Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC and Artificial Neural Network Analysis for Ganoderma lucidum
Abstract
1. Introduction
2. Results and Discussion
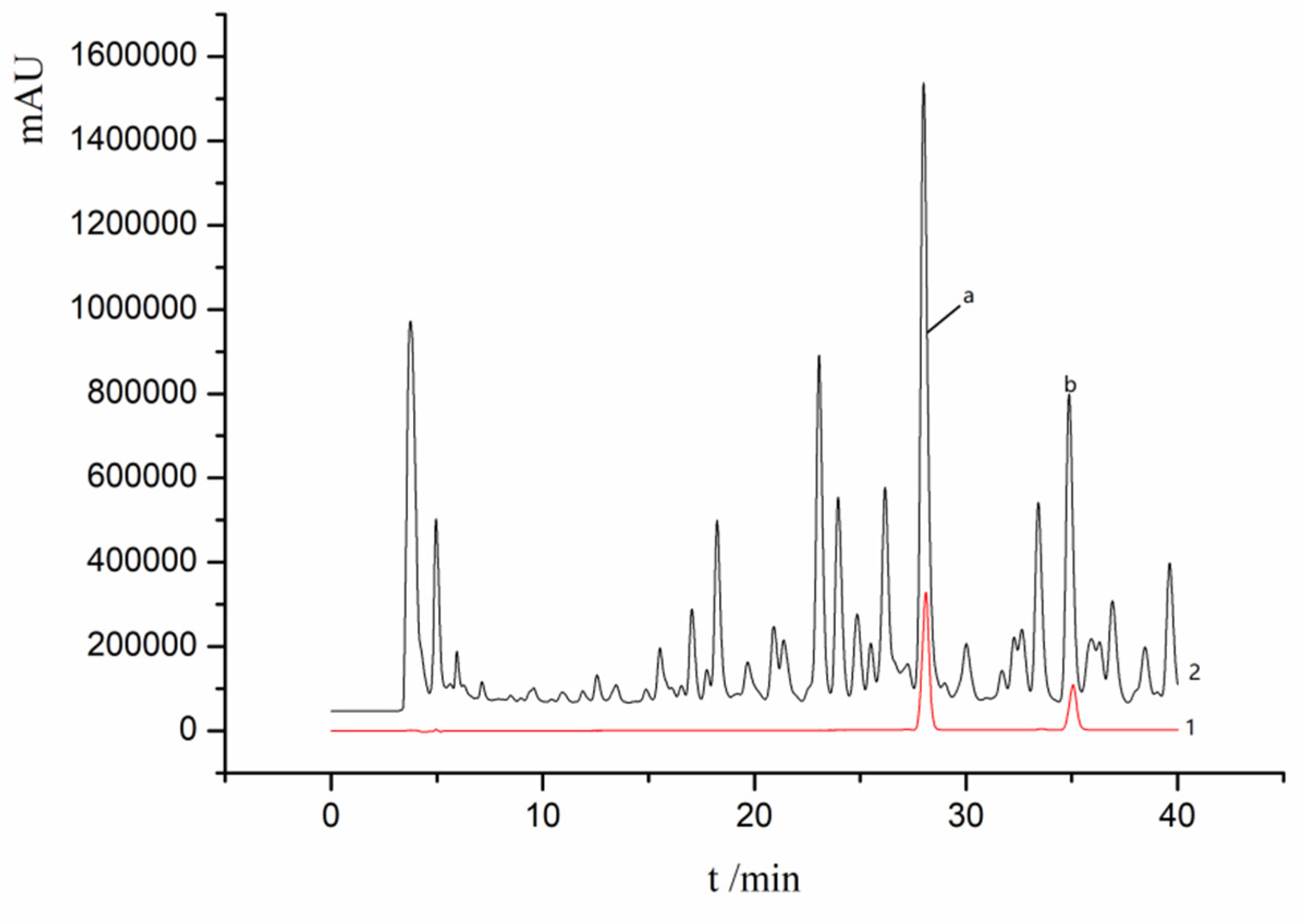
2.1. Linear Relationship
2.2. Selection Period of Ionic Liquids (ILs)
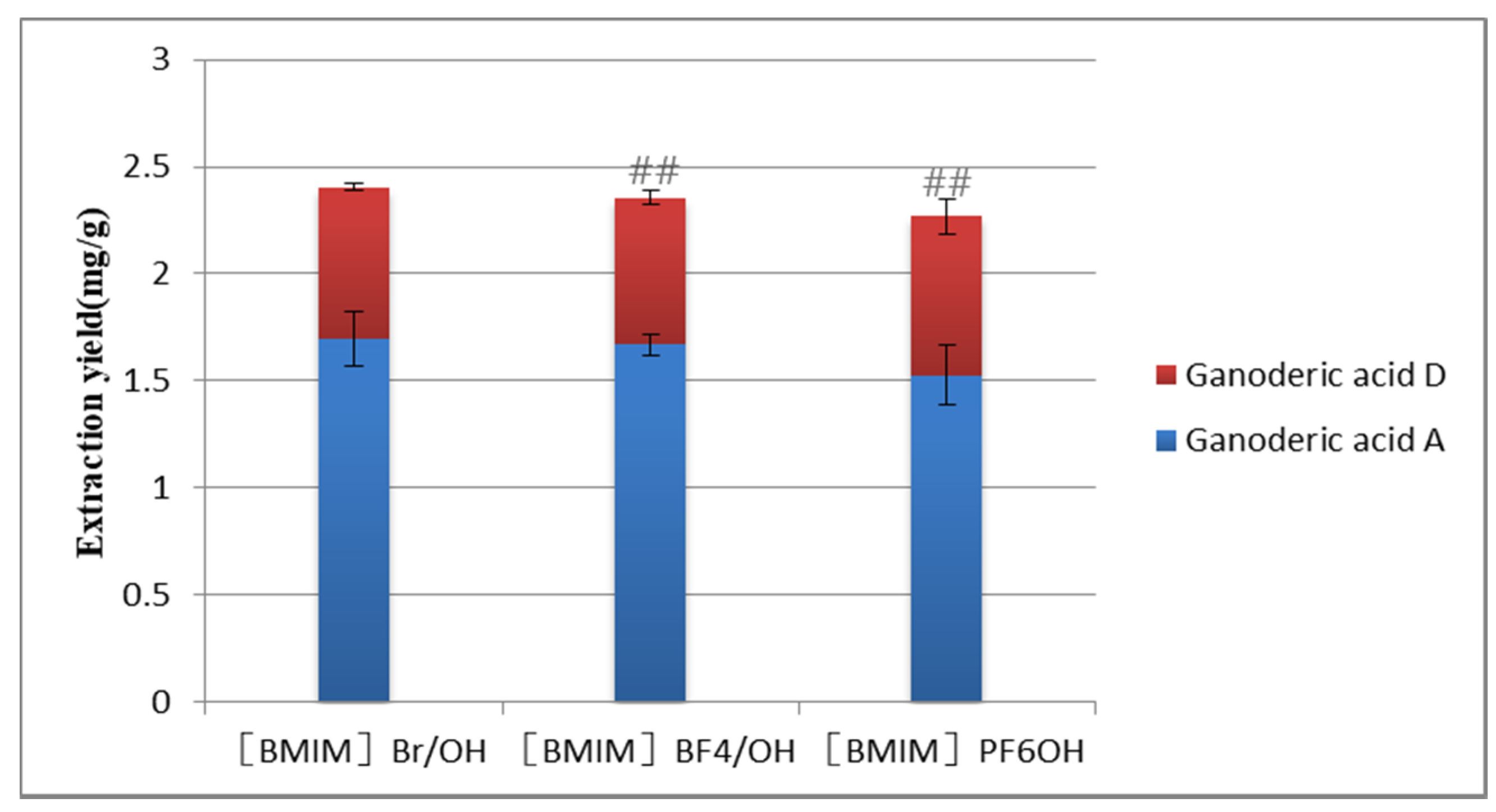
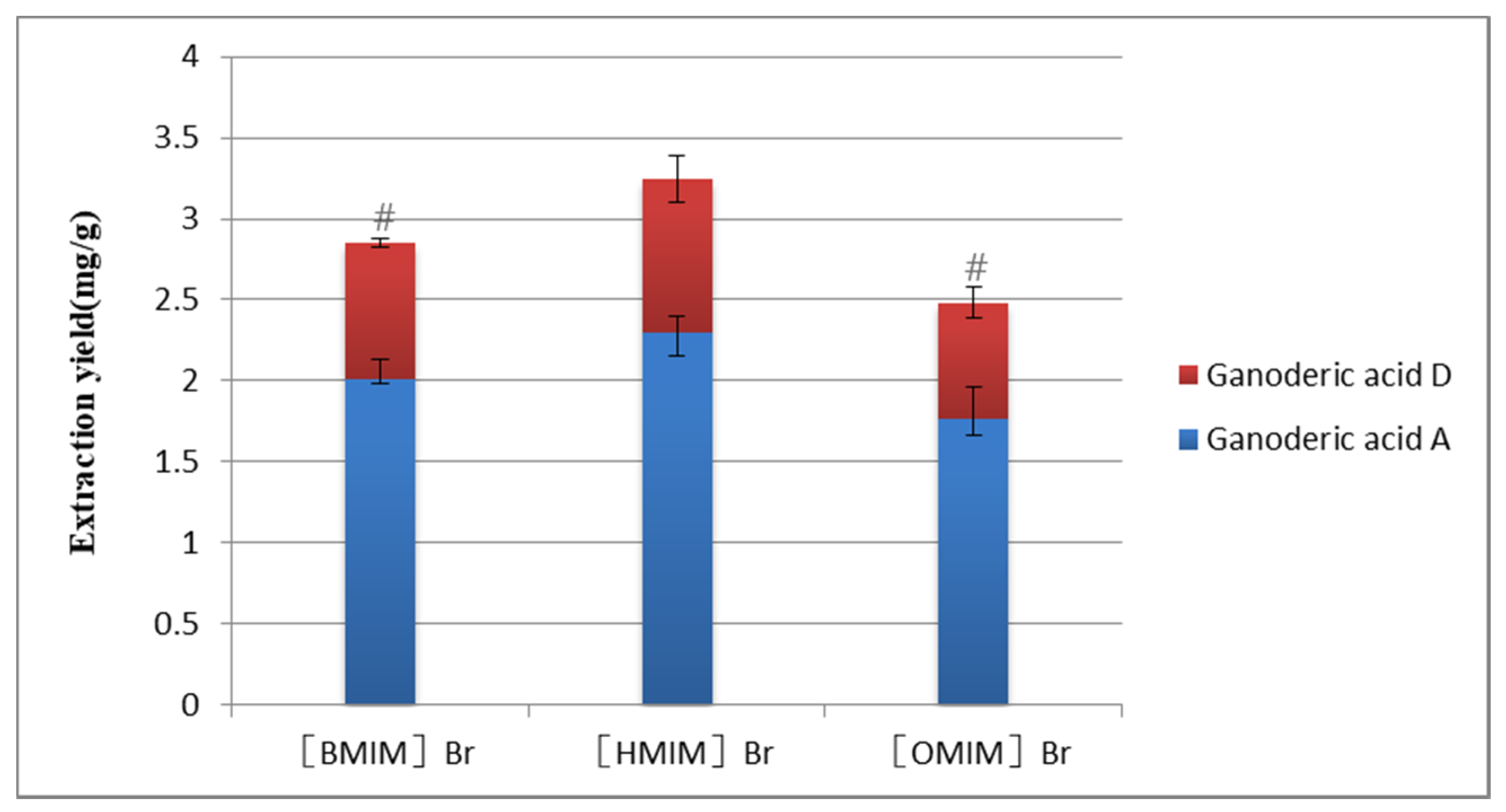
2.2.1. Selection of Concentration of ILs
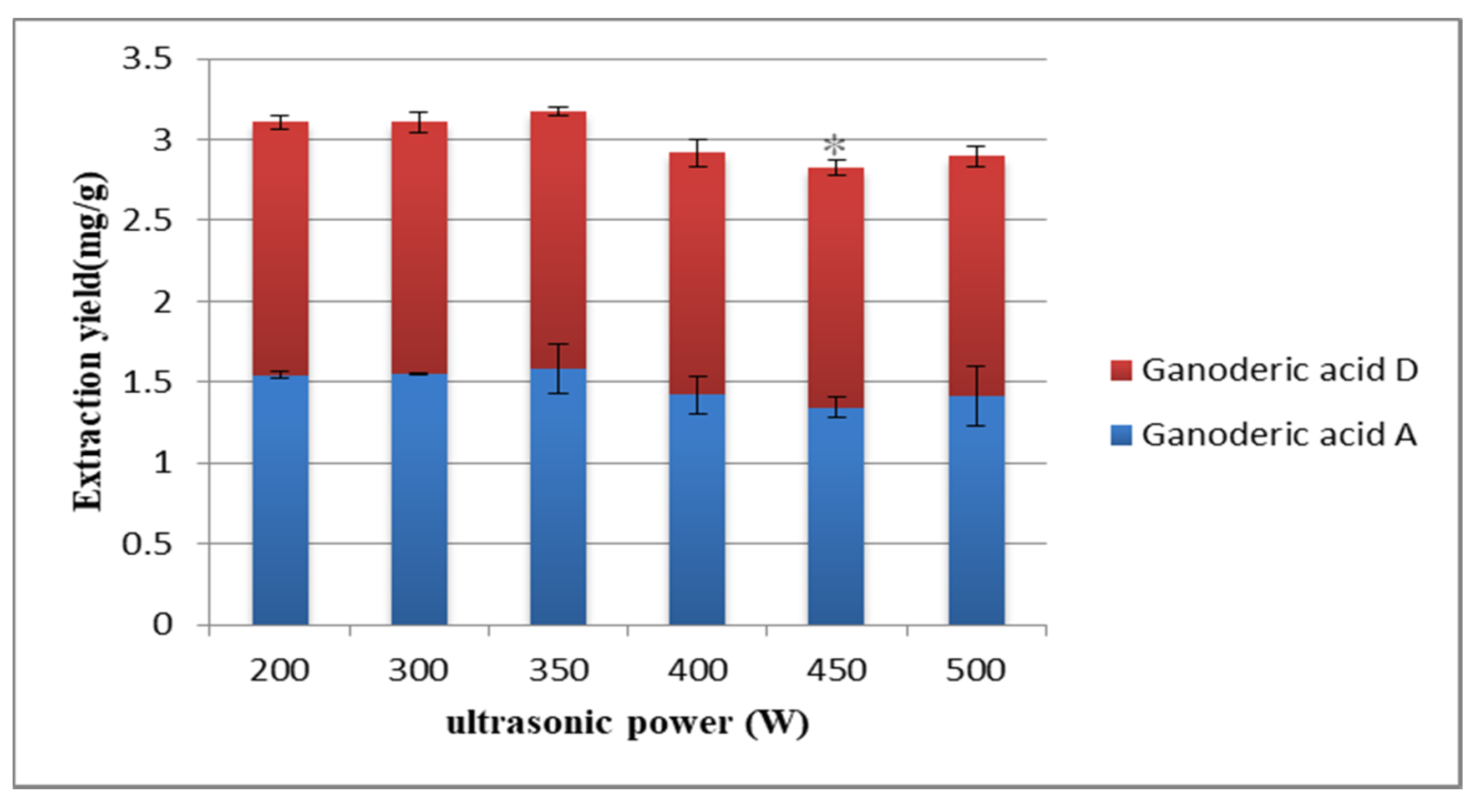
2.2.2. Selection of Ultrasonic Power
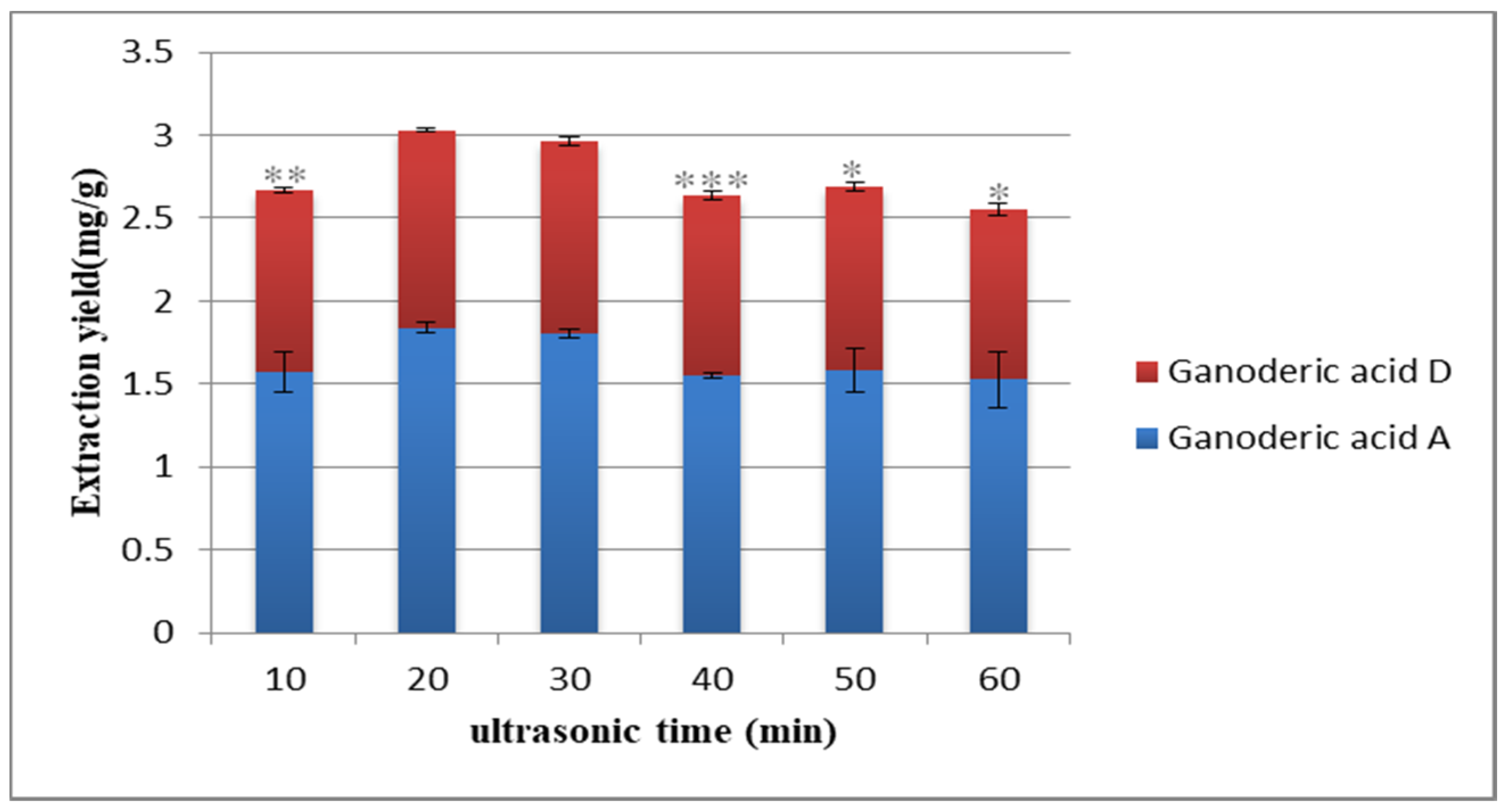
2.2.3. Selection of Ultrasonic Time
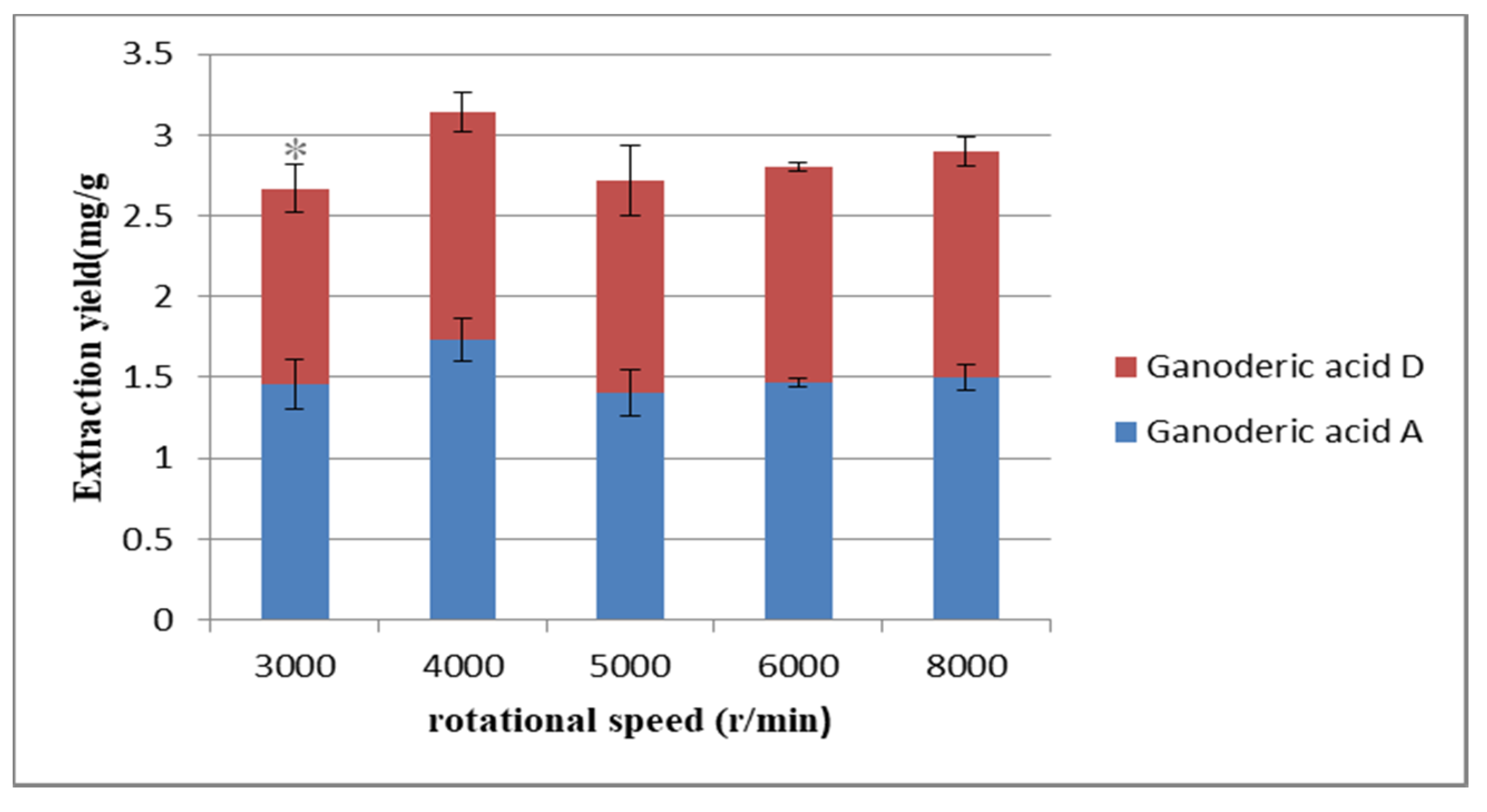
2.2.4. Selection of Rotational Speed
2.2.5. Selection of Solid–Liquid Ratio
2.3. Optimization Extraction Process in Ganoderma lucidum
2.3.1. The Results of the Intuitionistic Analysis
2.3.2. The Results of the Variance Analysis
2.3.3. Comparison between IL-UAE Approach and the Traditional Methods
2.4. ANN Model Development
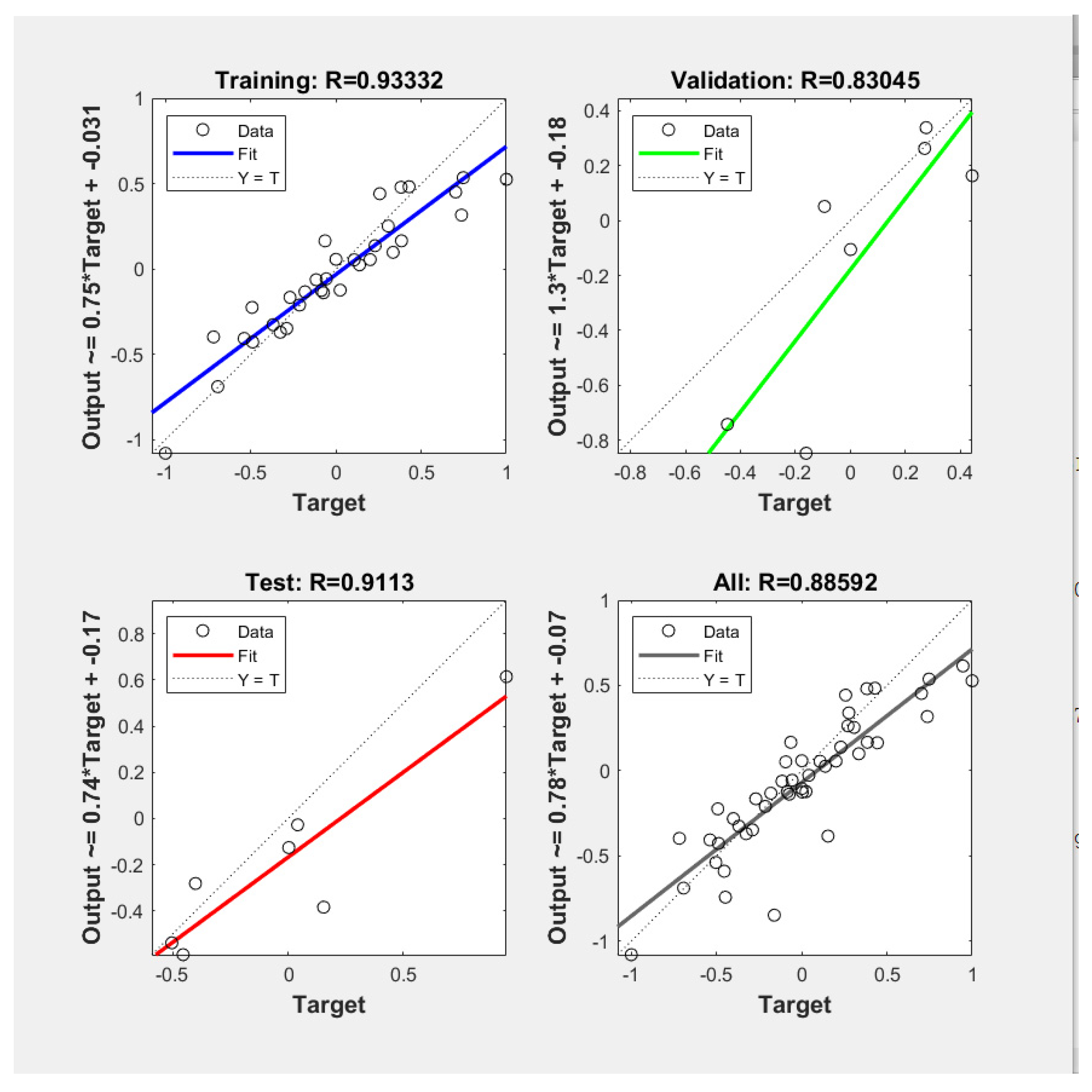
2.5. Method Validation
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Materials and Sample Preparation
3.3. Preparation of the Standard Solution
3.4. Preparation of Test Sample Solution
3.5. Chromatographic Conditions
3.6. Optimization Extraction Process in Ganoderma lucidum
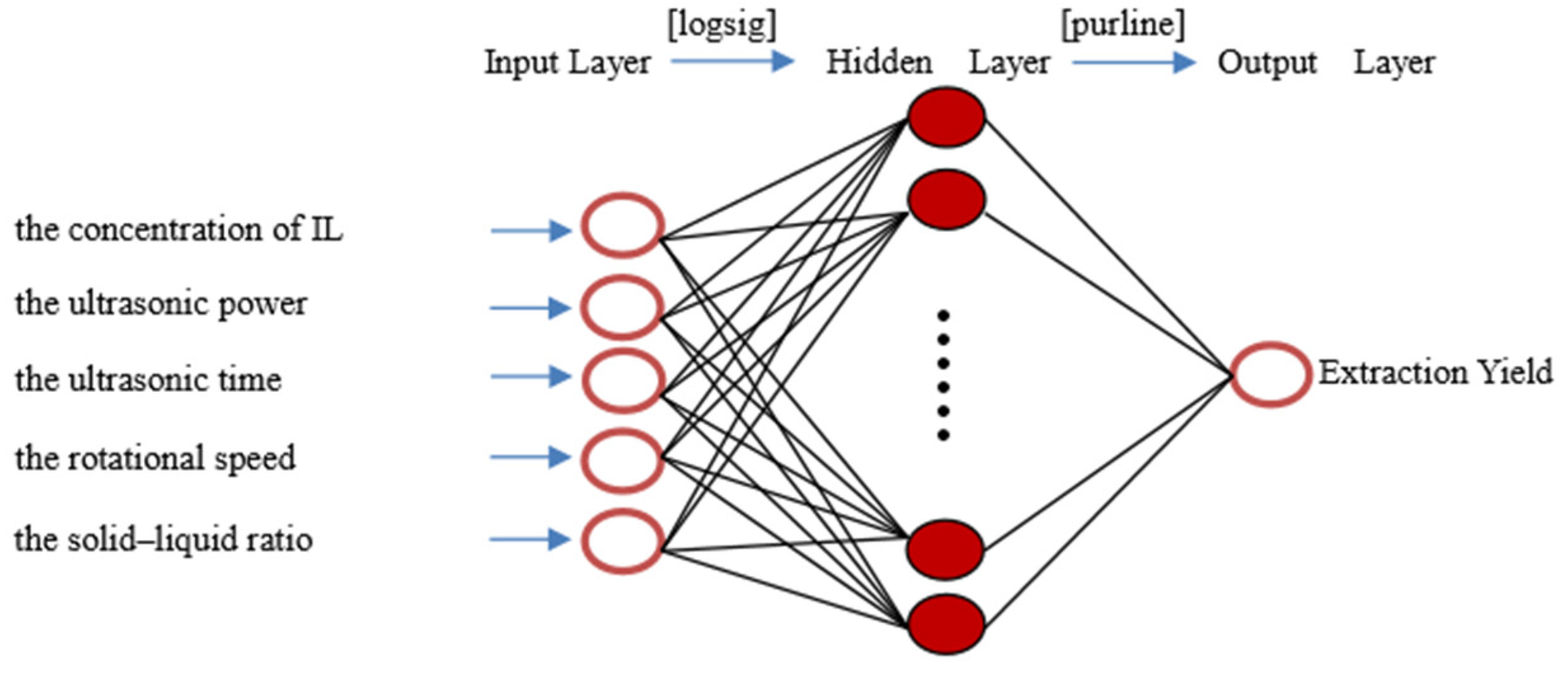
3.7. Artificial Neural Networks (ANNs)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, G.X.; Yang, W.J.; Zhao, L.Y.; Pei, F.; Fang, D.L.; Hu, Q.H. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness. 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Soccol, C.R.; Bissoqui, L.Y.; Rodrigues, C.; Rubel, R.; Sella, S.R.; Leifa, F.; de Souza Vandenberqhe, L.P.; Soccol, V.T. Pharmacological properties of biocompounds from spores of the Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes): A Review. Int. J. Med. Mushroomsw. 2016, 18, 757–767. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, C.R.; Ding, J.; Liang, X.Y.; Guo, D.A.; Guan, S.H.; Yang, Y. Pharmacokinetic studies of ganoderic acids from the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), by LC-MS/MS. Int. J. Med. Mushroomsw. 2016, 18, 405–412. [Google Scholar]
- Wu, L.; Liang, W.Y.; Chen, W.J.; Li, S.; Cui, Y.P.; Qi, Q.; Zhang, L.Z. Screening and analysis of the marker components in Ganoderma lucidum by HPLC and HPLC-MSn with the aid of Chemometrics. Molecules 2017, 22, 584. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Jiang, Q.; Liu, G.; Miao, X.; Zhong, D. Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int. J. Biol. Macromol. 2013, 55, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Sillapapongwarakorn, S.; Yanarojana, S.; Pinthong, D.; Thithapandha, A.; Unqwitayatorn, J.; Supavilai, P. Molecular docking based screening of triterpenoids as potential G-quadruplex stabilizing ligands with anti-cancer activity. Bioinformation 2017, 13, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Hai-Bang, T.; Zhu, Q.; Amen, Y.; Sakamoto, S.; Tanaka, H.; Morimoto, S.; Shimizu, K. Tubulin polymerization-stimulating activity of Ganoderma triterpenoids. J. Nat. Med. 2017, 71, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Fu, H.Y.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: A randomized, double-blind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm. Biol. 2017, 55, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Kondo, R.; Shimizu, K. Abstract: Structure-activity relationships of lanostane-type triterpenoids from Ganoderma lingzhi as α-glucosidase Inhibitors. ChemInform 2014, 45. [Google Scholar] [CrossRef]
- Kim, D.H.; Shim, S.B.; Kim, N.J.; Jang, I.S. Beta-Glucuronidase-Inhibitory activity and hepatoprotective effect of Ganoderma lucidum. Biol. Pharm. Bull. 1999, 22, 162–164. [Google Scholar] [CrossRef]
- Koyama, K.; Imaizumi, T.; Akiba, M.; Kinoshita, K.; Takahashi, K.; Suzuki, A.; Yano, S.; Horie, S.; Watanabe, K.; Naoi, Y. Antinociceptive components of Ganoderma lucidum. Planta Med. 1997, 63, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, Z.Z.; Jiao, G.L.; Sun, G.D.; Zhou, Z.G. Ganoderic acid A suppresses proliferation and invasion and induces apoptosis in human osteosarcoma cells. J. South. Med. Univ. 2015, 35, 619–624. [Google Scholar]
- Zhu, M.; Chang, Q.; Wong, L.K.; Chong, F.S.; Li, R.C. Triterpene antioxidants from Ganoderma lucidum. Phytother. Res. 1999, 13, 529–531. [Google Scholar] [CrossRef]
- Das, A.; Miller, R.; Lee, P.; Holden, C.A.; Lindhorst, S.M.; Jaboin, J.J.; Vandergrift, W.A.; Banik, N.L.; Giqlio, P.; Varma, A.; et al. A novel component from citrus, ginger, and mushroom family exhibits antitumor activity on human meningioma cells through suppressing the Wnt/β-catenin signaling pathway. Tumor Biol. 2015, 36, 7027–7034. [Google Scholar] [CrossRef]
- Yao, X.; Li, G.; Xu, H.; Lü, C. Inhibition of the JAK-STAT3 signaling pathway by ganoderic Acid A enhances chemosensitivity of HepG2 cells to cisplatin. Planta Med. 2012, 78, 1740–1748. [Google Scholar] [CrossRef]
- Jiang, J.; Grieb, B.; Thyagarajan, A.; Sliva, D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-κB signaling. Int. J. Mol. Med. 2008, 21, 577–584. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, M.; Yu, K.; Guan, S.; Tao, S.; Millar, A.; Pang, X.; Guo, D. Identification of metabolites of ganoderic Acid D by Ultra-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. Drug Metab. Dispos. 2012, 40, 2307–2314. [Google Scholar] [CrossRef]
- Radwan, F.F.; Hossain, A.; God, J.M.; Leaphart, N.; Elvington, M.; Nagarkatti, M.; Tomlinson, S.; Haque, A. Reduction of myeloid-derived suppressor cells and Lymphoma growth by a natural triterpenoid. J. Cell. Biochem. 2015, 116, 102–114. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Xue, B. Effect of ganoderic acid D on colon cancer warburg effect: Role of SIRT3/cyclophilin D. Eur. J. Pharmacol. 2018, 824, 72–77. [Google Scholar] [CrossRef]
- Dupont, J.; Souza, R.F.; Suarez, P.A. Ionic liquid (Molten Salt) phase organometallic catalysis. Chem. Rev. 2003, 102, 3667–3692. [Google Scholar] [CrossRef]
- Wu, H.; Chen, M.; Fan, Y.; Elsebaei, F.; Zhu, Y. Determination of rutin and quercetin in Chinese herbal medicine by ionic liquid-based pressurized liquid extraction-liquid chromatography-chemiluminescence detection. Talanta 2012, 88, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cheng, H.; Fu, L.; Ding, C.; Zhang, L.; Yang, R.; Zhou, Y. Ultrasonic-assisted extraction and antioxidant activities of polysaccharides from Camellia oleifera leaves. Int. J. Biol. Macromol. 2014, 68, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Xue, Y.; Xu, Y.; Shen, Y. Artificial neural network modeling and optimization of ultrahigh pressure extraction of green tea polyphenols. Food Chem. 2013, 141, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Passos, H.; Freire, M.G.; Coutinho, J.A. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef]
- You, X.; Chen, X.; Liu, F.; Hou, F.; Li, Y. Ionic liquid-based air-assisted liquid-liquid microextraction followed by high performance liquid chromatography for the determination of five fungicides in juice samples. Food Chem. 2018, 239, 354–359. [Google Scholar] [CrossRef]
- Altunay, N.; Yildirim, E.; Gürkan, R. Extraction and preconcentration of trace Al and Cr from vegetable samples by vortex-assisted ionic liquid-based dispersive liquid–liquid microextraction prior to atomic absorption spectrometric determination. Food Chem. 2018, 245, 586–594. [Google Scholar] [CrossRef]
- Bağda, E.; Tüzenb, M. A simple and sensitive vortex-assisted ionic liquid-dispersive microextraction and spectrophotometric determination of selenium in food samples. Food Chem. 2017, 232, 98–104. [Google Scholar] [CrossRef]
- Zhang, M.L.; Mallik, A.; Takafuji, M.; Ihara, H.; Qiu, H.D. Versatile ligands for high-performance liquid chromatography: An overview of ionic liquid-functionalized stationary phases. Anal. Chim. Acta 2015, 887, 1–16. [Google Scholar] [CrossRef]
- Wang, J.L.; Feng, J.; Xu, L.; Ma, J.P.; Li, J.L.; Ma, R.; Sun, K.; Wang, Z.B.; Zhang, H.Q. Ionic liquid-based salt-induced liquid-liquid extraction of polyphenols and anthraquinones in Polygonum cuspidatum. J. Pharm. Biomed. Anal. 2019, 163, 95–104. [Google Scholar] [CrossRef]
- Belkacem, S.; Bouafia, S.; Chabani, M. Study of oxytetracycline degradation by means of anodic oxidation process using platinized titanium (Ti/Pt) anode and modeling by artificial neural networks. Process. Saf. Environ. Prot. 2017, 111, 170–179. [Google Scholar] [CrossRef]
- Dudhagara, D.R.; Rajpara, R.K.; Bhatt, J.K.; Gosai, H.B.; Dave, B.P. Bioengineering for polycyclic aromatic hydrocarbon degradation by Mycobacterium litorale: Statistical and artificial neural network (ANN) approach. Chemom. Intell. Lab. Syst. 2016, 159, 155–163. [Google Scholar] [CrossRef]
- Piuleac, C.G.; Curteanu, S.; Rodrigo, M.A.; Saez, C.; Fernandez, F.J. Optimization methodology based on neural networks and genetic algorithms applied to electro-coagulation processes. Cent. Eur. J. Chem. 2013, 11, 1213–1224. [Google Scholar] [CrossRef]
- Pang, A.H.; Deeth, H.; Prakash, S.; Bansal, N. Development of rheological and sensory properties of combinations of milk proteins and gelling polysaccharides as potential gelatin replacements in the manufacture of stirred acid milk gels and yogurt. J. Food Eng. 2016, 169, 27–37. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, X.; Wei, B.; Zhao, Y.; Wang, J. Evaluation of adsorption potential of bamboo biochar for metal-complex dye: Equilibrium, kinetics and artificial neural network modeling. Int. J. Environ. Sci. Technol. 2014, 11, 1093–1100. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, H.; Zu, Y.G.; Zhao, C.; Zhang, L.; Chen, X.Q.; Zhang, Z.G. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. Chem. Eng. J. 2011, 172, 705–712. [Google Scholar]
- Sun, Y.S.; Li, W.; Wang, J.H. Ionic liquid based ultrasonic assisted extraction of isoflavones from Iris tectorum Maxim and subsequently separation and purification by high-speed counter-current chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 975–980. [Google Scholar] [CrossRef]
- Shi, M.J.; Zhang, J.j.; Liu, C.Y.; Cui, Y.P.; Li, C.Q.; Liu, Z.H.; Kang, W.Y. Ionic liquid-based ultrasonic-assisted extraction to analyze seven compounds in Psoralea Fructus coupled with HPLC. Molecules 2019, 24, 1699. [Google Scholar] [CrossRef]
- Tian, Z.J.; Li, Q.; Wang, C.Y.; Zhou, W.L.; Yang, Y.R.; Wang, H.Y.; Yi, Y.J.; Li, F.F. Ultrasonic assisted extraction of paclitaxel from Taxus media using ionic liquids as adjuvants: Optimization of the process by response surface methodology. Molecules 2017, 22, 1483. [Google Scholar] [CrossRef]
- Wang, Y.G.; OuYang, X.K.; Yang, L.Y.; Li, Q.L. Microwave-assisted extraction of Rutin from Tamarix Chinensis with ionic liquid. Chem. Eng. Chin. 2011, 3, 411–415. [Google Scholar]
- Wang, L.L.; Bai, M.; Qin, Y.C.; Liu, B.T.; Wang, Y.B.; Zhou, Y.F. Application of ionic liquid-based ultras- onic-assisted Extraction of flavonoids from Bamboo Leaves. Molecules 2018, 23, 2309. [Google Scholar] [CrossRef]
- Liao, X.Y.; Li, J.; Suo, Y.J. Multiple action sites of ultrasound on Escherichia coli and Staphylococcus aureus. Food Sci. Hum. Wellness. 2018, 7, 102–109. [Google Scholar] [CrossRef]
- Huang, H.; Song, Y.Q.; Yan, D.S. Investigation of ion liquid combined withultrasound-assisted extraction technology of total flavones. Chin. Tradit. Herb. Drugs. 2019, 50, 5995–6001. [Google Scholar]
- Su, S.; Li, L.; Wang, S.X.; Wang, B. Extraction of isoflavones from Black Soybean by ultrasound-assisted ionic liquids and antibacterial. Food Res. Dev. 2020, 41, 32–37. [Google Scholar]
- Li, C.Q.; Wang, P.Y.; Kang, W.Y.; Shi, M.J.; Guo, X.C.; Li, W.J. Efficient determination of three flavonoids in Malus pumila flowers by ionic liquid-HPLC. J. Mol. Liq. 2018, 263, 139–146. [Google Scholar] [CrossRef]
- Bao, S.W.; Liao, Z.H.; Chen, M.; Tan, F. Selecting the best medium for the Tufted-bud induction of Alove vera L. var Chinesis(Haw.) Berger by orthogonal Design. J. Southwest Nationalities Coll. 2000, 26, 177–180. [Google Scholar]
- Bennemla, M.; Chabani, M.; Amrane, A. Photocatalytic degradation of oxytetracycline in aqueous solutions with TiO2 in suspension and prediction by artificial neural networks. Int. J. Chem. Kinetics 2016, 48, 464–473. [Google Scholar] [CrossRef]
- Ikrng, E.G.; Umani, K.C. Optimization of process conditions for drying of catfish (Clarias gariepinus) using response surface methodology (RSM). Food Sci. Hum. Wellness. 2019, 8, 46–52. [Google Scholar] [CrossRef]
- Yu, H.H.; Hwang, J.N.; Perry, S.W. Handbook of neural network signal processing. J. Acoust. Soc. Am. 2002, 111, 780. [Google Scholar]
- Sinha, K.; Chowdhury, S.; Saha, P.D.; Datta, S. Modeling of microwave-assisted extraction of natural dye from seeds of Bixa orellana (Annatto) using response surface methodology (RSM) and artificial neural network (ANN). Ind. Crops Prod. 2013, 41, 165–171. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Zhang, B. Modeling of UV-Induced photodegradation of naphthalene in marine oily wastewater by artificial neural networks. Water Air Soil Pollut. 2014, 225, 1906–3353. [Google Scholar] [CrossRef]
- Shi, M.J.; He, N.; Li, W.J.; Li, C.Q.; Kang, W.Y. Simultaneous determination of myricetrin, quercitrin and afzelin in leaves of Cercis chinensis by a fast and effective method of ionic liquid microextraction coupled with HPLC. Chem Cent. J. 2018, 12, 23–31. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Ganoderic acid A and D are available from the authors. |





| Factor Level | A IL Concentration (mol/L) | B Ultrasonic Time (W) | C Ultrasonic Time (min) | D Rotational Speed (r/min) | E Solid—Liquid Ratio (g/mL) |
|---|---|---|---|---|---|
| 1 | 1 | 300 | 10 | 3000 | 1:14.2 |
| 2 | 1.2 | 350 | 20 | 4000 | 1:20 |
| 3 | 1.4 | 400 | 30 | 5000 | 1:33.3 |
| NO. | A | B | C | D | E | F | Extraction Yield mg/g |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 3 | 3 | 3 | 3.013 |
| 2 | 1 | 3 | 1 | 2 | 2 | 1 | 3.040 |
| 3 | 2 | 2 | 3 | 2 | 3 | 1 | 2.951 |
| 4 | 2 | 1 | 3 | 3 | 2 | 1 | 2.953 |
| 5 | 3 | 1 | 1 | 3 | 3 | 2 | 2.908 |
| 6 | 1 | 2 | 2 | 3 | 2 | 3 | 2.795 |
| 7 | 2 | 2 | 1 | 1 | 1 | 3 | 2.692 |
| 8 | 3 | 2 | 1 | 2 | 2 | 2 | 2.719 |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 2.660 |
| 10 | 3 | 2 | 2 | 3 | 1 | 1 | 3.083 |
| 11 | 2 | 1 | 2 | 2 | 1 | 2 | 3.067 |
| 12 | 2 | 3 | 2 | 1 | 2 | 2 | 2.784 |
| 13 | 1 | 3 | 3 | 3 | 1 | 2 | 2.975 |
| 14 | 3 | 1 | 3 | 1 | 2 | 3 | 2.738 |
| 15 | 1 | 2 | 3 | 1 | 3 | 2 | 2.846 |
| 16 | 3 | 3 | 3 | 2 | 1 | 3 | 3.198 |
| 17 | 3 | 3 | 2 | 1 | 3 | 1 | 2.951 |
| 18 | 1 | 1 | 2 | 2 | 3 | 3 | 3.207 |
| K1 | 17.523 | 17.533 | 17.032 | 16.673 | 17.675 | ||
| K2 | 17.459 | 17.087 | 17.887 | 18.182 | 17.029 | ||
| K3 | 17.598 | 17.961 | 17.661 | 17.726 | 17.876 | ||
| k1 | 5.841 | 5.844 | 5.677 | 5.558 | 5.892 | ||
| k2 | 5.82 | 5.696 | 5.962 | 6.061 | 5.676 | ||
| k3 | 5.866 | 5.987 | 5.887 | 5.909 | 5.959 | ||
| R | 0.046 | 0.291 | 0.285 | 0.503 | 0.067 |
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| A | 0.002 | 2 | 0.001 | 0.075 | 0.929 |
| B | 0.064 | 2 | 0.032 | 3.045 | 0.112 |
| C | 0.065 | 2 | 0.033 | 3.122 | 0.107 |
| D | 0.200 | 2 | 0.100 | 9.557 | 0.010 |
| E | 0.065 | 2 | 0.033 | 3.115 | 0.108 |
| Error | 0.073 | 7 | 0.01 | ||
| Total | 154.062 | 18 |
| NO | Observed Value | Predicted Yield | Relative Error % |
|---|---|---|---|
| 1 | 3.013 | 2.983 | 0.98 |
| 2 | 3.040 | 2.729 | 10.22 |
| 3 | 2.951 | 2.984 | 1.12 |
| 4 | 2.953 | 2.878 | 2.52 |
| 5 | 2.908 | 2.871 | 1.28 |
| 6 | 2.795 | 2.856 | 2.17 |
| 7 | 2.692 | 2.523 | 6.29 |
| 8 | 2.719 | 2.788 | 2.52 |
| 9 | 2.660 | 2.640 | 0.76 |
| 10 | 3.083 | 3.030 | 1.72 |
| 11 | 3.067 | 2.983 | 2.73 |
| 12 | 2.784 | 2.750 | 1.23 |
| 13 | 2.975 | 2.935 | 1.33 |
| 14 | 2.738 | 2.763 | 0.9 |
| 15 | 2.846 | 2.874 | 0.98 |
| 16 | 3.198 | 3.230 | 0.99 |
| 17 | 2.951 | 2.890 | 2.07 |
| 18 | 3.207 | 3.045 | 5.05 |
| Factor | Weight (%) |
|---|---|
| A | 16.90 |
| B | 21.80 |
| C | 20.19 |
| D | 21.90 |
| E | 19.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Cui, Y.; Lu, J.; Liu, C.; Chen, S.; Ma, C.; Liu, Z.; Wang, J.; Kang, W. Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC and Artificial Neural Network Analysis for Ganoderma lucidum. Molecules 2020, 25, 1309. https://doi.org/10.3390/molecules25061309
Li C, Cui Y, Lu J, Liu C, Chen S, Ma C, Liu Z, Wang J, Kang W. Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC and Artificial Neural Network Analysis for Ganoderma lucidum. Molecules. 2020; 25(6):1309. https://doi.org/10.3390/molecules25061309
Chicago/Turabian StyleLi, Changqin, Yiping Cui, Jie Lu, Cunyu Liu, Sitan Chen, Changyang Ma, Zhenhua Liu, Jinmei Wang, and Wenyi Kang. 2020. "Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC and Artificial Neural Network Analysis for Ganoderma lucidum" Molecules 25, no. 6: 1309. https://doi.org/10.3390/molecules25061309
APA StyleLi, C., Cui, Y., Lu, J., Liu, C., Chen, S., Ma, C., Liu, Z., Wang, J., & Kang, W. (2020). Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC and Artificial Neural Network Analysis for Ganoderma lucidum. Molecules, 25(6), 1309. https://doi.org/10.3390/molecules25061309






