water mixtures. Desalination 2008, 233, 166–172. [Google Scholar] [CrossRef]
- Zhua, T.; Lina, Y.; Luoa, Y.; Hub, X.; Lina, W.; Yua, P.; Huanga, C. Preparation and characterization of TiO2-regenerated cellulose inorganic-polymer hybrid membranes for dehydration of caprolactam. Carbohydr. Polym. 2012, 87, 901–909. [Google Scholar] [CrossRef]
- Zhu, T.; Luo, Y.; Lin, Y.; Li, Q.; Yu, P.; Zeng, M. Study of pervaporation for dehydration of caprolactum through blend NaAlg-poly(vinyl pyrrolidone) membranes on PAN supports. Sep. Purif. Technol. 2010, 74, 242–252. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, L.N.; Peng, T.; Zhong, W. Effects of Ca2+ bridge cross-linking on structure and pervaporation of cellulose/alginate blend membranes. J. Membr. Sci. 2000, 175, 53–60. [Google Scholar] [CrossRef]
- Karuppuchamy, S.; Jeong, J.M. Super-hydrophilic amorphous titanium dioxide thin film deposited by cathodic electrodeposition. Mater. Chem. Phys. 2005, 93, 251–254. [Google Scholar] [CrossRef]
- Gestel, T.V.; Vandecasteele, C.; Buekenhoudt, A.; Dotremont, C.; Luyten, J.; Leysen, R.; Bruggen, B.V.; Maes, G. Salt retention in nanofiltration with multilayer ceramic TiO2 membranes. J. Membr. Sci. 2002, 209, 379–389. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kulkarni, S.S.; Ramesh, K. Development of novel pervaporation membranes for the separation of water-isopropanol mixtures using sodium alginate and NaY zeolite. J. Membr. Sci. 2004, 238, 165–175. [Google Scholar] [CrossRef]
- Kurkuri, M.D.; Toti, U.S.; Aminabhavi, T.M. Syntheses and characterization of blend membranes of sodium alginate and poly(vinyl alcohol) for the pervaporation separation of water isopropanol mixtures. J. Appl. Polym. Sci. 2002, 86, 3642–3651. [Google Scholar] [CrossRef]
- Saraswathi, M.; Rao, K.M.; Prabhakar, M.N.; Prasad, C.V.; Sudakar, K.; Kumar, H.M.P.N.; Prasad, M.; Rao, K.C.; Subha, M.C.S. Pervaporation studies of sodium alginate (SA)/dextrin blend membranes for separation of water and isopropanol mixture. Desalination 2011, 269, 177–183. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Pervaporation separation of water isopropyl alcohol mixtures with blend membranes of sodium alginate and poly(acrylamide)-grafted guar gum. J. Appl. Polym. Sci. 2002, 85, 2014–2024. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Different viscosity grade sodium alginate and modified sodium alginate membranes in pervaporation separation of water + acetic acid and water + isopropanol mixtures. J. Membr. Sci. 2004, 228, 199–208. [Google Scholar] [CrossRef]
- Bhat, S.D.; Naidu, B.V.K.; Shanbhag, G.V.; Halligudi, S.B.; Sairam, M.; Aminabhavi, T.M. Mesoporous molecular sieve (MCM-41)-filled sodium alginate hybrid nanocomposite membranes for pervaporation separation of water-isopropanol mixtures. Sep. Purif. Technol. 2006, 49, 56–63. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Novel sodium alginate composite membranes incorporated with SBA-15 molecular sieves for the pervaporation dehydration of aqueous mixtures of isopropanol and 1,4-dioxane at 30 °C. Micropor. Mesopor. Mater. 2006, 91, 206–214. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Novel sodium alginate-Na+MMT hybrid composite membranes for pervaporation dehydration of isopropanol, 1,4-dioxane and tetrahydrofuran. Sep. Purif. Technol. 2006, 51, 85–94. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Jeevan Kumar, B.K.; Kittur, A.A.; Kariduraganavar, M.Y. Novel approach for the development of pervaporation membranes using sodium alginate and chitosan-wrapped multiwalled carbon nanotubes for the dehydration of isopropanol. J. Membr. Sci. 2013, 425-426, 77–88. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Rachipudi, P.S. Development of crosslinked sodium alginate membranes for using polystyrene sulphonic co-malic acid for pervaporation dehydration of isopropanol. Precedia Eng. 2012, 44, 884–889. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Bourgeois, D.; Belfort, G. Sorption, diffusion and pervaporation of organics in polymer membranes. J. Membr. Sci. 1989, 44, 161–181. [Google Scholar] [CrossRef]
- Hwang, S.T.; Kammermeyer, K. Membrane in Separation; Wiley-Interscience: New York, NY, USA, 1975. [Google Scholar]
- Yamasaki, A.; Iwatsubo, T.; Masuoka, T.; Mizoguchi, K. Pervaporation of ethanol/water through a poly(vinyl alcohol)/cyclodextrin (PVA/CD) membrane. J. Membr. Sci. 1994, 89, 111–117. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of GTMAC grafted chitosan membranes for the dehydration of low water content isopropanol by pervaporation. J. Ind. Eng. Chem. 2015, 25, 151–161. [Google Scholar] [CrossRef]
- Kusumocahyo, S.P.; Sudoh, M. Dehydration of acetic acid by pervaporation with charged membranes. J. Membr. Sci. 1999, 161, 77–83. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Premakshi, H.G.; Kariduraganavar, M.Y. Development of novel alginate-silica hybrid membranes for pervaporation dehydration of isopropanol. Polym. Bull. 2016, 73, 743–762. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.Y.M.; Yeom, C.K. Pervaporation separation of aqueous mixtures using crosslinked poly(vinyl alcohol) membranes. III. Permeation of acetic acid-water mixtures. J. Membr. Sci. 1991, 58, 33–47. [Google Scholar] [CrossRef]
- Weinkauf, D.H.; Paul, D.R. Effects of structural order on barrier properties. In Barrier Polymers and Structures; Koros, W.J., Ed.; ACS Symp. Ser.: Washington, DC, USA, 1990; Volume 423, pp. 61–91. [Google Scholar]
Sample Availability: Samples of the membranes are available from the authors. |



| Membrane | Tensile Strength (MPa) | Elongation at Break (%) | Glass Transition Temperature (Tg) °C | Crystallinity % w/w |
|---|---|---|---|---|
| M | 42.83 | 14.28 | 240 | 29.3 |
| M-1 | 53.21 | 9.50 | 244 | 24.3 |
| M-2 | 51.50 | 7.60 | 246 | 24.0 |
| M-3 | 50.43 | 7.17 | 248 | 22.9 |
| M-4 | 33.67 | 5.50 | 250 | 21.4 |
| Membrane | Thick-ness (µm) | Temp. (oC) | Water in the Feed (wt%) | Flux (kg/m2∙h) | Separation Factor | PSI | References |
|---|---|---|---|---|---|---|---|
| NaAlg/PVA (75/25) | 35–40 | 30 | 10.0 | 0.06 | 195 | 4.87 | [37] |
| Composite membranes of NaAlg and CS | - | 30 | 10.0 | 0.55 | 2010 | 1113 | [37] |
| NaAlg/De (60/40) | 35–40 | 30 | 10.0 | 0.07 | 8991 | 656 | [38] |
| NaAlg/De (50/50) | 35–40 | 30 | 10.0 | 0.10 | 8172 | 776 | [38] |
| NaAlg/PAAM-g-GG blend | 10 | 30 | 10.0 | 0.13 | 890 | - | [39] |
| NaAlg + 5 wt% PVA + 10 wt% PEG | 10 | 30 | 10.0 | 0.15 | 3600 | - | [40] |
| MCM-41 (10 wt%) filled NaAlg | 10 | 30 | 10.0 | 0.55 | 30,000 | - | [41] |
| SBA-15 (10 wt%) filled NaAlg | 10 | 30 | 10.0 | 0.11 | ∞ | - | [42] |
| Fe-SBA 15 (10 wt%) filled NaAlg | 10 | 30 | 10.0 | 0.18 | ∞ | - | [42] |
| Na+ MMT (10 wt%) filled NaAlg | 10 | 30 | 10.0 | 0.25 | ∞ | - | [43] |
| NaAlg/NaY zeolite (5 wt%) | 40 | 30 | 10.0 | 14.19 | 191 | 27 | [36] |
| NaAlg/NaY zeolite (15 wt%) | 40 | 30 | 10.0 | 16.07 | 211 | 32 | [36] |
| NaAlg/NaY zeolite (30 wt%) | 40 | 30 | 10.0 | 23.25 | 272 | 66 | [36] |
| NaAlg/CS wrapped MWCNTs (0.5 wt%) | 50 | 30 | 10.0 | 13.43 | 929 | 145 | [44] |
| NaAlg/CS wrapped MWCNTs (1.0 wt%) | 50 | 30 | 10.0 | 16.18 | 1397 | 226 | [44] |
| NaAlg/CS wrapped MWCNTs (1.5 wt%) | 50 | 30 | 10.0 | 19.67 | 2134 | 410 | [44] |
| NaAlg/CS wrapped MWCNTs (2.0 wt%) | 50 | 30 | 10.0 | 21.76 | 6420 | 1400 | [44] |
| SA/ PSSA-co-MA (0.5 wt%) | 40 | 30 | 10.0 | 6.38 | 6800 | 500 | [45] |
| SA/PSSA-co-MA (1.0 wt%) | 40 | 30 | 10.0 | 8.12 | 8023 | 750 | [45] |
| SA/PSSA-co-MA (2.0 wt%) | 40 | 30 | 10.0 | 9.46 | 14,894 | 1300 | [45] |
| NaAlg/PSSA-co-MA (3.0 wt%) | 40 | 30 | 10.0 | 10.53 | 22,312 | 2415 | [45] |
| Crosslinked NaAlg with 10 wt% TiO2 | 40 | 30 | 10.0 | 11.12 | 8,591 | 599 | Present work |
| Crosslinked NaAlg with 20 wt% TiO2 | 40 | 30 | 10.0 | 13.22 | 11,491 | 993 | Present work |
| Crosslinked NaAlg with 30 wt% TiO2 | 40 | 30 | 10.0 | 15.47 | 16,094 | 1627 | Present work |
| Crosslinked NaAlg with 40 wt% TiO2 | 40 | 30 | 10.0 | 18.61 | 24,092 | 2583 | Present work |
| Mass % of Water | Diffusion Coefficients of Water (Dw) ×108 (cm2/s) | Diffusion Coefficients of Isopropanol (DIPA) ×1010 (cm2/s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | M-1 | M-2 | M-3 | M-4 | M | M-1 | M-2 | M-3 | M-4 | |
| 5 | 3.18 | 3.56 | 4.43 | 4.95 | 6.53 | 1.06 | 0.89 | 0.72 | 0.56 | 0.52 |
| 10 | 2.22 | 2.31 | 2.76 | 3.26 | 3.74 | 2.39 | 2.06 | 1.96 | 1.72 | 1.31 |
| 15 | 1.80 | 1.96 | 2.23 | 2.65 | 3.09 | 4.14 | 3.92 | 3.80 | 3.74 | 3.46 |
| 20 | 1.59 | 1.72 | 1.94 | 2.36 | 2.61 | 22.01 | 21.12 | 19.01 | 15.24 | 8.37 |
| 25 | 1.46 | 1.58 | 1.91 | 2.15 | 2.38 | 35.61 | 34.11 | 32.80 | 32.00 | 23.11 |
| Temperature °C | Flux(J) × 102 (kg/m2∙h) | Separation Selectivity(αsep) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | M-1 | M-2 | M-3 | M-4 | M | M-1 | M-2 | M-3 | M-4 | |
| 30 | 10.14 | 11.12 | 13.22 | 15.47 | 18.61 | 5992 | 8591 | 11,491 | 16,094 | 24,092 |
| 40 | 11.63 | 13.66 | 14.29 | 16.13 | 19.15 | 3200 | 4800 | 4988 | 6242 | 7344 |
| 50 | 13.40 | 14.46 | 15.12 | 17.18 | 20.72 | 904 | 1050 | 2101 | 2606 | 2893 |
| Parameters (kJ/mol) | M | M-1 | M-2 | M-3 | M-4 |
|---|---|---|---|---|---|
| Total permeation (EP) | 10.60 | 6.41 | 5.55 | 5.17 | 3.96 |
| Total diffusion (ED) | 10.76 | 6.62 | 5.80 | 5.48 | 4.29 |
| Permeation of water (EPW) | 9.87 | 6.33 | 4.96 | 4.65 | 3.49 |
| Diffusion of water (EDW) | 10.12 | 7.56 | 5.27 | 4.89 | 3.75 |
| Permeation of isopropanol (EPIPA) | 93 | 104 | 104 | 110 | 125 |
| Heat of sorption (∆Hs) | −0.16 | −0.21 | −0.25 | −0.31 | −0.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premakshi, H.G.; Kariduraganavar, M.Y.; Mitchell, G.R. Crosslinked Nanocomposite Sodium Alginate-Based Membranes with Titanium Dioxide for the Dehydration of Isopropanol by Pervaporation. Molecules 2020, 25, 1298. https://doi.org/10.3390/molecules25061298
Premakshi HG, Kariduraganavar MY, Mitchell GR. Crosslinked Nanocomposite Sodium Alginate-Based Membranes with Titanium Dioxide for the Dehydration of Isopropanol by Pervaporation. Molecules. 2020; 25(6):1298. https://doi.org/10.3390/molecules25061298
Chicago/Turabian StylePremakshi, H.G., Mahadevappa Y. Kariduraganavar, and Geoffrey R. Mitchell. 2020. "Crosslinked Nanocomposite Sodium Alginate-Based Membranes with Titanium Dioxide for the Dehydration of Isopropanol by Pervaporation" Molecules 25, no. 6: 1298. https://doi.org/10.3390/molecules25061298
APA StylePremakshi, H. G., Kariduraganavar, M. Y., & Mitchell, G. R. (2020). Crosslinked Nanocomposite Sodium Alginate-Based Membranes with Titanium Dioxide for the Dehydration of Isopropanol by Pervaporation. Molecules, 25(6), 1298. https://doi.org/10.3390/molecules25061298








