Chitosan Oligosaccharides Attenuate Amyloid Formation of hIAPP and Protect Pancreatic β-Cells from Cytotoxicity
Abstract
:1. Introduction
2. Results
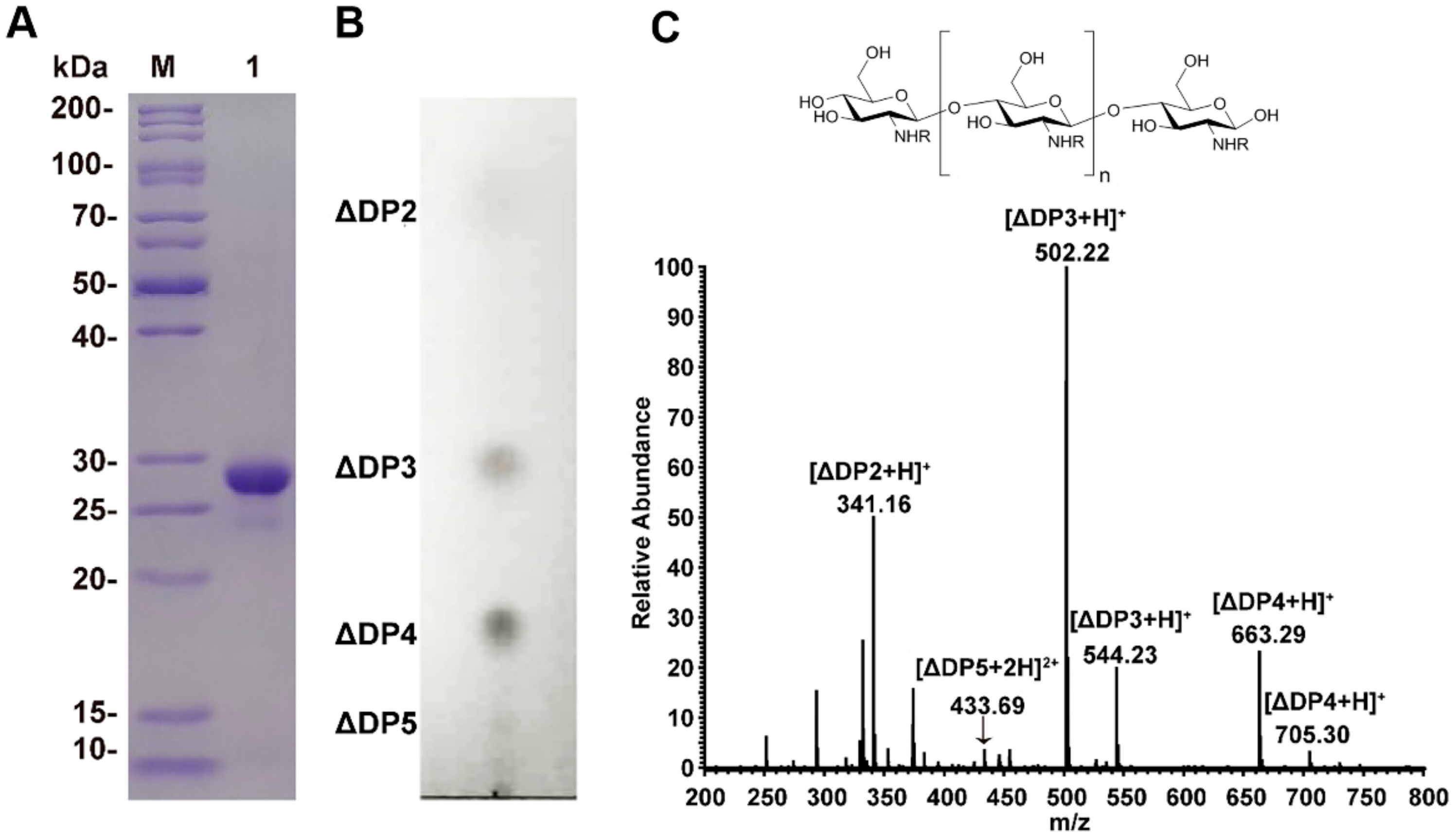
2.1. Chitosan Oligosaccharides Preparation
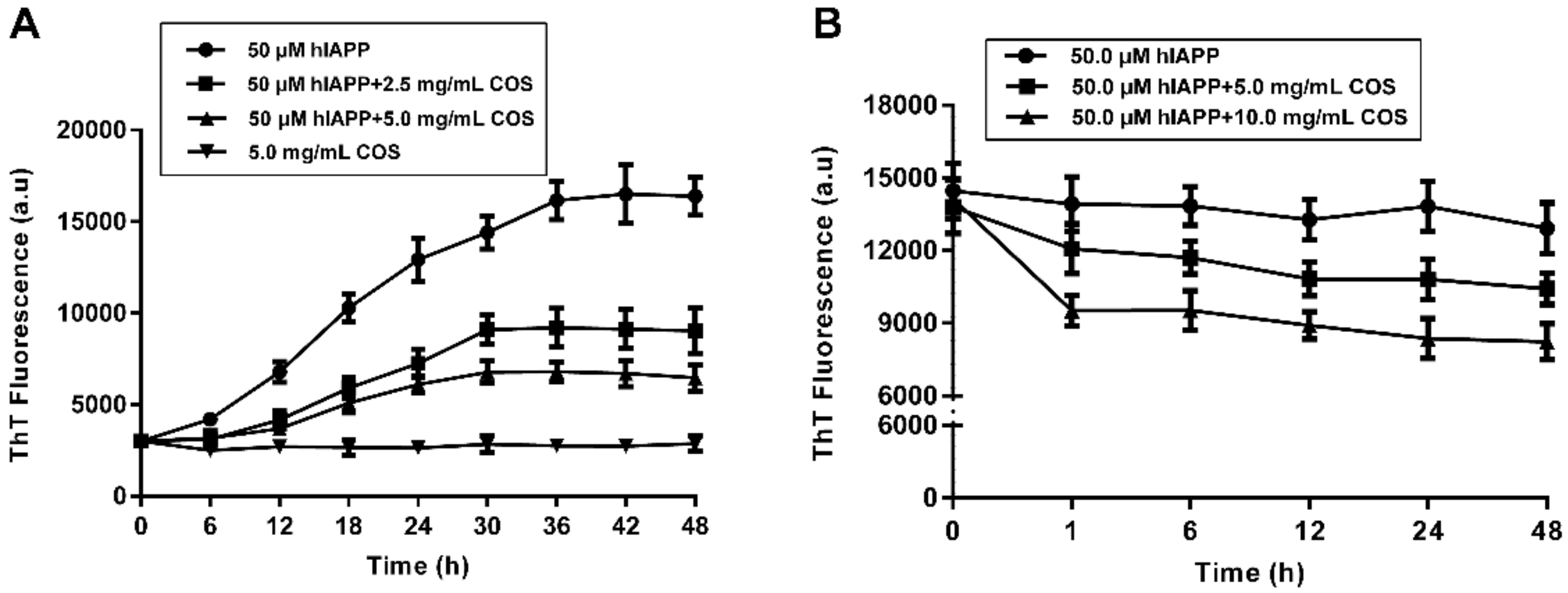
2.2. Fluorescent Assay of hIAPP Aggregation Influenced by COS
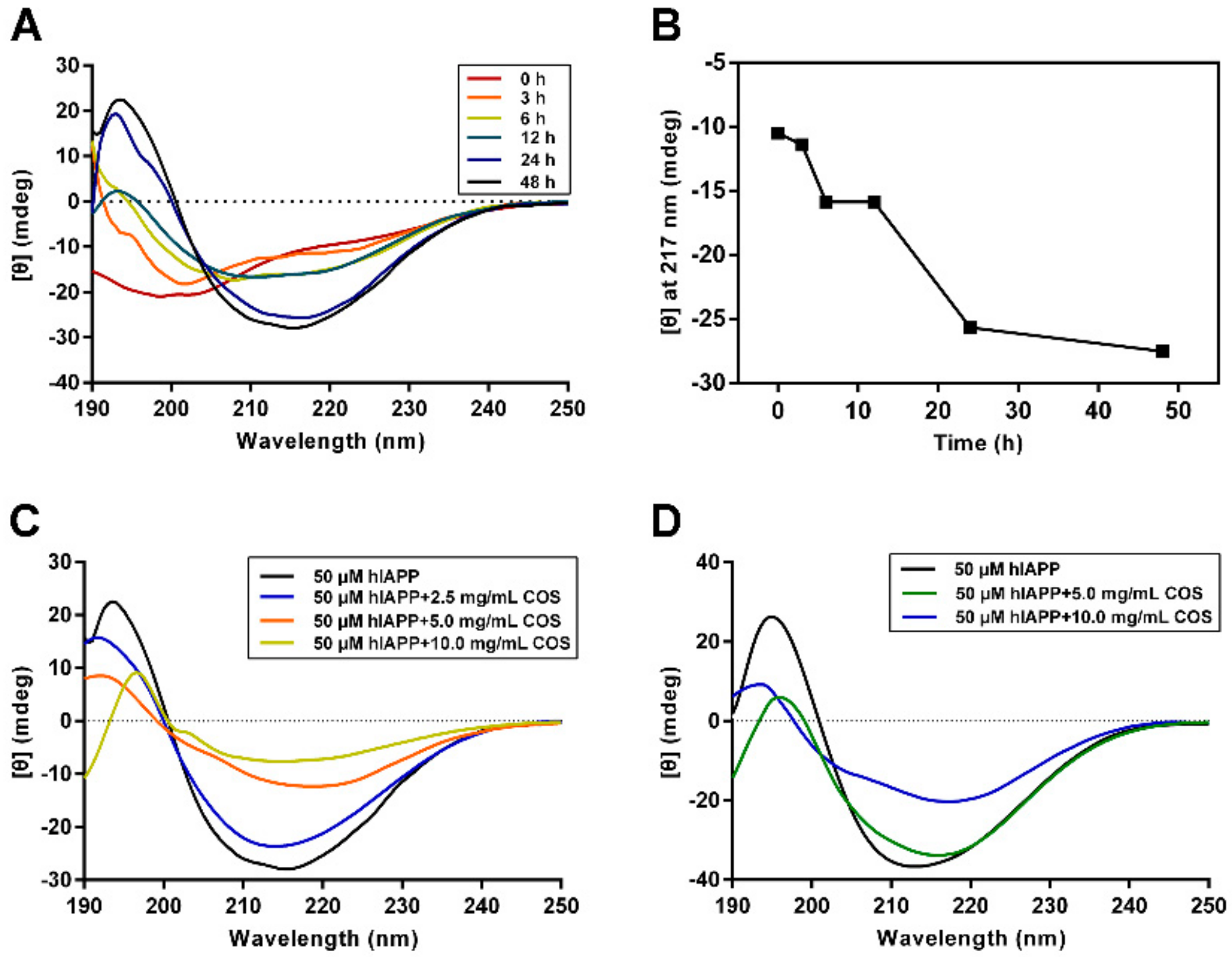
2.3. Secondary Structure Analysis of hIAPP Influenced by COS
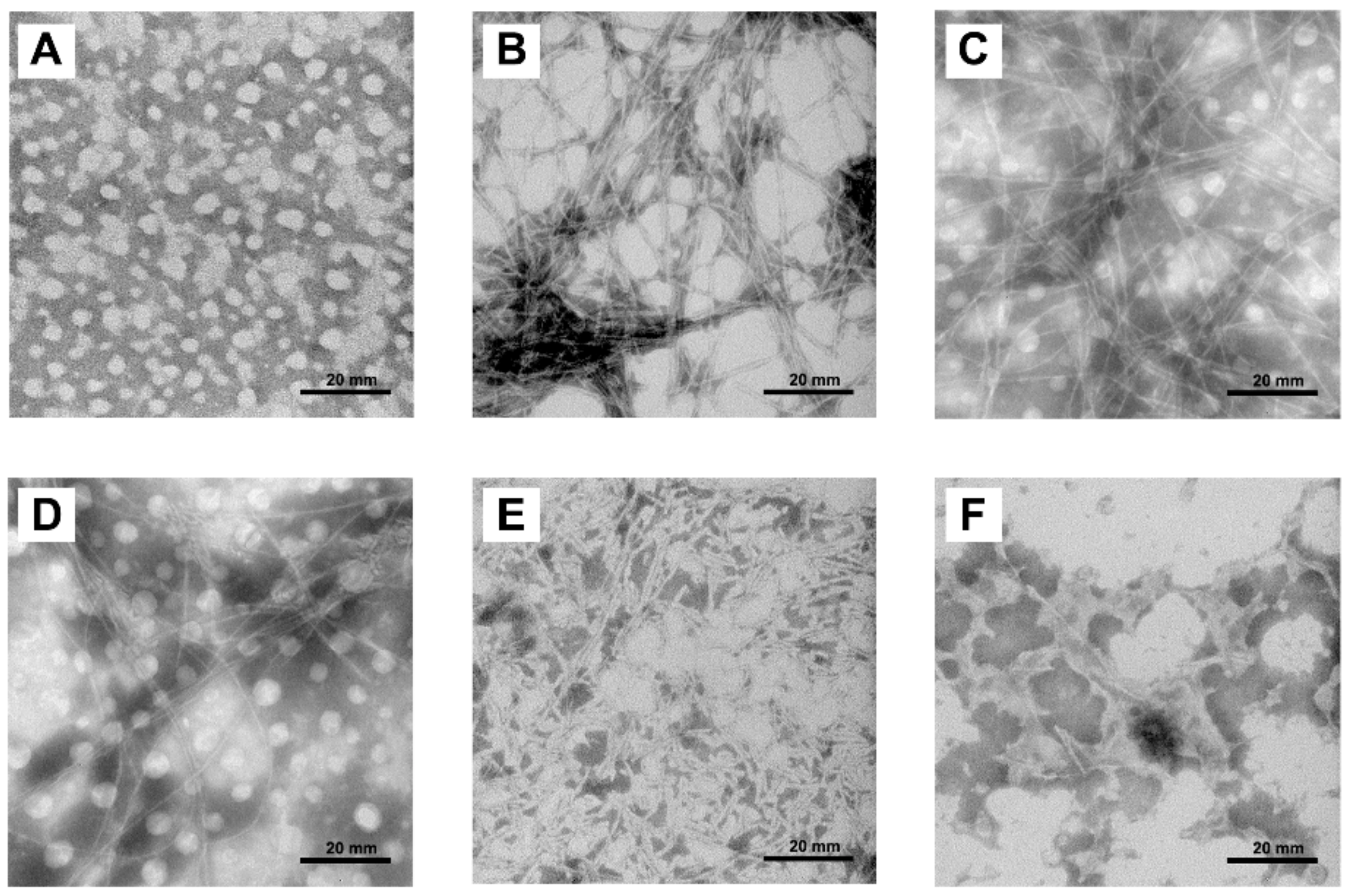
2.4. Morphologies of hIAPP Aggregates Visualized by Transmission Electron Microscope
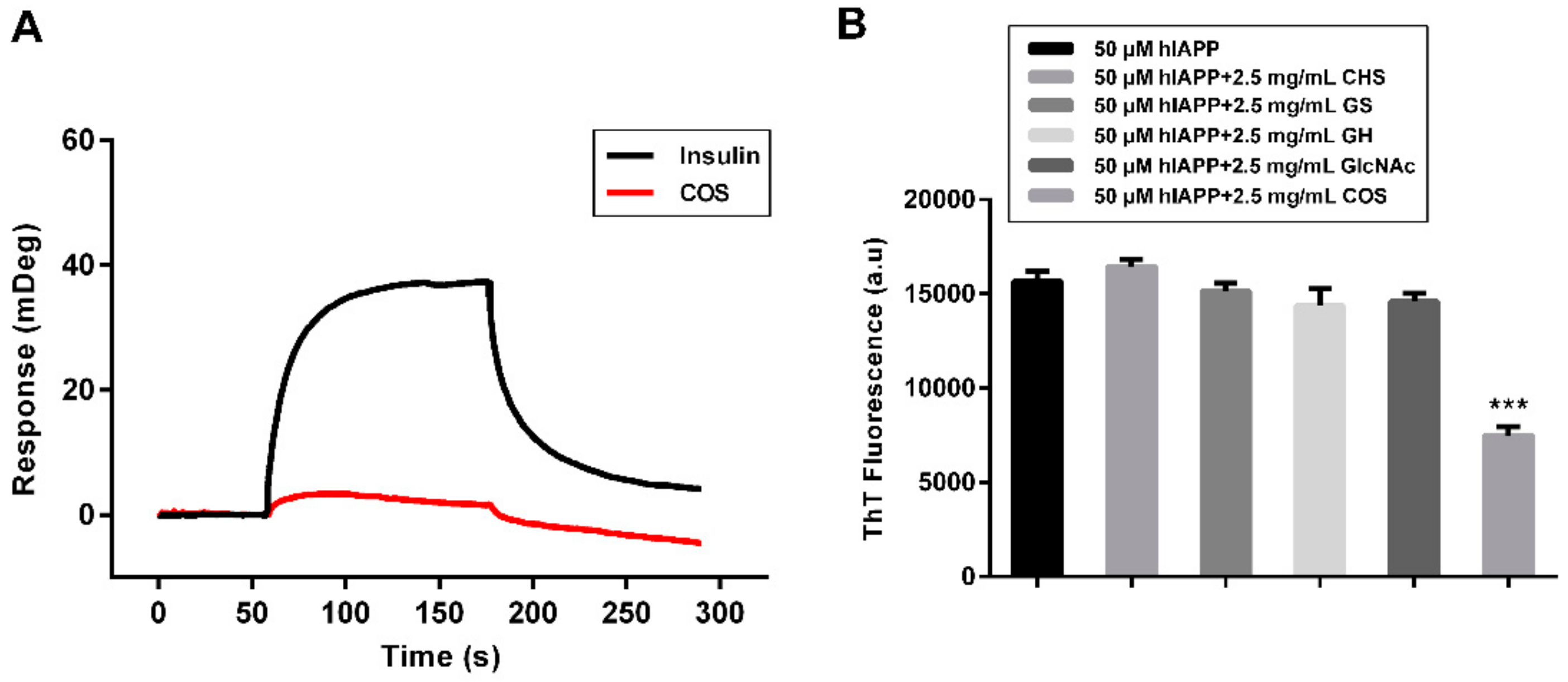
2.5. Mechanism Study of hIAPP Aggregation Influenced by COS
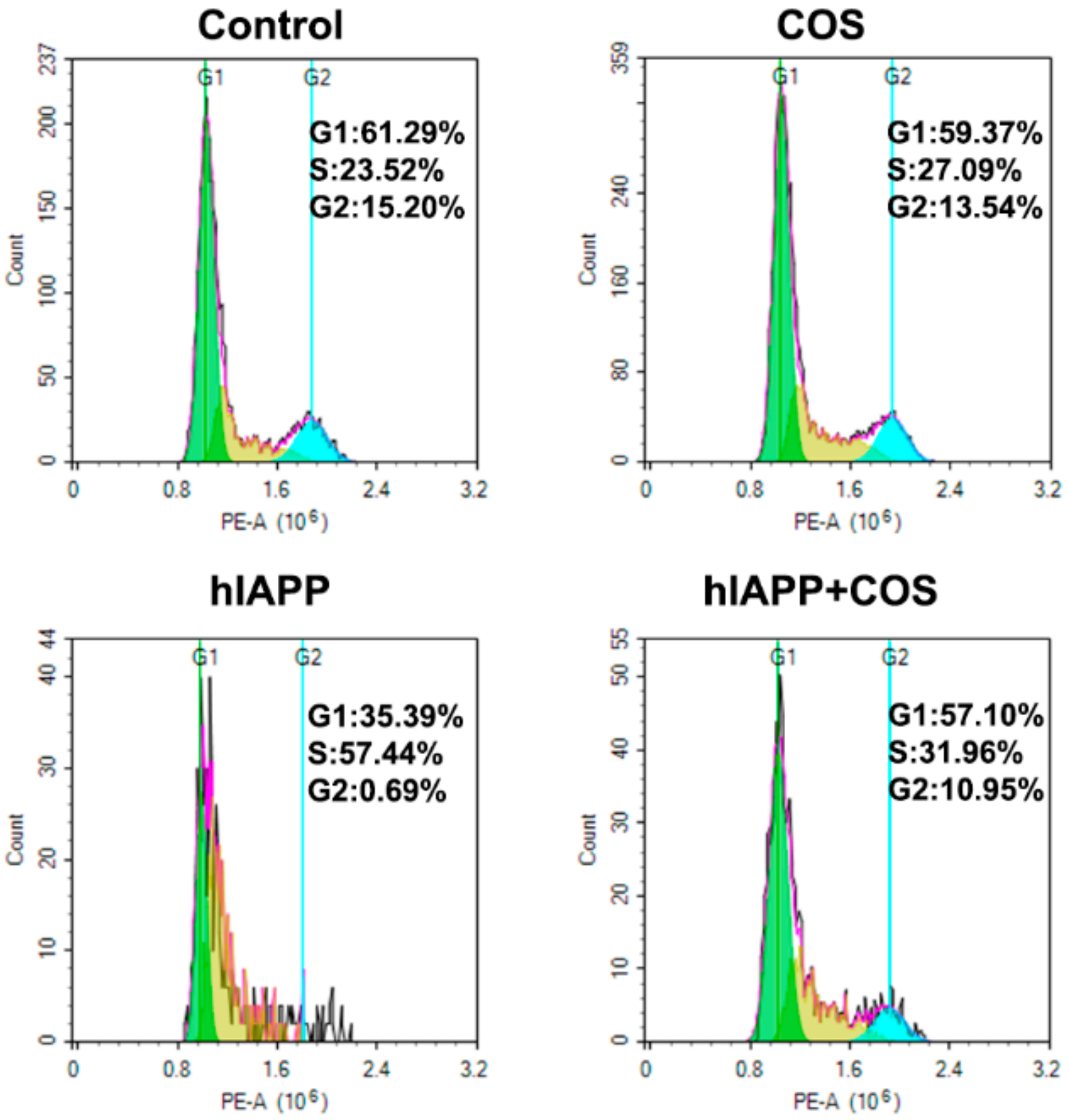
2.6. Effect of COS on hIAPP Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Purification of Recombinant Enzymes
4.3. Preparation of Chitosan Oligosaccharides
4.4. hIAPP Preparation and Aggregation
4.5. Thioflavine T (ThT) Fluorescence Assay
4.6. Circular Dichroism Spectroscopy
4.7. Transmission Electron Microscopy (TEM)
4.8. Cell Culture
4.9. Cytotoxicity Assay and Cell Cycle Analysis
4.10. Surface Plasmon Resonance (SPR)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spijker, H.S.; Song, H.; Ellenbroek, J.H.; Roefs, M.M.; Engelse, M.A.; Bos, E.; Koster, A.J.; Rabelink, T.J.; Hansen, B.C.; Clark, A.; et al. Loss of β-cell identity occurs in type 2 diabetes and is associated with islet amyloid deposits. Diabetes 2015, 64, 2928–2938. [Google Scholar] [CrossRef] [Green Version]
- Haataja, L.; Gurlo, T.; Huang, C.J.; Butler, P.C. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 2008, 29, 303–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes 2020. Diabetes Care 2020, 43, 14–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupi, R.; Del Prato, S. Beta-cell apoptosis in type 2 diabetes: Quantitative and functional consequences. Diabetes Metab. 2008, 34, 56–64. [Google Scholar] [CrossRef]
- Kanatsuka, A.; Kou, S.; Makino, H. IAPP/amylin and β-cell failure: Implication of the risk factors of type 2 diabetes. Diabetol. Int. 2018, 9, 143–157. [Google Scholar] [CrossRef]
- Lin, C.Y.; Gurlo, T.; Kayed, R.; Butler, A.E.; Haataja, L.; Glabe, C.G.; Butler, P.C. Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced beta-cell apoptosis in h-IAPP transgenic mice. Diabetes 2007, 56, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Jurgens, C.A.; Toukatly, M.N.; Fligner, C.L.; Udayasankar, J.; Subramanian, S.L.; Zraika, S.; Aston-Mourney, K.; Carr, D.B.; Westermark, P.; Westermark, G.T.; et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am. J. Pathol. 2011, 178, 2632–2640. [Google Scholar] [CrossRef] [Green Version]
- Raleigh, D.; Zhang, X.; Hastoy, B.; Clark, A. The β-cell assassin: IAPP cytotoxicity. J. Mol. Endocrinol. 2017, 59, 121–140. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, M.T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, Y.; Luo, X.; Wang, C.; Wang, N.; He, H.; Zhang, T.; Chen, L. Chitooligosaccharides modulate glucose-lipid metabolism by suppressing SMYD3 pathways and regulating gut microflora. Mar. Drugs 2020, 18, 69. [Google Scholar] [CrossRef] [Green Version]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Liu, S.H.; Cai, F.Y.; Chiang, M.T. Long-Term Feeding of chitosan ameliorates glucose and lipid metabolism in a high-fructose-diet-impaired rat model of glucose tolerance. Mar. Drugs 2015, 13, 7302–7313. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.N.; Chang, I.Y.; Kim, H.I.; Yoon, S.P. Long-term effects of chitosan oligosaccharide in streptozotocin-induced diabetic rats. Islets 2009, 1, 111–116. [Google Scholar] [CrossRef]
- Ju, C.; Yue, W.; Yang, Z.; Zhang, Q.; Yang, X.; Liu, Z.; Zhang, F. Antidiabetic effect and mechanism of chitooligosaccharides. Biol. Pharm. Bull. 2010, 33, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Han, Y.; Gao, P.; Yu, W.; Lu, X. N-Terminal seven-amino-acid extension simultaneously improves the pH stability, optimal temperature, thermostability and catalytic efficiency of chitosanase CsnA. Biotechnol. Lett. 2018, 40, 75–82. [Google Scholar] [CrossRef]
- Levine, H., III. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef]
- Di Natale, C.; La Manna, S.; Malfitano, A.M.; Di Somma, S.; Florio, D.; Scognamiglio, P.L.; Novellino, E.; Netti, P.A.; Marasco, D. Structural insights into amyloid structures of the C-terminal region of nucleophosmin 1 in type A mutation of acute myeloid leukemia. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 637–644. [Google Scholar] [CrossRef]
- Scognamiglio, P.L.; Di, N.C.; Leone, M.; Cascella, R.; Cecchi, C.; Lirussi, L.; Antoniali, G.; Riccardi, D.; Morelli, G.; Tell, G.; et al. Destabilisation, aggregation, toxicity and cytosolic mislocalisation of nucleophosmin regions associated with acute myeloid leukemia. Oncotarget 2016, 7, 59129–59143. [Google Scholar] [CrossRef] [Green Version]
- La Manna, S.; Scognamiglio, P.L.; Roviello, V.; Borbone, F.; Florio, D.; Di Natale, C.; Bigi, A.; Cecchi, C.; Cascella, R.; Giannini, C.; et al. The acute myeloid leukemia-associated Nucleophosmin 1 gene mutations dictate amyloidogenicity of the C-terminal domain. FEBS J. 2019, 286, 2311–2328. [Google Scholar] [CrossRef] [Green Version]
- Jarrett, J.T.; Lansbury, P.T., Jr. Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 1993, 73, 1055–1058. [Google Scholar] [CrossRef]
- Trusova, V.; Gorbenko, G. Modelization of amyloid fibril self-assembly. East Eur. J. Phys. 2018, 5, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Kumar, E.K.; Haque, N.; Prabhu, N.P. Kinetics of protein fibril formation: Methods and mechanisms. Int. J. Biol. Macromol. 2017, 100, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Lindman, S.; Minogue, A.M.; Thulin, E.; Walsh, D.M.; Dawson, K.A.; Linse, S. Inhibition of amyloid β protein fibrillation by polymeric nanoparticles. J. Am. Chem. Soc. 2008, 130, 15437–15443. [Google Scholar] [CrossRef]
- Dai, X.; Hou, W.; Sun, Y.; Gao, Z.; Zhu, S.; Jiang, Z. Chitosan oligosaccharides inhibit/disaggregate fibrils and attenuate amyloid β-mediated neurotoxicity. Int. J. Mol. Sci. 2015, 16, 10526–10536. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight into the kinetic of amyloid beta (1–42) peptide self-aggregation: Elucidation of inhibitors’ mechanism of action. Chembiochem 2007, 8, 2152–2161. [Google Scholar] [CrossRef]
- Bruggink, K.A.; Müller, M.; Kuiperij, H.B.; Verbeek, M.M. Methods for analysis of amyloid-β aggregates. J. Alzheimers Dis. 2012, 28, 735–758. [Google Scholar] [CrossRef]
- Blikstad, I.; Fägerstam, L.G.; Bhikhabhai, R.; Lindblom, H. Detection and characterization of oligosaccharides in column effluents using surface plasmon resonance. Anal. Biochem. 1996, 233, 42–49. [Google Scholar] [CrossRef]
- Wei, L.; Jiang, P.; Yau, Y.H.; Summer, H.; Shochat, S.G.; Mu, Y.; Pervushin, K. Residual structure in islet amyloid polypeptide mediates its interactions with soluble insulin. Biochemistry 2009, 48, 2368–2376. [Google Scholar] [CrossRef]
- Jaikaran, E.T.; Nilsson, M.R.; Clark, A. Pancreatic beta-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation. Biochem. J. 2004, 377, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Shamseddeen, H.; Getty, J.Z.; Hamdallah, I.N.; Ali, M.R. Epidemiology and economic impact of obesity and type 2 diabetes. Surg. Clin. N. Am. 2011, 91, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-β1-42-induced rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Chang, P.; Zhu, Q.; Liu, W.; Sun, Y.; Zhu, S.; Jiang, Z. Chitosan oligosaccharides protect rat primary hippocampal neurons from oligomeric β-amyloid 1-42-induced neurotoxicity. Neurosci. Lett. 2013, 554, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Saafi, E.L.; Cooper, G.J. Induction of apoptosis by human amylin in RINm5F islet β-cells is associated with enhanced expression of p53 and p21WAF1/CIP1. FEBS Lett. 1999, 455, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Ritzel, R.A.; Meier, J.J.; Lin, C.Y.; Veldhuis, J.D.; Butler, P.C. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes 2007, 56, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Song, Y.; Liu, W.; Wang, K.; Gao, Y.; Li, S.; Duan, Z.; Shao, Z.; Yang, S.; Yang, C. IAPP modulates cellular autophagy, apoptosis, and extracellular matrix metabolism in human intervertebral disc cells. Cell Death Discov. 2017, 30, 16107. [Google Scholar] [CrossRef]
- Yuan, W.P.; Liu, B.; Liu, C.H.; Wang, X.J.; Zhang, M.S.; Meng, X.M.; Xia, X.K. Antioxidant activity of chito-oligosaccharides on pancreatic islet cells in streptozotocin-induced diabetes in rats. World J. Gastroenterol. 2009, 15, 1339–1345. [Google Scholar] [CrossRef]
- Han, Y.; Gao, P.; Yu, W.; Lu, X. Thermostability enhancement of chitosanase CsnA by fusion a family 5 carbohydrate-binding module. Biotechnol. Lett. 2017, 39, 1895–1901. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds, COS are available from the authors. |

| Sample | t1/2 (h)1 | Lag Time (h)2 | k (h−1)3 | Maximum Intensity (a.u.) 4 |
|---|---|---|---|---|
| hIAPP | 14.83 ± 0.96 | 4.30 ± 0.36 | 0.19 ± 0.01 | 16060 ± 206.40 |
| hIAPP + COS-2.5 mg/mL | 17.63 ± 0.23 | 9.00 ± 0.43 | 0.23 ± 0.012 | 8640 ± 226.50 |
| hIAPP + COS-5.0 mg/mL | 15.91 ± 0.76 | 8.47 ± 0.80 | 0.28 ± 0.03 | 6410 ± 124.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.-Y.; Wang, H.; Cui, Z.-B.; Yu, W.-G.; Lu, X.-Z. Chitosan Oligosaccharides Attenuate Amyloid Formation of hIAPP and Protect Pancreatic β-Cells from Cytotoxicity. Molecules 2020, 25, 1314. https://doi.org/10.3390/molecules25061314
Meng Q-Y, Wang H, Cui Z-B, Yu W-G, Lu X-Z. Chitosan Oligosaccharides Attenuate Amyloid Formation of hIAPP and Protect Pancreatic β-Cells from Cytotoxicity. Molecules. 2020; 25(6):1314. https://doi.org/10.3390/molecules25061314
Chicago/Turabian StyleMeng, Qin-Yu, Hua Wang, Zi-Bo Cui, Wen-Gong Yu, and Xin-Zhi Lu. 2020. "Chitosan Oligosaccharides Attenuate Amyloid Formation of hIAPP and Protect Pancreatic β-Cells from Cytotoxicity" Molecules 25, no. 6: 1314. https://doi.org/10.3390/molecules25061314




