Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin
Abstract
:1. Introduction
2. Results
2.1. Identification of Compounds
2.2. Cytotoxic Activity
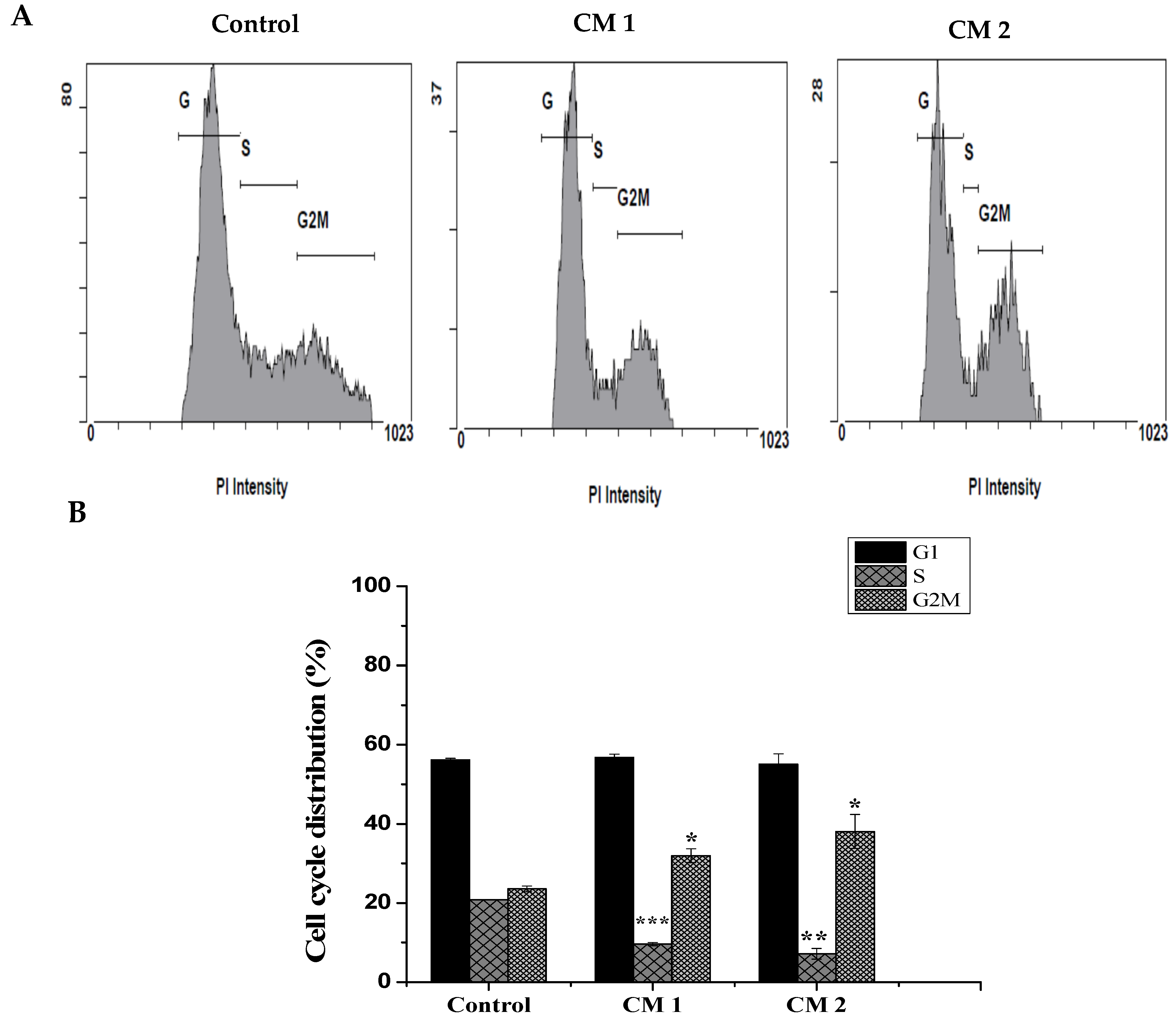
2.3. Cell Cycle Analysis
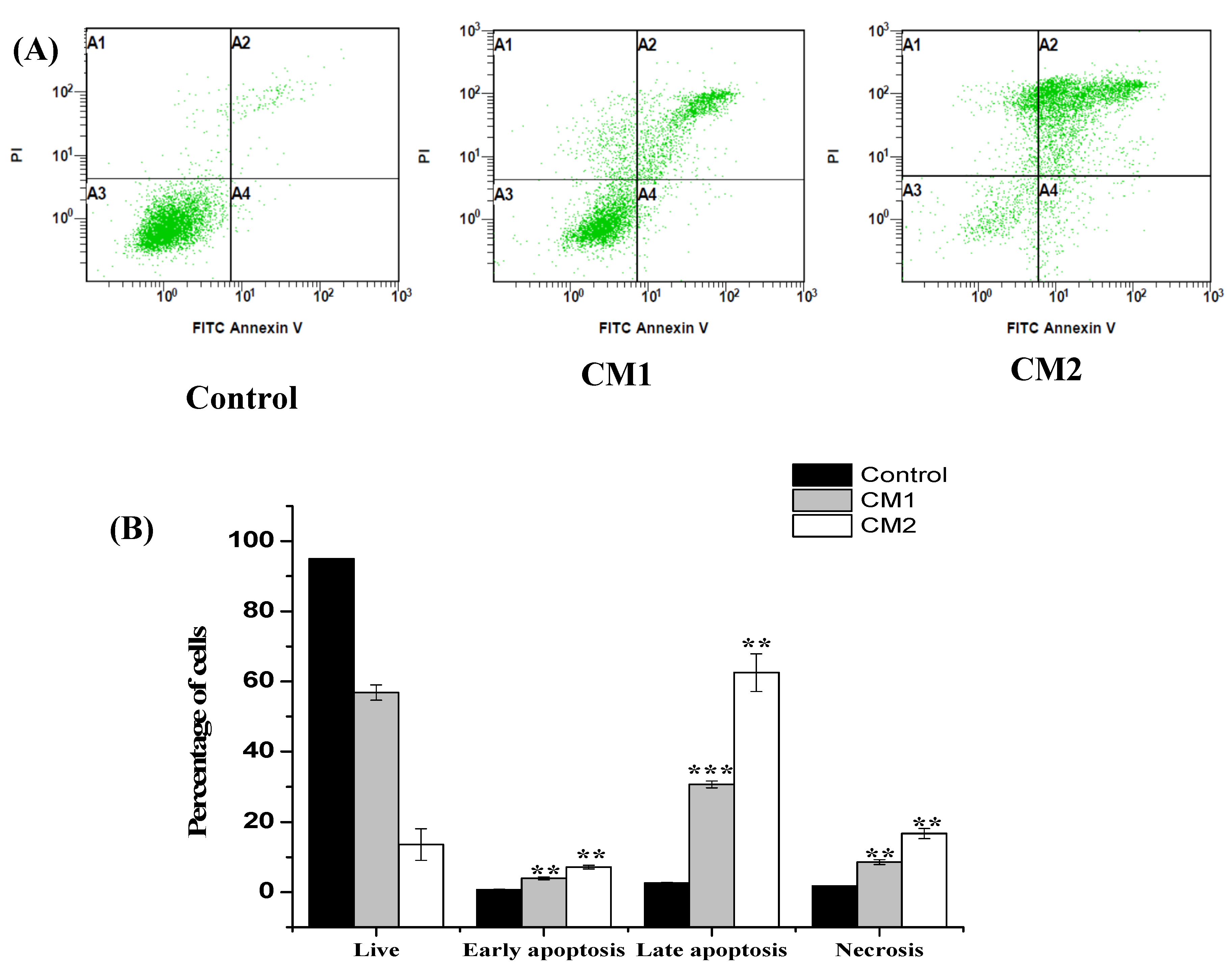
2.4. Apoptosis/Necrosis Assessment Using Flow Cytometry
2.5. Developmental Toxicity of 2-Methoxyfuranodiene and 2-Acetoxyfuranodiene in Zebrafish Embryos
2.6. 2-Methoxyfuranodiene and 2-Acetoxyfuranodiene Inhibited the Formation of Angiogenic Blood Vessels during Zebrafish Embryonic Development
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection and Compound Isolation
4.1.1. Preparation of the Crude Extracts
4.1.2. Compound Isolation and Identification
4.2. In Vitro Biochemical Studies
4.2.1. Cytotoxic Activity Test for the Isolated Compounds (MTT Assay)
4.2.2. Apoptosis Detection by Flow Cytometry (Annexin V-FITC/PI Staining)
4.2.3. Cell Cycle Phase Analysis
4.3. In Vivo Toxicity Assessment Using Zebrafish Embryos
4.3.1. Animals
4.3.2. Toxicity Screening of Zebrafish Embryos
4.3.3. Angiogenesis
4.3.4. Microscopy and Imaging
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- Poonia, P.; Mittal, S.; Gupta, V.; Singh, J.; Sweety. Gum guggul: An Ayurvedic boom. Int. J. Pharmacogn Phytochem Res. 2014, 6, 347–354. [Google Scholar]
- Ojha, S.; Bhatia, J.; Arora, S.; Golechha, M.; Kumari, S.; Arya, D.S. Cardioprotective effects of Commiphora mukul against isoprenaline-induced cardiotoxicity: A biochemical and histopathological evaluation. J. Environ. Biol. 2011, 32, 731–738. [Google Scholar]
- Gebrehiwot, M.; Asres, K.; Bisrat, D.; Mazumder, A.; Lindemann, P.; Bucar, F. Evaluation of the wound healing property of Commiphora guidottii Chiov. Ex. Guid. BMC Complement. Altern Med. 2015, 15, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamad, S.R.; Al-Ghadeer, A.R.; Ali, R.; Qamar, W.; Aljarboa, S. Analysis of inorganic and organic constituents of myrrh resin by GC-MS and ICP-MS: An emphasis on medicinal assets. Saudi Pharm. J. 2017, 25, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Al-Anazi, K.M.; Mahmoud, A.H.; Farah, M.A.; Allam, A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kweyamba, P.A.; Zofou, D.; Efange, N.; Assob, J.N.; Kitau, J.; Nyindo, M. In vitro and in vivo studies on anti-malarial activity of Commiphora africana and Dichrostachys cinerea used by the Maasai in Arusha region, Tanzania. Malar. J. 2019, 18, 119. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Li, G.H.; Wang, X.N.; Lou, H.X. The genus Commiphora: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2012, 142, 319–330. [Google Scholar] [CrossRef]
- Lamichhane, R.; Lee, K.H.; Pandeya, P.R.; Sung, K.K.; Lee, S.; Kim, Y.K.; Jung, H.J. Subcutaneous injection of myrrh essential oil in mice: Acute and subacute toxicity study. Evid Based Complem. Alternat Med. 2019, 2019, 8497980. [Google Scholar] [CrossRef] [Green Version]
- Al-Jaroudi, D.; Kaddour, O.; Al-Amin, N. Risks of myrrh usage in pregnancy. JBRA Assist. Reprod. 2016, 20, 257–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoganantharjah, P.; Gibert, Y. The use of the zebrafish model to aid in drug discovery and target validation. Curr. Top. Med. Chem. 2017, 17, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
- d’Amora, M.; Giordani, S. The utility of zebrafish as a model for screening developmental neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Koiwa, J. Next generation zebrafish-based drug discovery and precision medicine. Nihon Yakurigaku Zasshi 2019, 154, 78–83. [Google Scholar] [CrossRef]
- Heinz Brieskorn, C.; Noble, P. Furanosesquiterpenes from the essential oil of myrrh. Phytochemistry 1983, 22, 1207–1211. [Google Scholar] [CrossRef]
- Tonkal, A.M.; Morsy, T.A. An update review on Commiphora molmol and related species. J. Egypt. Soc. Parasitol. 2008, 38, 763–796. [Google Scholar]
- Shaik, J.; Vishakha, K.; Ramyasree, D. Evaluation of antibacterial activity of Commiphora myrrha against antibiotic resistant clinical pathogens. IJPBR 2015, 3, 7. [Google Scholar] [CrossRef]
- Tao, S.; Hong-xiang, L. Chemical constituents from resin of Commiphora species and their biological activities. NPRD 2008, 20. [Google Scholar]
- Su, S.; Wang, T.; Duan, J. Distribution, chemical components and bioactivity of resin herbs: Research advances. IJPR 2009, 36, 109–114. [Google Scholar]
- Zhu, N.; Sheng, S.; Sang, S.; Rosen, R.T.; Ho, C.T. Isolation and characterization of several aromatic sesquiterpenes from Commiphora myrrha. Flavour Fragr. J. 2003, 18, 282–285. [Google Scholar] [CrossRef]
- Maradufu, A. Furanosesquiterpenoids of Commiphora erythraea and C. myrrh. Phytochemistry 1982, 21, 677–680. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19. [Google Scholar]
- Safarzadeh, E.; Sandoghchian Shotorbani, S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and beyond: Cytometry in studies of programmed cell death. Methods Cell Biol. 2011, 103, 55–98. [Google Scholar]
- Gabrielli, B.; Brooks, K.; Pavey, S. Defective cell cycle checkpoints as targets for anti-cancer therapies. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Su, X.; Dong, X.; Chen, Y.; Zhou, C.; Xin, P.; Yu, C.; Wei, T. Cycloartan-24-ene-1alpha,2alpha,3beta-triol, a cycloartane-type triterpenoid from the resinous exudates of Commiphora myrrha, induces apoptosis in human prostatic cancer PC-3 cells. Oncol Rep. 2015, 33, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Dolara, P.; Luceri, C.; Ghelardini, C.; Monserrat, C.; Aiolli, S.; Luceri, F.; Lodovici, M.; Menichetti, S.; Romanelli, M.N. Analgesic effects of myrrh. Nature 1996, 379, 29. [Google Scholar] [CrossRef]
- Fraternale, D.; Sosa, S.; Ricci, D.; Genovese, S.; Messina, F.; Tomasini, S.; Montanari, F.; Marcotullio, M.C. Anti-inflammatory, antioxidant and antifungal furanosesquiterpenoids isolated from Commiphora erythraea (Ehrenb). Engl. Resin. Fitoterapia 2011, 82, 654–661. [Google Scholar] [CrossRef]
- Rajaram, S.; Ramulu, U.; Ramesh, D.; Srikanth, D.; Bhattacharya, P.; Prabhakar, P.; Kalivendi, S.V.; Babu, K.S.; Venkateswarlu, Y.; Navath, S. Anti-cancer evaluation of carboxamides of furano-sesquiterpene carboxylic acids from the soft coral Sinularia kavarattiensis. Bioorg. Med. Chem. Lett. 2013, 23, 6234–6238. [Google Scholar] [CrossRef] [PubMed]
- Arepalli, S.K.; Sridhar, V.; Venkateswara Rao, J.; Kavin Kennady, P.; Venkateswarlu, Y. Furano-sesquiterpene from soft coral, Sinularia kavarittiensis: Induces apoptosis via the mitochondrial-mediated caspase-dependent pathway in THP-1, leukemia cell line. Apoptosis 2009, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Kithcart, A.; MacRae, C.A. Using zebrafish for high-throughput screening of novel cardiovascular drugs. JACC Basic Transl. Sci. 2017, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.S.; Redfield, S.E.; Zon, L.I. Chemical screening in zebrafish for novel biological and therapeutic discovery. Methods Cell Biol. 2017, 138, 651–679. [Google Scholar] [PubMed] [Green Version]
- Dang, M.; Fogley, R.; Zon, L.I. Identifying novel cancer therapies using chemical genetics and zebrafish. Adv. Exp. Med. Biol. 2016, 916, 103–124. [Google Scholar] [PubMed]
- Allocco, A.A.; Jin, S.C.; Duy, P.Q.; Furey, C.G.; Zeng, X.; Dong, W.; Nelson-Williams, C.; Karimy, J.K.; DeSpenza, T.; Hao, L.T.; et al. Recessive inheritance of congenital hydrocephalus with other structural brain abnormalities caused by compound heterozygous mutations in ATP1A3. Front. Cell Neurosci. 2019, 13, 425. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Capela, R.; Rodolfo, J.; Fonseca, E.; Montes, R.; Andre, A.; Capitao, A.; Carvalho, A.P.; Quintana, J.B.; Castro, L.F.C.; et al. Linking chemical exposure to lipid homeostasis: A municipal waste water treatment plant influent is obesogenic for zebrafish larvae. Ecotoxicol. Environ. Saf. 2019, 182, 109406. [Google Scholar] [CrossRef]
- Castro-Sanchez, S.; Suarez-Bregua, P.; Novas, R.; Alvarez-Satta, M.; Badano, J.L.; Rotllant, J.; Valverde, D. Functional analysis of new human Bardet-Biedl syndrome loci specific variants in the zebrafish model. Sci. Rep. 2019, 9, 12936. [Google Scholar] [CrossRef]
- Cavodeassi, F.; Wilson, S.W. Looking to the future of zebrafish as a model to understand the genetic basis of eye disease. Hum. Genet. 2019, 138, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Poujol de Molliens, M.; Jamadagni, P.; Letourneau, M.; Devost, D.; Hebert, T.E.; Patten, S.A.; Fournier, A.; Chatenet, D. Design and biological assessment of membrane-tethering neuroprotective peptides derived from the pituitary adenylate cyclase-activating polypeptide type 1 receptor. BBA-Gen. Subj. 2019, 1863, 129398. [Google Scholar] [CrossRef]
- Doro, E.; Jacobs, S.H.; Hammond, F.R.; Schipper, H.; Pieters, R.P.; Carrington, M.; Wiegertjes, G.F.; Forlenza, M. Visualizing trypanosomes in a vertebrate host reveals novel swimming behaviours, adaptations and attachment mechanisms. Elife 2019, 8. [Google Scholar]
- Fulmer, D.; Toomer, K.; Guo, L.; Moore, K.; Glover, J.; Moore, R.; Stairley, R.; Lobo, G.; Zuo, X.; Dang, Y.; et al. Defects in the exocyst-cilia machinery cause bicuspid aortic valve disease and aortic stenosis. Circulation 2019, 140, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, S.; Kersseboom, S.; van den Berge, A.; Dolcetta-Capuzzo, A.; van Geest, F.S.; van Heerebeek, R.E.A.; Arjona, F.J.; Meima, M.E.; Peeters, R.P.; Visser, W.E.; et al. In vitro characterization of human, mouse, and zebrafish MCT8 orthologues. Thyroid 2019, 29, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Haerlingen, B.; Opitz, R.; Vandernoot, I.; Trubiroha, A.; Gillotay, P.; Giusti, N.; Costagliola, S. Small-molecule screening in zebrafish embryos identifies signaling pathways regulating early thyroid development. Thyroid 2019, 29, 1683–1703. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Zha, J. Fish behavior: A promising model for aquatic toxicology research. Sci. Total Environ. 2019, 686, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wen Jun, L.; Pit Foong, C.; Abd Hamid, R. Ardisia crispa root hexane fraction suppressed angiogenesis in human umbilical vein endothelial cells (HUVECs) and in vivo zebrafish embryo model. Biomed. Pharmacother 2019, 118, 109221. [Google Scholar] [CrossRef]
- Konantz, M.; Schurch, C.; Hanns, P.; Muller, J.S.; Sauteur, L.; Lengerke, C. Modeling hematopoietic disorders in zebrafish. Dis. Model. Mech. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Kwon, R.Y.; Watson, C.J.; Karasik, D. Using zebrafish to study skeletal genomics. Bone 2019, 126, 37–50. [Google Scholar] [CrossRef]
- Lin, Y.; Xiang, X.; Chen, T.; Mao, G.; Deng, L.; Zeng, L.; Zhang, J. In vivo monitoring the dynamic process of acute retinal hemorrhage and repair in zebrafish with spectral-domain optical coherence tomography. J. Biophotonics 2019, e201900235. [Google Scholar] [CrossRef]
- Pott, A.; Rottbauer, W.; Just, S. Streamlining drug discovery assays for cardiovascular disease using zebrafish. Expert Opin. Drug Dis. 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Yimer, T.A.; Xie, S.; Wong, F.; Yu, S.; Liu, X.; Han, S.; Ma, J.; Lu, Z.; Hu, X.; et al. Knocking out lca5 in zebrafish causes cone-rod dystrophy due to impaired outer segment protein trafficking. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2694–2705. [Google Scholar] [CrossRef] [PubMed]
- Schutera, M.; Just, S.; Gierten, J.; Mikut, R.; Reischl, M.; Pylatiuk, C. Machine learning methods for automated quantification of ventricular dimensions. Zebrafish 2019, 16, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, X.; Qin, W.; Jiang, J.; Cheng, L. Emerging regulatory mechanisms and functions of autophagy in fish. Aquaculture 2019, 511, 734212. [Google Scholar] [CrossRef]
- Cao, B.; Wei, X.C.; Xu, X.R.; Zhang, H.Z.; Luo, C.H.; Feng, B.; Xu, R.C.; Zhao, S.Y.; Du, X.J.; Han, L.; et al. Seeing the unseen of the combination of two natural resins, frankincense and myrrh: Changes in chemical constituents and pharmacological activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Zaki, A.R.; Hassan, M.E.; Mostafa-Hedeab, G. Commiphora molmol resin attenuates diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis by modulating oxidative stress, inflammation, angiogenesis and Nrf2/ARE/HO-1 signaling. Chem. Biol. Interact. 2017, 270, 41–50. [Google Scholar] [CrossRef]
- Pang, X.; Yi, Z.; Zhang, X.; Sung, B.; Qu, W.; Lian, X.; Aggarwal, B.B.; Liu, M. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009, 69, 5893–5900. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.R.; Prasad, S.; Sung, B.; Gelovani, J.G.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by downregulating inflammatory, proliferative, invasive and angiogenic biomarkers. Int. J. Cancer 2012, 130, 2176–2184. [Google Scholar] [CrossRef] [Green Version]
- Chavez, M.N.; Aedo, G.; Fierro, F.A.; Allende, M.L.; Egana, J.T. Zebrafish as an emerging model organism to study angiogenesis in development and regeneration. Front. Physiol. 2016, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Serbedzija, G.N.; Flynn, E.; Willett, C.E. Zebrafish angiogenesis: A new model for drug screening. Angiogenesis 1999, 3, 353–359. [Google Scholar] [CrossRef]
- Dhoble, S.; Patravale, V. Development of anti-angiogenic erlotinib liposomal formulation for pulmonary hypertension: A QbD approach. Drug Deliv. Transl. Res. 2019, 9, 980–996. [Google Scholar] [CrossRef]
- Lu, F.I.; Wang, Y.T.; Wang, Y.S.; Wu, C.Y.; Li, C.C. Involvement of BIG1 and BIG2 in regulating VEGF expression and angiogenesis. Faseb. J. 2019, 33, 9959–9973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish Danio (“Brachydanio Rerio”); University of Oregon: Eugene, Oregon, 2007. [Google Scholar]
- Khan, M.F.; Abutaha, N.; Nasr, F.A.; Alqahtani, A.S.; Noman, O.M.; Wadaan, M.A.M. Bitter gourd (Momordica charantia) possess developmental toxicity as revealed by screening the seeds and fruit extracts in zebrafish embryos. BMC Complement. Altern. Med. 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

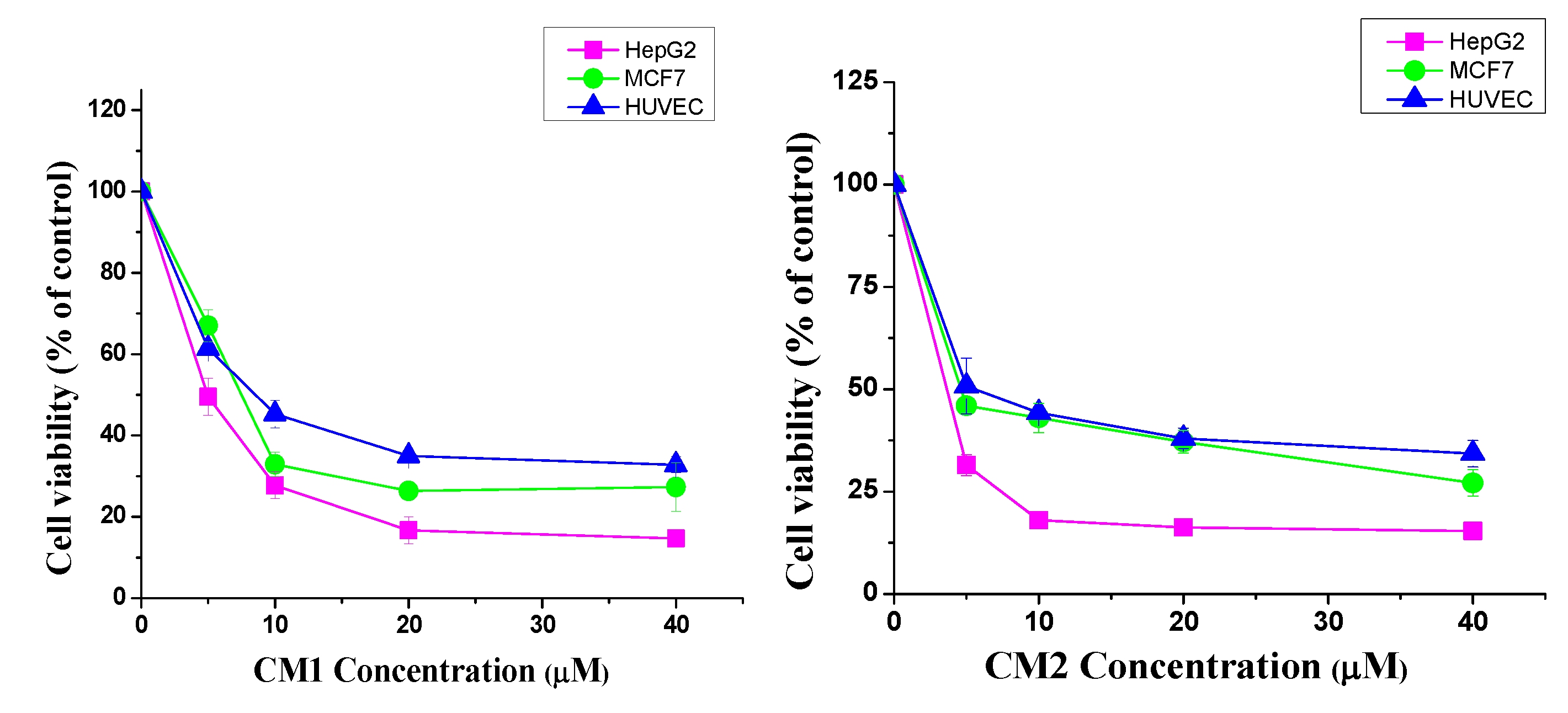


| Extract/Compound | Cell Lines and IC50 (µM) | ||
|---|---|---|---|
| HepG2 | MCF-7 | HUVEC | |
| CM1 | 4.8 ± 0.3 | 7.5 ± 0.2 | 8.5 ± 0.9 |
| CM2 | 3.6 ± 0.6 | 4.4 ± 0.2 | 4.9 ± 1.1 |
| Vinblastine | 1.4 ± 0.2 | 1.2 ± 0.3 | 3.1 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. Alqahtani, A.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alhawassi, T.; Qamar, W.; El-Gamal, A. Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin. Molecules 2020, 25, 1318. https://doi.org/10.3390/molecules25061318
S. Alqahtani A, Nasr FA, Noman OM, Farooq M, Alhawassi T, Qamar W, El-Gamal A. Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin. Molecules. 2020; 25(6):1318. https://doi.org/10.3390/molecules25061318
Chicago/Turabian StyleS. Alqahtani, Ali, Fahd A. Nasr, Omar M. Noman, Muhammad Farooq, Tariq Alhawassi, Wajhul Qamar, and Ali El-Gamal. 2020. "Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin" Molecules 25, no. 6: 1318. https://doi.org/10.3390/molecules25061318
APA StyleS. Alqahtani, A., Nasr, F. A., Noman, O. M., Farooq, M., Alhawassi, T., Qamar, W., & El-Gamal, A. (2020). Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin. Molecules, 25(6), 1318. https://doi.org/10.3390/molecules25061318






