A Halogen Bonding Perspective on Iodothyronine Deiodinase Activity
Abstract
:1. Introduction
2. Summary of XB Models Related to Dio Activity
2.1. Thyroid Hormone (TH) I···Se XB Interactions
2.2. Polybrominated Diphenyl Ether (PBDE) Br···Se XB Interactions
2.3. Polychlorinated Biphenyl (PCB) Cl···Se XB Interactions
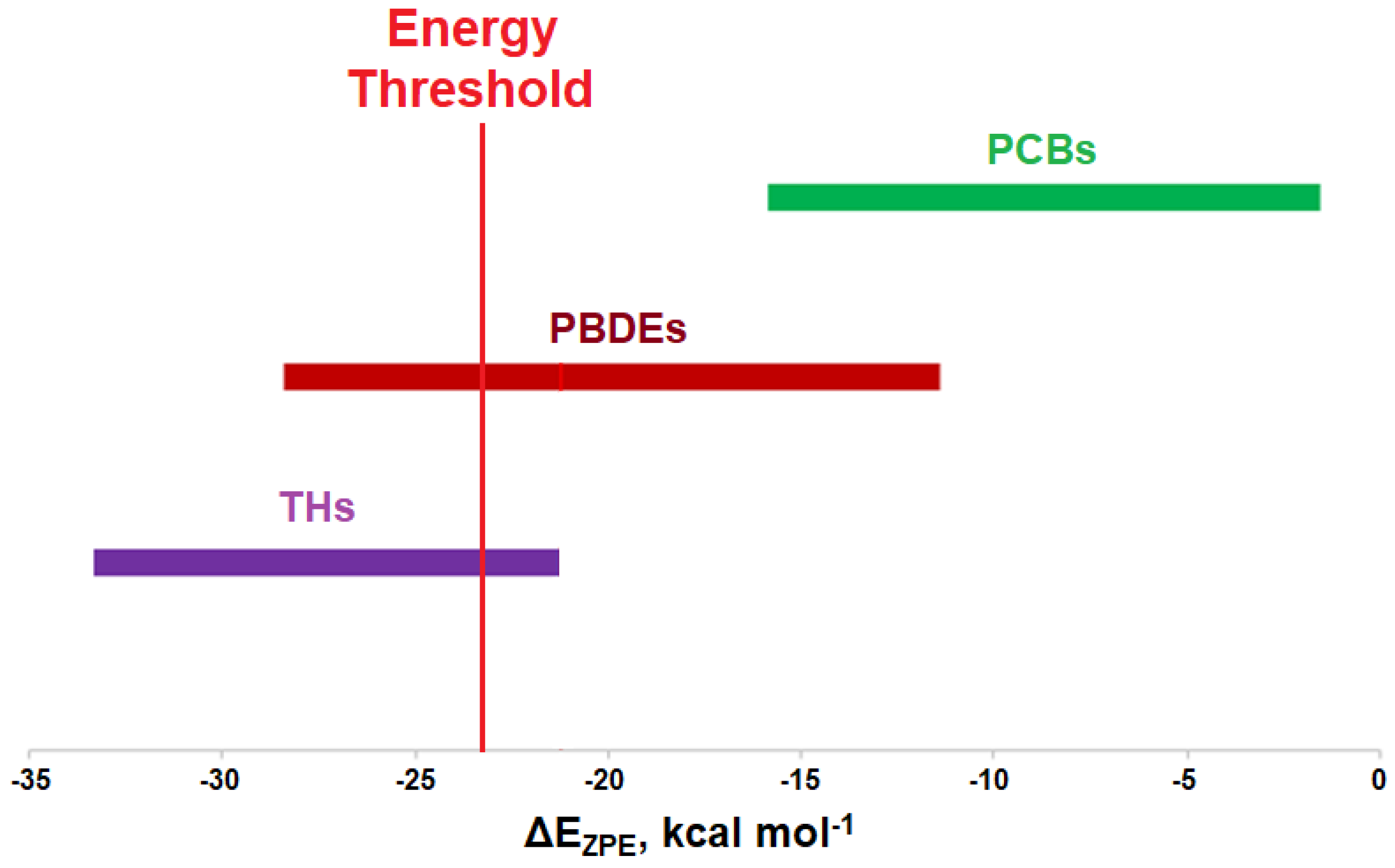
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mondal, S.; Raja, K.; Schweizer, U.; Mugesh, G. Chemistry and Biology in the Biosynthesis and Action of Thyroid Hormones. Angew. Chem. Int. Edit. 2016, 55, 7606–7630. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, U.; Steegborn, C. New insights into the structure and mechanism of iodothyronine deiodinases. J. Mol. Endocrinol. 2015, 55, 37–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, Cellular and Molecular Biology, and Physiological Roles of the Iodothyronine Selenodeiodinases. Endocr. Rev. 2002, 23, 38. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Endocrinol. Invest. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [Green Version]
- Köhrle, J. The Colorful Diversity of Thyroid Hormone Metabolites. Eur. Thyroid J. 2019, 8, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, M.; Libert, F.; Maenhaut, C.; Lefort, A.; Gerard, C.; Perret, J.; Van Sande, J.; Dumont, J.; Vassart, G. Molecular cloning of the thyrotropin receptor. Science 1989, 246, 1620–1622. [Google Scholar] [CrossRef]
- Schweizer, U.; Schlicker, C.; Braun, D.; Köhrle, J.; Steegborn, C. Crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 10526–10531. [Google Scholar] [CrossRef] [Green Version]
- Fekete, C.; Lechan, R.M. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: Role of neuronal afferents and type 2 deiodinase. Front. Neuroendocrinol. 2007, 28, 97–114. [Google Scholar] [CrossRef] [Green Version]
- Jinhui, L.; Yuan, C.; Wenjing, X. Polybrominated diphenyl ethers in articles: A review of its applications and legislation. Environ. Sci. Pollut. Res. 2017, 24, 4312–4321. [Google Scholar] [CrossRef]
- Chen, D.; Hale, R.C. A global review of polybrominated diphenyl ether flame retardant contamination in birds. Environ. Int. 2010, 36, 800–811. [Google Scholar] [CrossRef]
- Dorman, D.C.; Chiu, W.; Hales, B.F.; Hauser, R.; Johnson, K.J.; Mantus, E.; Martel, S.; Robinson, K.A.; Rooney, A.A.; Rudel, R.; et al. Polybrominated diphenyl ether (PBDE) neurotoxicity: A systematic review and meta-analysis of animal evidence. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Lorber, M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 2–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Rogers, M.J.; Ding, C.; He, J. Reductive Debromination of Polybrominated Diphenyl Ethers—Microbes, Processes and Dehalogenases. Front. Microbiol. 2018, 9, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hites, R.A. Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ. Sci. Technol. 2004, 38, 945–956. [Google Scholar] [CrossRef]
- Gibson, E.A.; Siegel, E.L.; Eniola, F.; Herbstman, J.B.; Factor-Litvak, P. Effects of Polybrominated Diphenyl Ethers on Child Cognitive, Behavioral, and Motor Development. Int. J. Environ. Res. Public Health 2018, 15, 1636. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.R. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr. Opin. Pharmacol. 2014, 19, 134–144. [Google Scholar] [CrossRef] [Green Version]
- McGovern, V. PCBs Are Endocrine Disruptors: Mixture Affects Reproductive Development in Female Mice. Environ. Health Perspect. 2006, 114, A368–A369. [Google Scholar] [CrossRef] [Green Version]
- Yang, O.; Kim, H.L.; Weon, J.-I.; Seo, Y.R. Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis. J. Cancer Prev. 2015, 20, 12–24. [Google Scholar] [CrossRef]
- Matthiessen, P.; Wheeler, J.R.; Weltje, L. A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations. Crit. Rev. Toxicol. 2018, 48, 195–216. [Google Scholar] [CrossRef] [Green Version]
- Vuong, A.M.; Braun, J.M.; Webster, G.M.; Zoeller, R.T.; Hoofnagle, A.N.; Sjödin, A.; Yolton, K.; Lanphear, B.P.; Chen, A. Polybrominated diphenyl ether (PBDE) exposures and thyroid hormones in children at age 3 years. Environ. Int. 2018, 117, 339–347. [Google Scholar] [CrossRef]
- Roberts, S.C.; Bianco, A.C.; Stapleton, H.M. Disruption of Type 2 Iodothyronine Deiodinase Activity in Cultured Human Glial Cells by Polybrominated Diphenyl Ethers. Chem. Res. Toxicol. 2015, 28, 1265–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y.; Ikushiro, S.; Haraguchi, K.; Yamazaki, T.; Ito, Y.; Suzuki, H.; Kimura, R.; Yamada, S.; Inoue, T.; Degawa, M. A Possible Mechanism for Decrease in Serum Thyroxine Level by Polychlorinated Biphenyls in Wistar and Gunn Rats. Toxicol. Sci. 2004, 81, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J.G.; Celis, N.; Klaren, P.H.M.; Blust, R.; Dirtu, A.C.; Covaci, A.; Das, K. Thyroid dysfunction in sea bass (Dicentrarchus labrax): Underlying mechanisms and effects of polychlorinated biphenyls on thyroid hormone physiology and metabolism. Aquat. Toxicol. 2011, 105, 438–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, H.M.; Kelly, S.M.; Pei, R.; Letcher, R.J.; Gunsch, C. Metabolism of Polybrominated Diphenyl Ethers (PBDEs) by Human Hepatocytes in Vitro. Environ. Health Perspect. 2009, 117, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Curtis, S.W.; Terrell, M.L.; Jacobson, M.H.; Cobb, D.O.; Jiang, V.S.; Neblett, M.F.; Gerkowicz, S.A.; Spencer, J.B.; Marder, M.E.; Barr, D.B.; et al. Thyroid hormone levels associate with exposure to polychlorinated biphenyls and polybrominated biphenyls in adults exposed as children. Environ. Health 2019, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- Domingo, J.L. Polychlorinated diphenyl ethers (PCDEs): Environmental levels, toxicity and human exposure: A review of the published literature. Environ. Int. 2006, 32, 121–127. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Darrow, L.A.; Barr, D.B.; Howards, P.P.; Lyles, R.H.; Terrell, M.L.; Smith, A.K.; Conneely, K.N.; Marder, M.E.; Marcus, M. Serum Polybrominated Biphenyls (PBBs) and Polychlorinated Biphenyls (PCBs) and Thyroid Function among Michigan Adults Several Decades after the 1973–1974 PBB Contamination of Livestock Feed. Environ. Health Perspect. 2017, 125, 097020. [Google Scholar] [CrossRef] [Green Version]
- Marsan, E.S.; Bayse, C.A. Halogen-Bonding Interactions of Polybrominated Diphenyl Ethers and Thyroid Hormone Derivatives: A Potential Mechanism for the Inhibition of Iodothyronine Deiodinase. Chem. Eur. J. 2017, 23, 6625–6633. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Hu, X.; Zhang, X.; Zhang, Q.; Jiang, H.; Shi, W.; Yu, H. Effects of HO-/MeO-PBDEs on Androgen Receptor: In Vitro Investigation and Helix 12-Involved MD Simulation. Environ. Sci. Technol. 2013, 47, 11802–11809. [Google Scholar] [CrossRef]
- Siddiqi, M.A.; Laessig, R.H.; Reed, K.D. Polybrominated Diphenyl Ethers (PBDEs): New Pollutants–Old Diseases. Clin. Med. Res. 2003, 1, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Schecter, A.; Pavuk, M.; Päpke, O.; Ryan, J.J.; Birnbaum, L.; Rosen, R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ. Health Perspect. 2003, 111, 1723–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, E.N.; Braverman, L.E. Environmental pollutants and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, C.; Tagliaferri, S.; Caglieri, A.; Goldoni, M.; Giordano, G.; Mutti, A.; Costa, L.G. Synergistic interactions between PBDEs and PCBs in human neuroblastoma cells. Environ. Toxicol. 2014, 29, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Streets, S.S.; Henderson, S.A.; Stoner, A.D.; Carlson, D.L.; Simcik, M.F.; Swackhamer, D.L. Partitioning and Bioaccumulation of PBDEs and PCBs in Lake Michigan. Environ. Sci. Technol. 2006, 40, 7263–7269. [Google Scholar] [CrossRef] [PubMed]
- Holma-Suutari, A.; Ruokojärvi, P.; Komarov, A.A.; Makarov, D.A.; Ovcharenko, V.V.; Panin, A.N.; Kiviranta, H.; Laaksonen, S.; Nieminen, M.; Viluksela, M.; et al. Biomonitoring of selected persistent organic pollutants (PCDD/Fs, PCBs and PBDEs) in Finnish and Russian terrestrial and aquatic animal species. Environ. Sci. Eur. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, A.; Rüdel, H.; Jürling, H.; Müller, J.; Neugebauer, F.; Schröter-Kermani, C. Levels and trends of industrial chemicals (PCBs, PFCs, PBDEs) in archived herring gull eggs from German coastal regions. Environ. Sci. Eur. 2012, 24, 7. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.C.; Noyes, P.D.; Gallagher, E.P.; Stapleton, H.M. Species-Specific Differences and Structure−Activity Relationships in the Debromination of PBDE Congeners in Three Fish Species. Environ. Sci. Technol. 2011, 45, 1999–2005. [Google Scholar] [CrossRef]
- Yu, H.; Wondrousch, D.; Li, F.; Chen, J.; Lin, H.; Ji, L. In Silico Investigation of the Thyroid Hormone Activity of Hydroxylated Polybrominated Diphenyl Ethers. Chem. Res. Toxicol. 2015, 28, 1538–1545. [Google Scholar] [CrossRef]
- Qin, W.-P.; Li, C.-H.; Guo, L.-H.; Ren, X.-M.; Zhang, J.-Q. Binding and activity of polybrominated diphenyl ether sulfates to thyroid hormone transport proteins and nuclear receptors. Environ. Sci.-Proc. Imp. 2019, 21, 950–956. [Google Scholar] [CrossRef]
- Noyes, P.D.; Hinton, D.E.; Stapleton, H.M. Accumulation and Debromination of Decabromodiphenyl Ether (BDE-209) in Juvenile Fathead Minnows (Pimephales promelas) Induces Thyroid Disruption and Liver Alterations. Toxicol. Sci. 2011, 122, 265–274. [Google Scholar] [CrossRef] [Green Version]
- François, A.; Verreault, J. Interaction between deca-BDE and hepatic deiodinase in a highly PBDE-exposed bird. Environ. Res. 2018, 163, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.B.; Fini, J.-B.; Demeneix, B.A. Thyroid-disrupting chemicals and brain development: An update. Endocrin. Connect. 2018, 7, R160–R186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossberg, M.; Lendle, W.; Pfleiderer, G.; Tögel, A.; Dreher, E.-L.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack, H.; Cook, R.; et al. Chlorinated Hydrocarbons. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 978-3-527-30673-2. [Google Scholar]
- Hens, B.; Hens, L. Persistent Threats by Persistent Pollutants: Chemical Nature, Concerns and Future Policy Regarding PCBs–What Are We Heading For? Toxics. 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, V.A.; Clarke, J.U. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: Considerations for a congener-specific analysis. Environ. Health Perspect. 1989, 81, 225–239. [Google Scholar] [CrossRef]
- Mennigen, J.A.; Thompson, L.M.; Bell, M.; Tellez Santos, M.; Gore, A.C. Transgenerational effects of polychlorinated biphenyls: 1. Development and physiology across 3 generations of rats. Environ. Health 2018, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Chevrier, J.; Eskenazi, B.; Holland, N.; Bradman, A.; Barr, D.B. Effects of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy. Am. J. Epidemiol. 2008, 168, 298–310. [Google Scholar] [CrossRef]
- Pavuk, M.; Schecter, A.J.; Akhtar, F.Z.; Michalek, J.E. Serum 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Levels and Thyroid Function in Air Force Veterans of the Vietnam War. Ann. Epidemol. 2003, 13, 335–343. [Google Scholar] [CrossRef]
- Gordon, A.; Surks, M.I.; Oppenheimer, J.H. Thyroxine Stimulation of Amino Acid Incorporation Into Mitochondrial Protein: Differences Between In Vivo and In Vitro Effects. Acta Endocrinol. 1973, 72, 684–696. [Google Scholar] [CrossRef]
- Lans, M.C.; Spiertz, C.; Brouwer, A.; Koeman, J.H. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur. J. Pharm. Environ. 1994, 270, 129–136. [Google Scholar] [CrossRef]
- McKinney, J.D.; Waller, C.L. Polychlorinated biphenyls as hormonally active structural analogues. Environ. Health Perspect. 1994, 102, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Cheek, A.O.; Kow, K.; Chen, J.; McLachlan, J.A. Potential mechanisms of thyroid disruption in humans: Interaction of organochlorine compounds with thyroid receptor, transthyretin, and thyroid-binding globulin. Environ. Health Perspect. 1999, 107, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.T.M.; van Zanden, J.J.; Luijks, E.A.C.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, Å.; Brouwer, A. Potent Competitive Interactions of Some Brominated Flame Retardants and Related Compounds with Human Transthyretin in Vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katarzyńska, D.; Hrabia, A.; Kowalik, K.; Sechman, A. Comparison of the in vitro effects of TCDD, PCB 126 and PCB 153 on thyroid-restricted gene expression and thyroid hormone secretion by the chicken thyroid gland. Environ. Toxicol. Phar. 2015, 39, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, H.; Guo, H.; Li, W.; Tang, J.; Xu, B.; Sun, M.; Ding, G.; Jiang, L.; Cui, D.; et al. Molecular Mechanisms of 2, 3′, 4, 4′, 5-Pentachlorobiphenyl-Induced Thyroid Dysfunction in FRTL-5 Cells. Plos ONE 2015, 10, e0120133. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Chen, H.; Li, W.; Tang, J.; Cheng, P.; Xie, Y.; Liu, Y.; Ding, G.; Cui, D.; et al. Molecular mechanisms of human thyrocyte dysfunction induced by low concentrations of polychlorinated biphenyl 118 through the Akt/FoxO3a/NIS pathway. J. App. Toxicol. 2015, 35, 992–998. [Google Scholar] [CrossRef]
- Soechitram, S.D.; Berghuis, S.A.; Visser, T.J.; Sauer, P.J.J. Polychlorinated biphenyl exposure and deiodinase activity in young infants. Sci. Total Environ. 2017, 574, 1117–1124. [Google Scholar] [CrossRef]
- Morse, D.C.; Wehler, E.K.; Wesseling, W.; Koeman, J.H.; Brouwer, A. Alterations in Rat Brain Thyroid Hormone Status Following Pre- and Postnatal Exposure to Polychlorinated Biphenyls (Aroclor 1254). Toxicol. App. Pharmacol. 1996, 136, 269–279. [Google Scholar] [CrossRef]
- Darras, V.M. Endocrine disrupting polyhalogenated organic pollutants interfere with thyroid hormone signalling in the developing brain. Cerebellum 2008, 7, 26–37. [Google Scholar] [CrossRef]
- Butt, C.M.; Wang, D.; Stapleton, H.M. Halogenated Phenolic Contaminants Inhibit the In Vitro Activity of the Thyroid-Regulating Deiodinases in Human Liver. Toxicol. Sci. 2011, 124, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Parisini, E.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in halocarbon–protein complexes: A structural survey. Chem. Soc. Rev. 2011, 40, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, M.R.; Zanden, C.M.V.; Carter, M.; Ho, P.S. Halogen bonding (X-bonding): A biological perspective. Prot. Sci. 2013, 22, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.; Henriquez, G.; Sirimulla, S.; Narayan, M. Looking Back, Looking Forward at Halogen Bonding in Drug Discovery. Molecules 2017, 22, 1397. [Google Scholar] [CrossRef]
- Bayse, C.A. Halogen bonding from the bonding perspective with considerations for mechanisms of thyroid hormone activation and inhibition. New J. Chem. 2018, 42, 10623–10632. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef]
- Costa, P.J.; Nunes, R.; Vila-Viçosa, D. Halogen bonding in halocarbon-protein complexes and computational tools for rational drug design. Expert Opin. Drug Discov. 2019, 14, 805–820. [Google Scholar] [CrossRef]
- Scholfield, M.R.; Ford, M.C.; Carlsson, A.-C.C.; Butta, H.; Mehl, R.A.; Ho, P.S. Structure–Energy Relationships of Halogen Bonds in Proteins. Biochemistry 2017, 56, 2794–2802. [Google Scholar] [CrossRef]
- Bayse, C.A.; Rafferty, E.R. Is Halogen Bonding the Basis for Iodothyronine Deiodinase Activity? Inorg. Chem. 2010, 49, 5365–5367. [Google Scholar] [CrossRef]
- Manna, D.; Mugesh, G. Regioselective Deiodination of Thyroxine by Iodothyronine Deiodinase Mimics: An Unusual Mechanistic Pathway Involving Cooperative Chalcogen and Halogen Bonding. J. Am. Chem. Soc. 2012, 134, 4269–4279. [Google Scholar] [CrossRef]
- Marsan, E.S.; Bayse, C.A. Halogen Bonding Interactions of Polychlorinated Biphenyls and the Potential for Thyroid Disruption. Chem. Eur. J. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Li, G.-X.; He, G.; Chen, G. Halogen-Bond-Promoted Photoactivation of Perfluoroalkyl Iodides: A Photochemical Protocol for Perfluoroalkylation Reactions. Org. Lett. 2017, 19, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Brammer, L. Halogen bonding, chalcogen bonding, pnictogen bonding, tetrel bonding: Origins, current status and discussion. Faraday Discuss. 2017, 203, 485–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs. π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.C.; Wright, J.S.; Carrington, E.J.; Perutz, R.N.; Hunter, C.A.; Brammer, L. Hydrogen bonding vs. halogen bonding: The solvent decides. Chem. Sci. 2017, 8, 5392–5398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Y.-C.; Yeung, Y.-Y. Halogen Bond Catalyzed Bromocarbocyclization. Angew. Chem. Int. Edit. 2018, 57, 3483–3487. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. WIRES Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Wolters, L.P.; Schyman, P.; Pavan, M.J.; Jorgensen, W.L.; Bickelhaupt, F.M.; Kozuch, S. The many faces of halogen bonding: A review of theoretical models and methods. WIRES Comput. Mol. Sci. 2014, 4, 523–540. [Google Scholar] [CrossRef]
- Oliveira, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid function disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019, 62, R1–R19. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-C.; Yu, S.; Wu, D.; Huang, J.; Liu, T.; Xiao, J.; Huang, W.; Gao, Y.; Li, X.; Zeng, W.; et al. Disruption of thyroid hormone regulated proteins and gene expression by polychlorinated biphenyls, polybrominated diphenyl ethers and new flame retardants in residents of an e-waste region. Environ. Pollut. 2019, 254, 112925. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Murray, J.S.; Politzer, P. A perspective on quantum mechanics and chemical concepts in describing noncovalent interactions. Phys. Chem. Chem. Phys. 2018, 20, 30076–30082. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Hathwar, V.R.; Chopra, D.; Panini, P.; Guru Row, T.N. Revealing the Polarizability of Organic Fluorine in the Trifluoromethyl Group: Implications in Supramolecular Chemistry. Cryst. Growth Des. 2014, 14, 5366–5369. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. Fluorine-Centered Halogen Bonding: A Factor in Recognition Phenomena and Reactivity. Cryst. Growth Des. 2011, 11, 4238–4246. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. The fluorine atom as a halogen bond donor, viz. a positive site. Cryst. Eng. Comm. 2011, 13, 6593–6596. [Google Scholar] [CrossRef] [Green Version]
- Wolters, L.P.; Bickelhaupt, F.M. Halogen Bonding versus Hydrogen Bonding: A Molecular Orbital Perspective. ChemistryOpen 2012, 1, 96–105. [Google Scholar] [CrossRef]
- Bigoli, F.; Deplano, P.; Ienco, A.; Mealli, C.; Mercuri, M.L.; Pellinghelli, M.A.; Pintus, G.; Saba, G.; Trogu, E.F. Structure and Bonding of Diiodine Adducts of the Sulfur-Rich Donors 1,3-Dithiacyclohexane-2-thione (ptc) and 4,5-Ethylenedithio-1,3-dithiole-2-thione (ttb). Inorg. Chem. 1999, 38, 4626–4636. [Google Scholar] [CrossRef]
- Manca, G.; Ienco, A.; Mealli, C. Factors Controlling Asymmetrization of the Simplest Linear I3– and I42– Polyiodides with Implications for the Nature of Halogen Bonding. Cryst. Growth Des. 2012, 12, 1762–1771. [Google Scholar] [CrossRef]
- Arman, H.D.; Rafferty, E.R.; Bayse, C.A.; Pennington, W.T. Complementary Selenium···Iodine Halogen Bonding and Phenyl Embraces: Cocrystals of Triphenylphosphine Selenide with Organoiodides. Cryst. Growth Des. 2012, 12, 4315–4323. [Google Scholar] [CrossRef]
- Mulliken, R.S.; Person, W.B. Molecular compounds and their spectra. XXI. Some general considerations. J. Am. Chem. Soc. 1969, 91, 3409–3413. [Google Scholar] [CrossRef]
- Wang, C.; Danovich, D.; Shaik, S.; Mo, Y. A Unified Theory for the Blue- and Red-Shifting Phenomena in Hydrogen and Halogen Bonds. J. Chem. Theory Comput. 2017, 13, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen and halogen bonds are ruled by the same mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 7249–7259. [Google Scholar] [CrossRef] [PubMed]
- Cesario, D.; Fortino, M.; Marino, T.; Nunzi, F.; Russo, N.; Sicilia, E. The role of the halogen bond in iodothyronine deiodinase: Dependence on chalcogen substitution in naphthyl-based mimetics. J. Comp. Chem. 2019, 40, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.; Mugesh, G. A Chemical Model for the Inner-Ring Deiodination of Thyroxine by Iodothyronine Deiodinase. Angew. Chem. Int. Edit. 2010, 49, 9246–9249. [Google Scholar] [CrossRef]
- Pierangelo, M.; Giuseppe, R. Halogen Bonding: A Paradigm in Supramolecular Chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar]
- Pimentel, G.C. The Bonding of Trihalide and Bifluoride Ions by the Molecular Orbital Method. J. Chem. Phys. 1951, 19, 446–448. [Google Scholar] [CrossRef]
- Hach, R.J.; Rundle, R.E. The Structure of Tetramethylammonium Pentaiodide1,1a. J. Am. Chem. Soc. 1951, 73, 4321–4324. [Google Scholar] [CrossRef]
- Munzarová, M.L.; Hoffmann, R. Electron-Rich Three-Center Bonding: Role of s,p Interactions across the p-Block. J. Am. Chem. Soc. 2002, 124, 4787–4795. [Google Scholar] [CrossRef]
- Sirimulla, S.; Bailey, J.B.; Vegesna, R.; Narayan, M. Halogen Interactions in Protein–Ligand Complexes: Implications of Halogen Bonding for Rational Drug Design. J. Chem. Inf. Model. 2013, 53, 2781–2791. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Strength and Character of Halogen Bonds in Protein–Ligand Complexes. Cryst. Growth Des. 2011, 11, 4272–4278. [Google Scholar] [CrossRef]
- Zhou, P.; Tian, F.; Zou, J.; Shang, Z. Rediscovery of halogen bonds in protein-ligand complexes. Mini. Rev. Med. Chem. 2010, 10, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, U.; Towell, H.; Vit, A.; Rodriguez-Ruiz, A.; Steegborn, C. Structural aspects of thyroid hormone binding to proteins and competitive interactions with natural and synthetic compounds. Mol. Cell. Endocrinol. 2017, 458, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Eneqvist, T.; Lundberg, E.; Karlsson, A.; Huang, S.; Santos, C.R.A.; Power, D.M.; Sauer-Eriksson, A.E. High Resolution Crystal Structures of Piscine Transthyretin Reveal Different Binding Modes for Triiodothyronine and Thyroxine. J. Biol. Chem. 2004, 279, 26411–26416. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Wei, Z.; Read, R.J.; Carrell, R.W. Structural mechanism for the carriage and release of thyroxine in the blood. Proc. Natl. Acad. Sci. 2006, 103, 13321–13326. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Baase, W.A.; Matthews, B.W. Halogenated Benzenes Bound within a Non-polar Cavity in T4 Lysozyme Provide Examples of I⋯S and I⋯Se Halogen-bonding. J. Mol. Biol. 2009, 385, 595–605. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Invest. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [Green Version]
- Sorimachi, K.; Cahnmann, H.J. Formation and metabolism of 3′,5′-diiodothyronine and 3,5-diiodothyronine by cultured monkey hepatocarcinoma cells. Horm. Metab. Res. 1979, 11, 233–237. [Google Scholar] [CrossRef]
- Butt, C.M.; Stapleton, H.M. Inhibition of Thyroid Hormone Sulfotransferase Activity by Brominated Flame Retardants and Halogenated Phenolics. Chem. Res. Toxicol. 2013, 26, 1692–1702. [Google Scholar] [CrossRef]
- Byrne, S.C.; Miller, P.; Seguinot-Medina, S.; Waghiyi, V.; Buck, C.L.; von Hippel, F.A.; Carpenter, D.O. Associations between serum polybrominated diphenyl ethers and thyroid hormones in a cross sectional study of a remote Alaska Native population. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Steen, E.; den Steen, E.V.; Covaci, A.; Jaspers, V.L.B.; Dauwe, T.; Voorspoels, S.; Eens, M.; Pinxten, R. Accumulation, tissue-specific distribution and debromination of decabromodiphenyl ether (BDE 209) in European starlings (Sturnus vulgaris). Environ. Pollut. 2007, 148, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.M.; Reis-Henriques, M.A.; Darras, V.M. Circulating thyroid hormone levels and iodothyronine deiodinase activities in Nile tilapia (Oreochromis niloticus) following dietary exposure to Endosulfan and Aroclor 1254. Comp. Biochem. Phys. C 2005, 141, 8–14. [Google Scholar] [CrossRef]
- Chauhan, K.R.; Kodavanti, P.R.S.; McKinney, J.D. Assessing the Role of ortho-Substitution on Polychlorinated Biphenyl Binding to Transthyretin, a Thyroxine Transport Protein. Toxicol. App. Pharmacol. 2000, 162, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, R.P.; Visser, T.J. Metabolism of Thyroid Hormone. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Jorgensen, W.L.; Schyman, P. Treatment of Halogen Bonding in the OPLS-AA Force Field; Application to Potent Anti-HIV Agents. J. Chem. Theory Comput. 2012, 8, 3895–3901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolář, M.; Hobza, P.; Bronowska, A.K. Plugging the explicit σ-holes in molecular docking. Chem. Commun. 2013, 49, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.A. Molecular mechanical study of halogen bonding in drug discovery. J. Comp. Chem. 2011, 32, 2564–2574. [Google Scholar] [CrossRef]
- Rendine, S.; Pieraccini, S.; Forni, A.; Sironi, M. Halogen bonding in ligand–receptor systems in the framework of classical force fields. Phys. Chem. Chem. Phys. 2011, 13, 19508–19516. [Google Scholar] [CrossRef]
- Gutiérrez, I.S.; Lin, F.-Y.; Vanommeslaeghe, K.; Lemkul, J.A.; Armacost, K.A.; Brooks, C.L., III; MacKerell, A.D., Jr. Parametrization of halogen bonds in the CHARMM general force field: Improved treatment of ligand–protein interactions. Bioorg. Med. Chem. 2016, 24, 4812–4825. [Google Scholar] [CrossRef] [Green Version]
- Koebel, M.R.; Schmadeke, G.; Posner, R.G.; Sirimulla, S. AutoDock VinaXB: Implementation of XBSF, new empirical halogen bond scoring function, into AutoDock Vina. J. Cheminformatics 2016, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. Comparison of the Efficiency of the LIE and MM/GBSA Methods to Calculate Ligand-Binding Energies. J. Chem. Theory Comput. 2011, 7, 3768–3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaura, K.; Ikeo, E.; Asada, T.; Nakano, T.; Uebayasi, M. Fragment molecular orbital method: An approximate computational method for large molecules. Chem. Phys. Lett. 1999, 313, 701–706. [Google Scholar] [CrossRef]
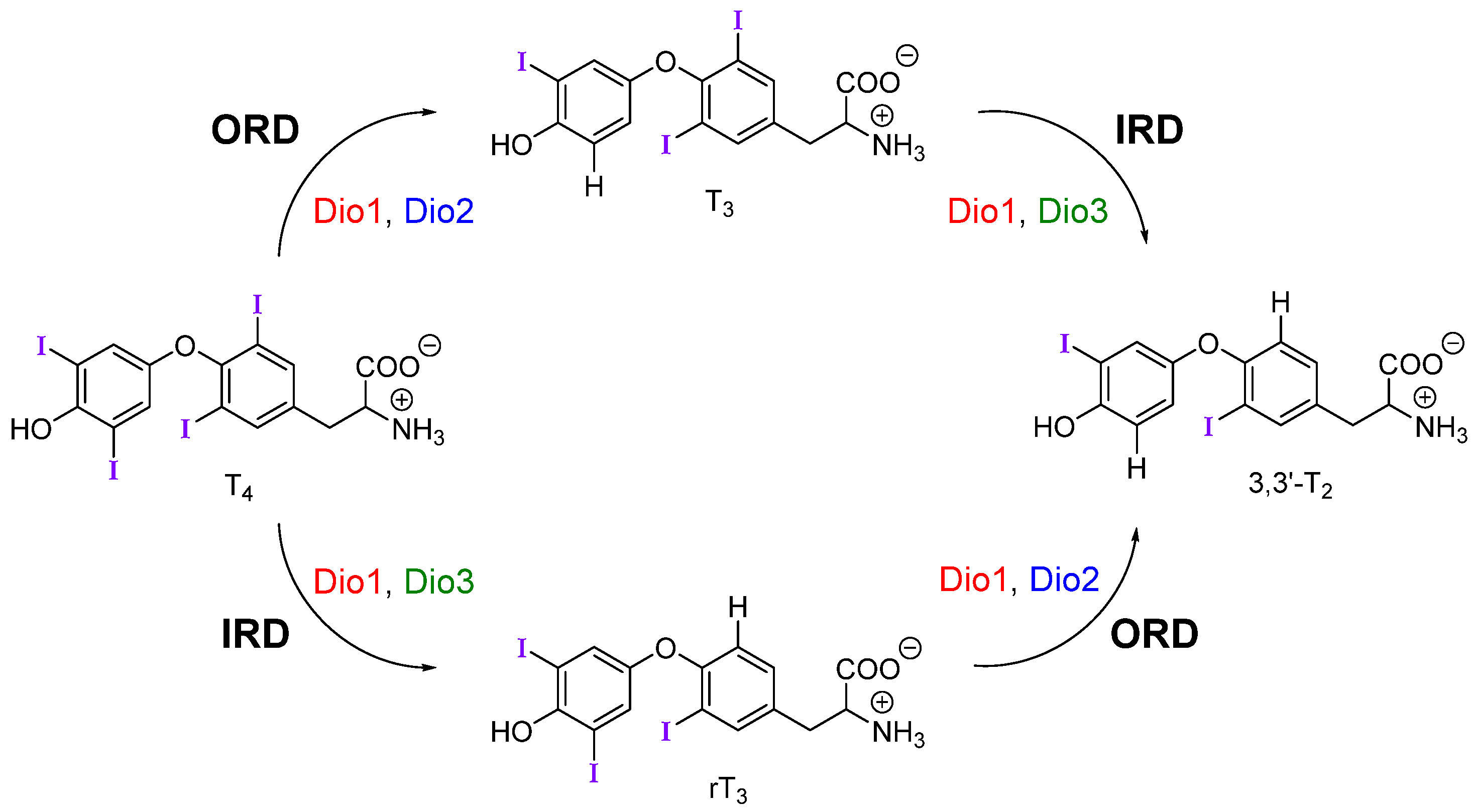


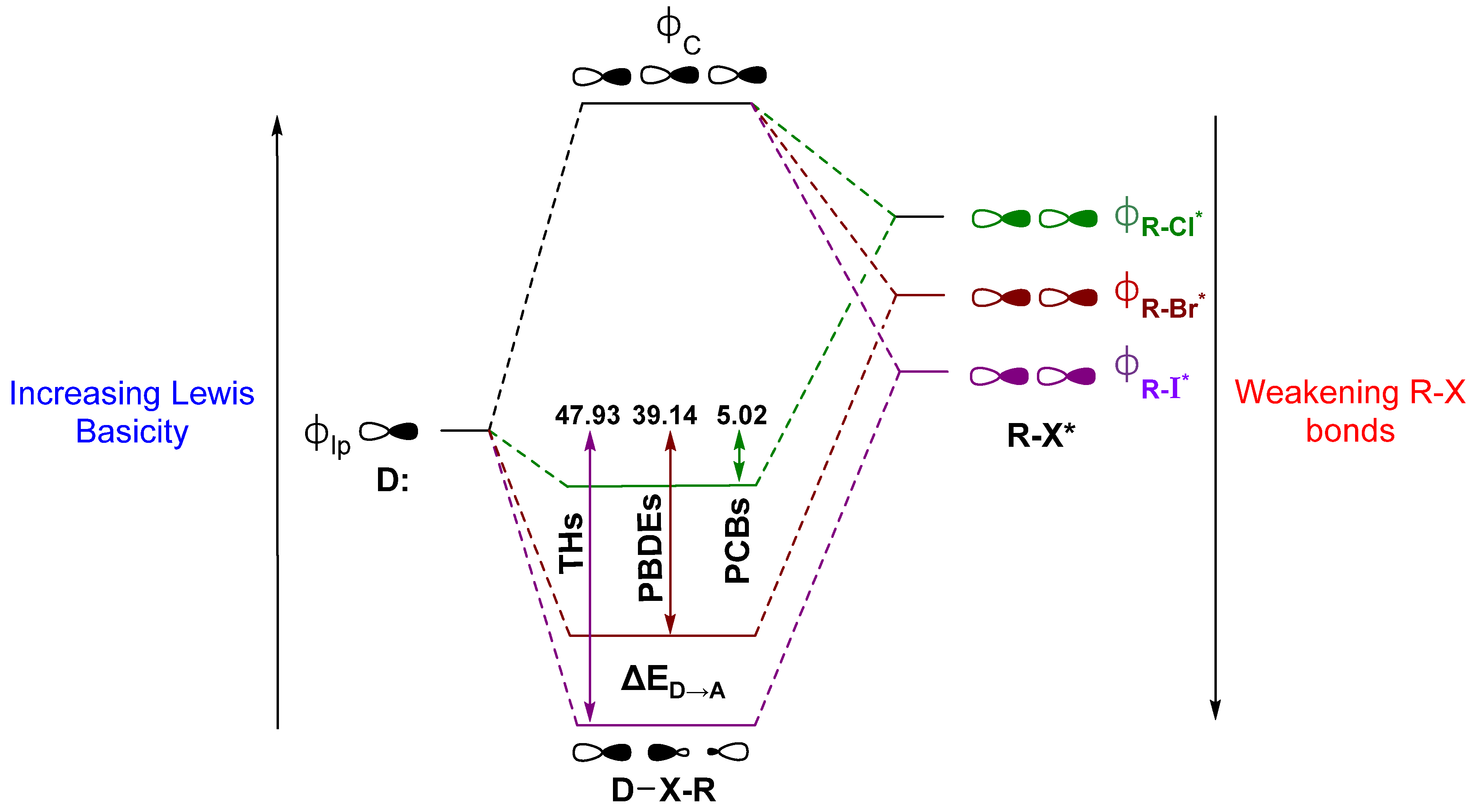
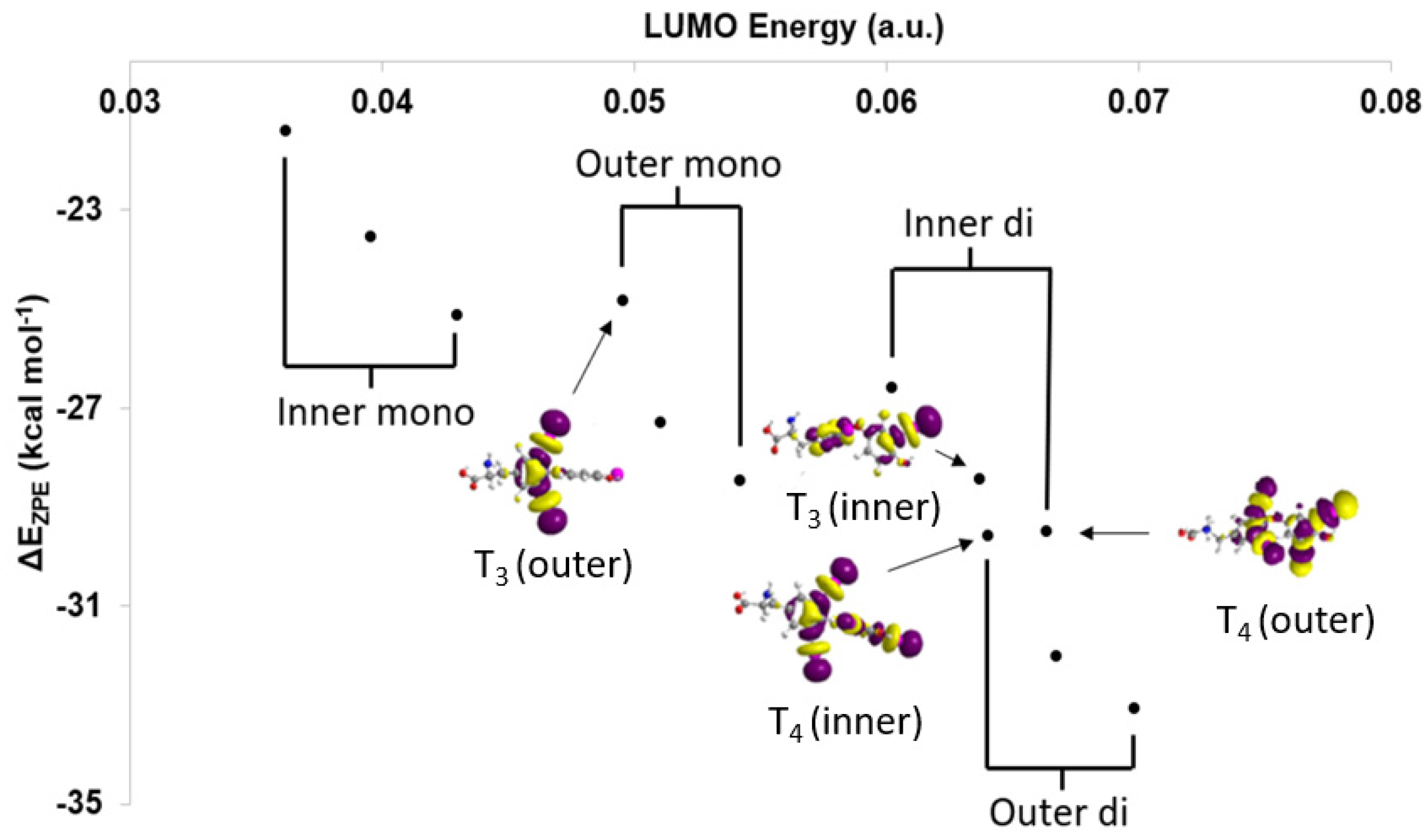
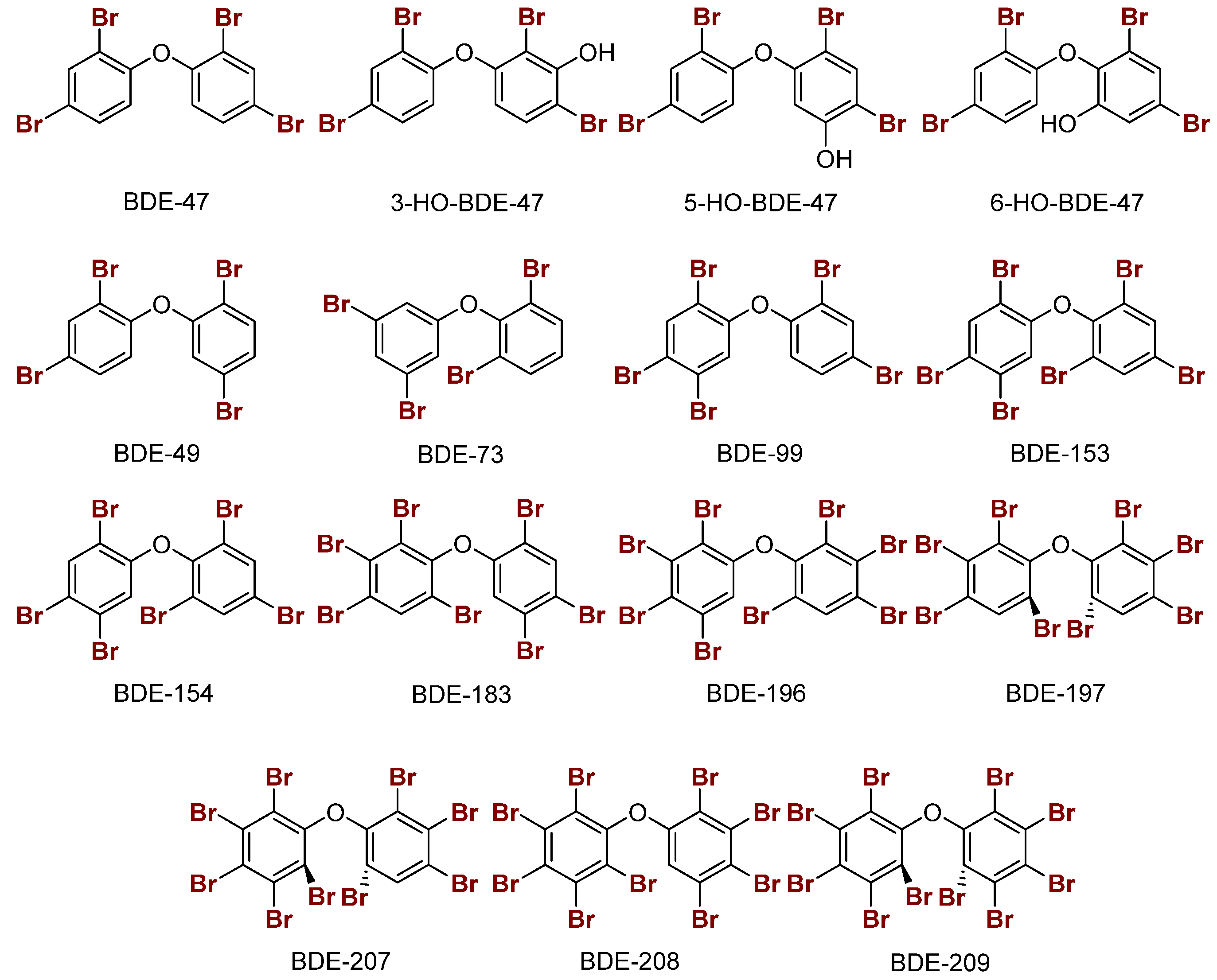
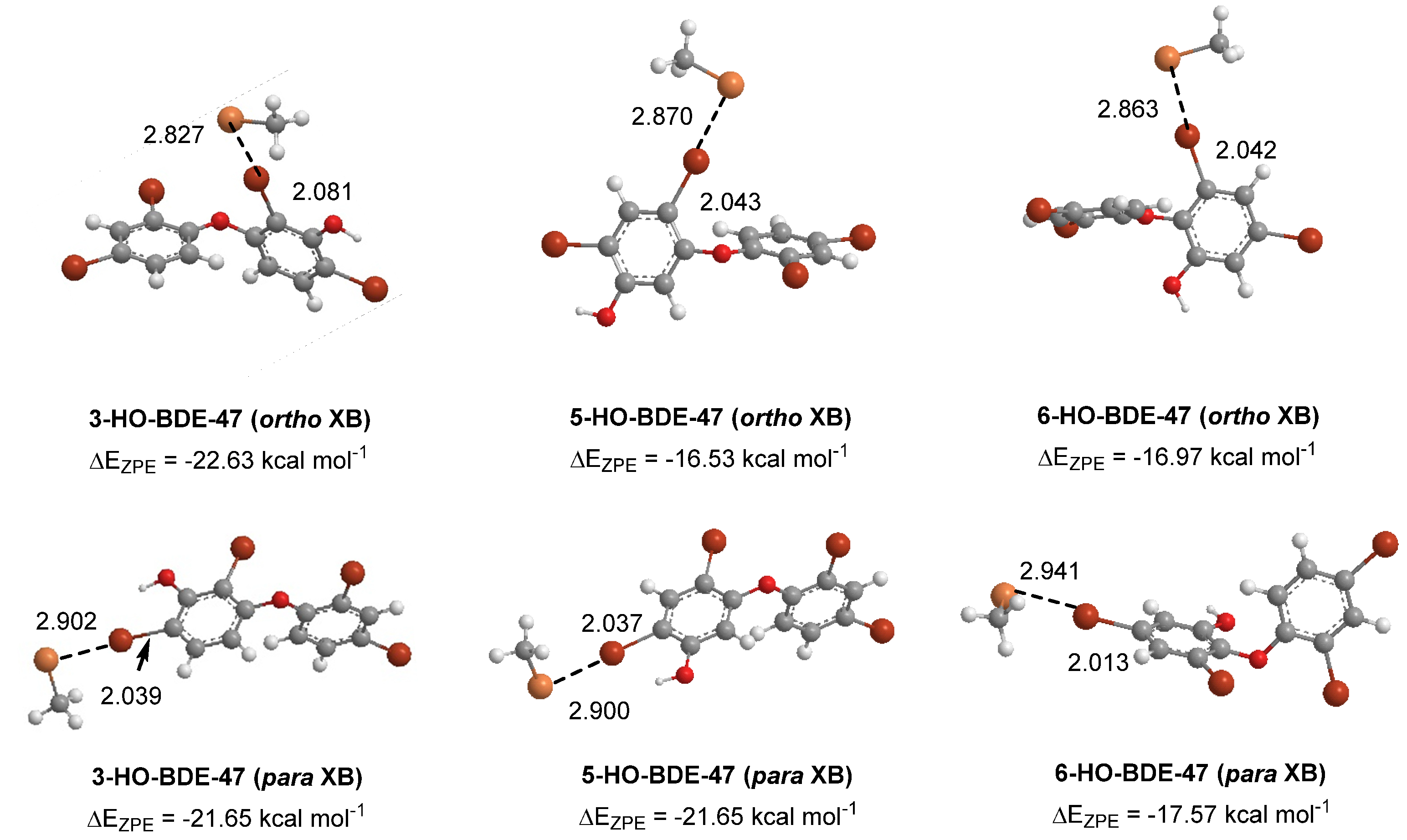



| Compound | XB Position | d(C–I), Å; (Δd(C–I), Å) | d(I···Se), Å | ΔEZPE, kcal mol−1 | ΔED→A, kcal mol−1 |
|---|---|---|---|---|---|
| T4 | Inner | 2.300 (+0.198) | 2.917 | −29.59 | 53.58, 4.18 [a] |
| T4 | Outer | 2.282 (+0.169) | 2.960 | −29.50 | 45.93, 3.83 [a] |
| T3 | Inner | 2.299 (+0.197) | 2.922 | −28.43 | 52.87, 3.96 [a] |
| T3 | Outer | 2.256 (+0.144) | 3.006 | −24.84 | 40.25, 3.03 [a] |
| rT3 | Inner | 2.276 (+0.174) | 2.953 | −25.14 | 48.43, 3.17 [a] |
| rT3 | Outer | 2.291 (+0.190) | 2.946 | −33.07 | 48.98, 4.12 [a] |
| 3,3′-T2 | Inner | 2.272 (+0.169) | 2.960 | −23.56 | 46.89, 3.20 [a] |
| 3,3′-T2 | Outer | 2.265 (+0.153) | 2.990 | −28.46 | 43.06, 3.61 [a] |
| 3,5-T2 | Inner | 2.286 (+0.184) | 2.942 | −26.61 | 48.51 |
| 3′,5′-T2 | Outer | 2.286 (+0.173) | 2.954 | −32.02 | 47.48 |
| 3-T1 | Inner | 2.262 (+0.158) | 2.980 | −21.41 | 43.43 |
| 3′-T1 | Outer | 2.260 (+0.158) | 3.002 | −27.29 | 41.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsan, E.S.; Bayse, C.A. A Halogen Bonding Perspective on Iodothyronine Deiodinase Activity. Molecules 2020, 25, 1328. https://doi.org/10.3390/molecules25061328
Marsan ES, Bayse CA. A Halogen Bonding Perspective on Iodothyronine Deiodinase Activity. Molecules. 2020; 25(6):1328. https://doi.org/10.3390/molecules25061328
Chicago/Turabian StyleMarsan, Eric S., and Craig A. Bayse. 2020. "A Halogen Bonding Perspective on Iodothyronine Deiodinase Activity" Molecules 25, no. 6: 1328. https://doi.org/10.3390/molecules25061328
APA StyleMarsan, E. S., & Bayse, C. A. (2020). A Halogen Bonding Perspective on Iodothyronine Deiodinase Activity. Molecules, 25(6), 1328. https://doi.org/10.3390/molecules25061328






