Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Emulsion Characterization
2.2. Powder Characterization
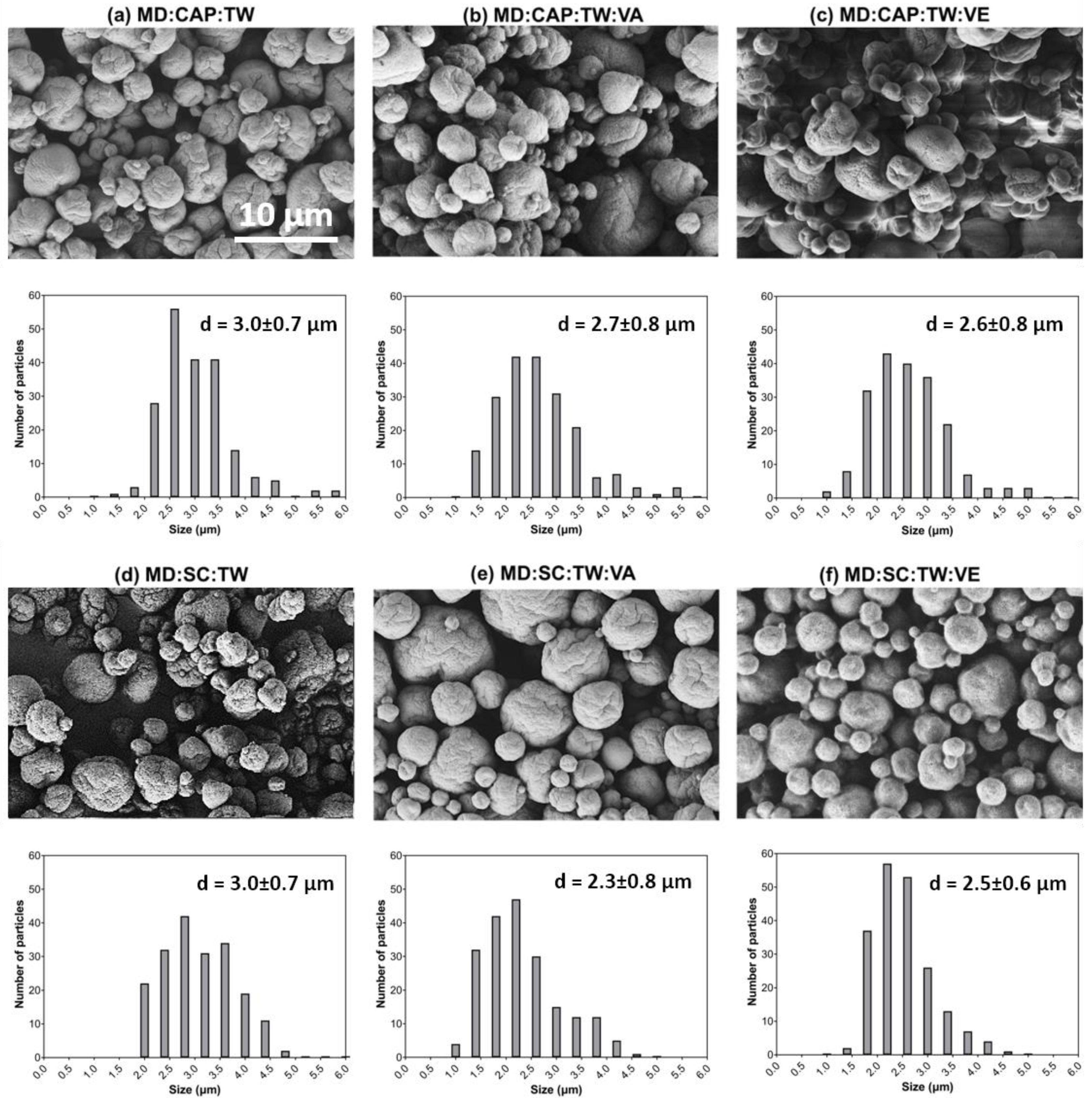
2.2.1. Surface Morphology
2.2.2. Product Yield
2.2.3. Encapsulation Efficiency
2.2.4. Moisture Content
2.2.5. Porosity
2.2.6. Structural Analysis
2.2.7. Thermal Properties
2.2.8. Vitamin A Retention after One-Month Storage
2.2.9. Thermo-Oxidative and Thermal Stability
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.2.1. Nanoemulsions
3.2.2. Spray Drying Conditions
3.3. Emulsion Characterization by Droplet Size and Polydispersity Index
3.4. Powder Characterization
3.4.1. Product Yield and Encapsulation Efficiency
3.4.2. Moisture Content
3.4.3. Porosity
3.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.5. Differential Scanning Calorimetry (DSC)
3.4.6. Thermogravimetric Analysis (TGA)
3.4.7. Particle Morphology by Field-emission Scanning Electron Microscopy (FE-SEM)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sauvant, P.; Cansell, M.; Hadj Sassi, A.; Atgié, C. Vitamin A enrichment: Caution with encapsulation strategies used for food applications. Food Res. Int. 2012, 46, 469–479. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef] [Green Version]
- NIH—National Institute of Health Vitamin A. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 13 May 2019).
- NIH—National Institute of Health Vitamin E. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 13 May 2019).
- Tomas, M.; Jafari, S.M. Influence of Food Processing Operations on Vitamins; Elsevier: London, UK, 2018; ISBN 9780081005965. [Google Scholar]
- Loewen, A.; Chan, B.; Li-Chan, E.C.Y. Optimization of vitamins A and D3 loading in re-assembled casein micelles and effect of loading on stability of vitamin D3 during storage. Food Chem. 2018, 240, 472–481. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P.C. Spray- or freeze-drying of casein micelles loaded with Vitamin D2: Studies on storage stability and in vitro digestibility. LWT 2018, 97, 87–93. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin E-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pezeshky, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Moghadam, M.; Babazadeh, A. Vitamin A palmitate-bearing nanoliposomes: Preparation and characterization. Food Biosci. 2016, 13, 49–55. [Google Scholar] [CrossRef]
- Quintanilla-Carvajal, M.X.; Hernández-Sánchez, H.; Alamilla-Beltrán, L.; Zepeda-Vallejo, G.; Jaramillo-Flores, M.E.; de Jesús Perea-Flores, M.; Jimenez-Aparicio, A.; Gutiérrez-López, G.F. Effects of microfluidisation process on the amounts and distribution of encapsulated and non-encapsulated α-tocopherol microcapsules obtained by spray drying. Food Res. Int. 2014, 63, 2–8. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, H.; Liang, X.; He, B.; Han, X. Study on the Morphology, Particle Size and Thermal Properties of Vitamin A Microencapsulated by Starch Octenylsucciniate. Agric. Sci. China 2010, 9, 1058–1064. [Google Scholar] [CrossRef]
- Zhao, L.; Xiong, H.; Peng, H.; Wang, Q.; Han, D.; Bai, C.; Liu, Y.; Shi, S.; Deng, B. PEG-coated lyophilized proliposomes: Preparation, characterizations and in vitro release evaluation of vitamin E. Eur. Food Res. Technol. 2011, 232, 647–654. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mundra, S.; Ramalingam, C.; Kumar, A. Fabrication of Food Grade Vitamin E Nanoemulsion by Low Energy Approach, Characterization and Its Application. Int. J. Food Prop. 2016, 19, 700–708. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Encapsulation of vitamin E in edible emulsions fabricated using a natural surfactant. Food Hydrocoll. 2013, 30, 712–720. [Google Scholar] [CrossRef]
- García, P.; Vega, J.; Jimenez, P.; Santos, J.; Robert, P. Alpha-tocopherol microspheres with cross-linked and acetylated inulin and their release profile in a hydrophilic model. Eur. J. Lipid Sci. Technol. 2013, 115, 811–819. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Influence of soy protein’s structural modifications on their microencapsulation properties: α-Tocopherol microparticle preparation. Food Res. Int. 2012, 48, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.N.; Asif, M.; Ali, R. Stability of Vitamins during Extrusion. Crit. Rev. Food Sci. Nutr. 2009, 49, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Ranjan, S. An Introduction to Food Grade Nanoemulsions; Environmental Chemistry for a Sustainable World; Springer: Singapore, 2018; ISBN 978-981-10-6985-7. [Google Scholar]
- Arancibia, C.; Miranda, M.; Matiacevich, S.; Troncoso, E. Physical properties and lipid bioavailability of nanoemulsion-based matrices with different thickening agents. Food Hydrocoll. 2017, 73, 243–254. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429154034. [Google Scholar]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-drying performance and thermal stability of L-ascorbic acid microencapsulated with sodium alginate and gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Navarro, R.; Arancibia, C.; Herrera, M.L.; Matiacevich, S. Effect of type of encapsulating agent on physical properties of edible films based on alginate and thyme oil. Food Bioprod. Process. 2016, 97, 63–75. [Google Scholar] [CrossRef]
- Patel, A.R.; Bhandari, B. Nano-and Microencapsulation of Vitamins. In Nano- and Microencapsulation for Foods; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 223–248. [Google Scholar]
- Cheng, H.; Fan, Q.; Liu, T.; Liang, L. Co-encapsulation of α-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2020, 264, 109685. [Google Scholar] [CrossRef]
- Haham, M.; Ish-Shalom, S.; Nodelman, M.; Duek, I.; Segal, E.; Kustanovich, M.; Livney, Y.D. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct. 2012, 3, 737. [Google Scholar] [CrossRef] [PubMed]
- SEMO, E.; KESSELMAN, E.; DANINO, D.; LIVNEY, Y. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein–polysaccharide interactions. Soft Matter 2008, 4, 932. [Google Scholar] [CrossRef]
- Evans, M.; Ratcliffe, I.; Williams, P.A. Emulsion stabilisation using polysaccharide–protein complexes. Curr. Opin. Colloid Interface Sci. 2013, 18, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Harnsilawat, T.; Pongsawatmanit, R.; McClements, D.J. Influence of pH and Ionic Strength on Formation and Stability of Emulsions Containing Oil Droplets Coated by β-Lactoglobulin−Alginate Interfaces. Biomacromolecules 2006, 7, 2052–2058. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Stabilization of Vitamin E-Enriched Nanoemulsions: Influence of Post-Homogenization Cosurfactant Addition. J. Agric. Food Chem. 2014, 62, 1625–1633. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Zhong, Q. Thyme Oil Nanoemulsions Coemulsified by Sodium Caseinate and Lecithin. J. Agric. Food Chem. 2014, 62, 9900–9907. [Google Scholar] [CrossRef]
- de Figueiredo Furtado, G.; Mantovani, R.A.; Consoli, L.; Hubinger, M.D.; da Cunha, R.L. Structural and emulsifying properties of sodium caseinate and lactoferrin influenced by ultrasound process. Food Hydrocoll. 2017, 63, 178–188. [Google Scholar] [CrossRef]
- Campelo, P.H.; Junqueira, L.A.; de Resende, J.V.; Zacarias, R.D.; de Barros Fernandes, R.V.; Botrel, D.A.; Borges, S.V. Stability of lime essential oil emulsion prepared using biopolymers and ultrasound treatment. Int. J. Food Prop. 2017, 20, S564–S579. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Sharif, H.R.; Sharif, M.K.; Zhong, F. Preparation, characterization and rheological properties of vitamin E enriched nanoemulsion. Pakistan J. Food Sci. 2017, 27, 7–14. [Google Scholar]
- Selamat, S.N.; Muhamad, I.I.; Sarmidi, M.R. Encapsulation of Tocopherol and Tocotrienol in Vitamin-E Using Spray Drying Technique. In Proceedings of the South East Asian Technical Universities Consortium(SEATUC)-3rd SEATUC Symposium Proceeding, Johor Bahru, Malaysia, 25–26 February 2009; pp. 143–147. [Google Scholar]
- Gangurde, A.B.; Ali, M.T.; Pawar, J.N.; Amin, P.D. Encapsulation of vitamin E acetate to convert oil to powder microcapsule using different starch derivatives. J. Pharm. Investig. 2017, 47, 559–574. [Google Scholar] [CrossRef]
- Hogan, S.A.; McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Microencapsulating Properties of Sodium Caseinate. J. Agric. Food Chem. 2001, 49, 1934–1938. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems Associated With Spray Drying Of Sugar-Rich Foods. Dry. Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Ishwarya, P. Spray Drying Techniques for Food Ingredient Encapsulation; Institute of Food Technologists Series; Wiley: Hoboken, NJ, USA, 2015; ISBN 9781118864197. [Google Scholar]
- Jafari, S.M.; Ghalegi Ghalenoei, M.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Wang, W.; Dufour, C.; Zhou, W. Impacts of spray-drying conditions on the physicochemical properties of soy sauce powders using maltodextrin as auxiliary drying carrier. CyTA J. Food 2015, 13, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials—Physics and Chemistry—New Edition; InTech: London, UK, 2018. [Google Scholar]
- Samborska, K.; Langa, E.; Kamińska-Dwórznicka, A.; Witrowa-Rajchert, D. The influence of sodium caseinate on the physical properties of spray-dried honey. Int. J. Food Sci. Technol. 2015, 50, 256–262. [Google Scholar] [CrossRef]
- Wang, B. Development of spray drying technology for microencapsulation of bioactive materials. Ph.D. Thesis, Degree-Aubum University, Auburn, AL, USA, 12 December 2015. [Google Scholar]
- Keshani, S.; Daud, W.R.W.; Nourouzi, M.M.; Namvar, F.; Ghasemi, M. Spray drying: An overview on wall deposition, process and modeling. J. Food Eng. 2015, 146, 152–162. [Google Scholar] [CrossRef]
- Roos, Y.H.; Drusch, S. Food components and polymers. In Phase Transitions in Foods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 115–172. [Google Scholar]
- Negrão-Murakami, A.N.; Nunes, G.L.; Pinto, S.S.; Murakami, F.S.; Amante, E.R.; Petrus, J.C.C.; Prudêncio, E.S.; Amboni, R.D.M.C. Influence of DE-value of maltodextrin on the physicochemical properties, antioxidant activity, and storage stability of spray dried concentrated mate (Ilex paraguariensis A. St. Hil.). LWT Food Sci. Technol. 2017, 79, 561–567. [Google Scholar] [CrossRef]
- Lacerda, E.C.Q.; de Araújo Calado, V.M.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, inulin and maltodextrin as encapsulating agents affect the quality and stability of jussara pulp microparticles. Carbohydr. Polym. 2016, 151, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.A.; Martin, L.G.P.; de Oliveira, R.A.; Kurozawa, L.E.; Park, K.J. Spray drying of babassu coconut milk using different carrier agents. Dry. Technol. 2017, 35, 76–87. [Google Scholar] [CrossRef]
- Zotarelli, M.F.; da Silva, V.M.; Durigon, A.; Hubinger, M.D.; Laurindo, J.B. Production of mango powder by spray drying and cast-tape drying. Powder Technol. 2017, 305, 447–454. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Sritham, E.; Gunasekaran, S. FTIR spectroscopic evaluation of sucrose-maltodextrin-sodium citrate bioglass. Food Hydrocoll. 2017, 70, 371–382. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Selva, M.D.C.G.; Migallon, A.J. Desarrollo de un Sistema de Desarrollo de un Sistema de Envasado Activo: Biopelículas de Proteínas Lácteas para un Desarrollo Sostenible. 2009. Available online: https://docplayer.es/10439151-Desarrollo-de-un-sistema-de-envasado-activo-biopeliculas-de-proteinas-lacteas-para-un-desarrollo-sostenible.html (accessed on 27 December 2019).
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Characterization of chitosan/caseinate films. J. Appl. Polym. Sci. 2008, 107, 1080–1090. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.D.C.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Kura, A.U.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Fakurazi, S. Preparation of Tween 80-Zn/Al-Levodopa-Layered Double Hydroxides Nanocomposite for Drug Delivery System. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.; Tian, G.; Jian, S.; Gu, Z.; Zhou, L.; Yan, L.; Jin, S.; Yin, W.; Zhao, Y. TWEEN coated NaYF4:Yb,Er/NaYF4 core/shell upconversion nanoparticles for bioimaging and drug delivery. RSC Adv. 2012, 2, 7037. [Google Scholar] [CrossRef]
- Khan, Y.; Durrani, S.K.; Siddique, M.; Mehmood, M. Hydrothermal synthesis of alpha Fe2O3 nanoparticles capped by Tween-80. Mater. Lett. 2011, 65, 2224–2227. [Google Scholar] [CrossRef]
- Fahami, A.; Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin A delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Vilanova, N.; Solans, C. Vitamin A Palmitate–β-cyclodextrin inclusion complexes: Characterization, protection and emulsification properties. Food Chem. 2015, 175, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Ghanbarzadeh, B.; Mohammadi, M.; Fathollahi, I.; Hamishehkar, H. Encapsulation of Vitamin A Palmitate in Nanostructured Lipid Carrier (NLC)-Effect of Surfactant Concentration on the Formulation Properties. Adv. Pharm. Bull. 2014, 4, 563–568. [Google Scholar] [PubMed]
- Che Man, Y.B.; Ammawath, W.; Mirghani, M.E.S. Determining α-tocopherol in refined bleached and deodorized palm olein by Fourier transform infrared spectroscopy. Food Chem. 2005, 90, 323–327. [Google Scholar] [CrossRef]
- Fathi, M.; Nasrabadi, M.N.; Varshosaz, J. Characteristics of vitamin E-loaded nanofibres from dextran. Int. J. Food Prop. 2017, 20, 2665–2674. [Google Scholar] [CrossRef] [Green Version]
- de la Cruz-Gavia, A.; Pérez-Alonso, C.; Barrera-Díaz, C.E.; Alvarez-Ramírez, J.; Carrillo-Navas, H.; Guadarrama-Lezama, A.Y. Survival of Saccharomyces cerevisiae microencapsulated with complex coacervate after freezing process. Food Hydrocoll. 2018, 82, 45–52. [Google Scholar] [CrossRef]
- Teixeira, Z.; Dreiss, C.A.; Lawrence, M.J.; Heenan, R.K.; Machado, D.; Justo, G.Z.; Guterres, S.S.; Durán, N. Retinyl palmitate polymeric nanocapsules as carriers of bioactives. J. Colloid Interface Sci. 2012, 382, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullio Gamboa, G.V.; Benedini, L.A.; Schulz, P.C.; Allemandi, D.A. Phase Behavior of Ascorbyl Palmitate Coagels Loaded with Oligonucleotides as a New Carrier for Vaccine Adjuvants. J. Surfactants Deterg. 2016, 19, 747–757. [Google Scholar] [CrossRef]
- Villacrez, J.L.; Carriazo, J.G.; Osorio, C. Microencapsulation of Andes Berry (Rubus glaucus Benth.) Aqueous Extract by Spray Drying. Food Bioprocess Technol. 2014, 7, 1445–1456. [Google Scholar] [CrossRef]
- Borde, B.; Bizot, H.; Vigier, G.; Buleon, A. Calorimetric analysis of the structural relaxation in partially hydrated amorphous polysaccharides. I. Glass transition and fragility. Carbohydr. Polym. 2002, 48, 83–96. [Google Scholar] [CrossRef]
- Nurhadi, B.; Roos, Y.H.; Maidannyk, V. Physical properties of maltodextrin DE 10: Water sorption, water plasticization and enthalpy relaxation. J. Food Eng. 2016, 174, 68–74. [Google Scholar] [CrossRef]
- Korang-Yeboah, M.; Gorantla, Y.; Paulos, S.A.; Sharma, P.; Chaudhary, J.; Palaniappan, R. Polycaprolactone/maltodextrin nanocarrier for intracellular drug delivery: Formulation, uptake mechanism, internalization kinetics, and subcellular localization. Int. J. Nanomed. 2015, 10, 4763–4781. [Google Scholar]
- Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y.; Ebian, A.E.R. Maltodextrin: A novel excipient used in sugar-based orally disintegrating tablets and phase transition process. AAPS PharmSciTech 2010, 11, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Delgado, K.L.; Varona, G.A.; Montilla, C.E.; Villada, H.S. Efecto del Aditivo Tween 80 y de la Temperatura de Procesamiento en las Propiedades Mecánicas de Tensión de Películas Flexibles Elaboradas a Partir de Almidón de Yuca Termoplástico. Inf. Tecnol. 2016, 27, 145–152. [Google Scholar] [CrossRef]
- Liu, P.; Yu, L.; Liu, H.; Chen, L.; Li, L. Glass transition temperature of starch studied by a high-speed DSC. Carbohydr. Polym. 2009, 77, 250–253. [Google Scholar] [CrossRef]
- Wang, S.; Ye, F.; Wei, F.; Zhao, G. Spray-drying of curcumin-loaded octenylsuccinated corn dextrin micelles stabilized with maltodextrin. Powder Technol. 2017, 307, 56–62. [Google Scholar] [CrossRef]
- Ponce Cevallos, P.A.; Buera, M.P.; Elizalde, B.E. Encapsulation of cinnamon and thyme essential oils components (cinnamaldehyde and thymol) in β-cyclodextrin: Effect of interactions with water on complex stability. J. Food Eng. 2010, 99, 70–75. [Google Scholar] [CrossRef]
- Matiacevich, S.; Riquelme, N.; Herrera, M.L. Conditions to Prolonged Release of Microencapsulated Carvacrol on Alginate Films as Affected by Emulsifier Type and PH. Int. J. Polym. Sci. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dennis, M.J.; Heaton, K.; Rhodes, C.; Kelly, S.D.; Hird, S.; Brereton, P.A. Investigation into the use of pyrolysis-elemental analysis for the measurement of carbohydrates in foodstuffs. Anal. Chim. Acta 2006, 555, 175–180. [Google Scholar] [CrossRef]
- Castro, N.; Durrieu, V.; Raynaud, C.; Rouilly, A. Influence of DE-value on the physicochemical properties of maltodextrin for melt extrusion processes. Carbohydr. Polym. 2016, 144, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Anastasakis, K.; Ross, A.B.; Jones, J.M. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel 2011, 90, 598–607. [Google Scholar] [CrossRef]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J.; Mcphee, D.J. Technological Application of Maltodextrins According to the Degree of Polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, M.M.; Rosana, C.; De Castro Lima, R.; Santos Quenca-Guillen, J.; Filho, M.; Mercuri, L.P.; Rocha, M.I.; Santoro, M.; Rosa, E.; Kedor-Hackmann, M. Stability evaluation of tocopheryl acetate and ascorbyl tetraisopalmitate in isolation and incorporated in cosmetic formulations using thermal analysis. Braz. J. Pharm. Sci. 2010, 46, 129–134. [Google Scholar] [CrossRef]
- He, H.; Hong, Y.; Gu, Z.; Liu, G.; Cheng, L.; Li, Z. Improved stability and controlled release of CLA with spray-dried microcapsules of OSA-modified starch and xanthan gum. Carbohydr. Polym. 2016, 147, 243–250. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Storebakken, T. Effects of dietary cellulose level on pellet quality and nutrient digestibilities in rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 272, 458–465. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.A.; Calderas, F.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Ochoa-Martínez, L.A.; Bernad-Bernad, M.J. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Artiaga, R.; Naya, S.; García, A.; Barbadillo, F.; García, L. Subtracting the water effect from DSC curves by using simultaneous TGA data. Thermochim. Acta 2005, 428, 137–139. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |






| Sample | Z-Average Size (nm) | PDI |
|---|---|---|
| MD:CAP:TW:VA | 102.5 ± 1.3 a | 0.473 ± 0.091 a |
| MD:CAP:TW:VE | 75.1 ± 0.1 b | 0.286 ± 0.050 b |
| MD:SC:TW:VA | 100.4 ± 0.2 a | 0.431 ± 0.009 a |
| MD:SC:TW:VE | 80.1 ± 2.7 c | 0.263 ± 0.004 b |
| Sample | Product Yield (%) | Encapsulation Efficiency (%) | Moisture (%db) | Bulk Density (g·cm−3) | Particle Density (g·cm−3) | Porosity (%) |
|---|---|---|---|---|---|---|
| MD:CAP:TW | 80.3 ± 1.2 ab | - | 8.1 ± 1.0 b | 0.44 ± 0.01 a | 1.14 ± 0.01 a | 62 ab |
| MD:CAP:TW:VA | 81.3 ± 2.0 abc | 100 ± 5 | 5.9 ± 2.2 ab | 0.40 ± 0.01 a | 1.12 ± 0.02 a | 64 a |
| MD:CAP:TW:VE | 79.7 ± 0.7 ab | 48 ± 3 | 8.2 ± 1.2 b | 0.37 ± 0.01 a | 1.06 ± 0.01 b | 65 a |
| MD:SC:TW | 77.3 ± 1.6 a | - | 6.5 ± 1.7 b | 0.44 ± 0.05 a | 1.09 ± 0.01 ab | 60 b |
| MD:SC:TW:VA | 83.1 ± 1.4 bc | 23 ± 4 | 1.9 ± 0.8 a | 0.35 ± 0.04 a | 1.25 ± 0.21 ab | 69 c |
| MD:SC:TW:VE | 84.6 ± 0.6 c | 29 ± 5 | 4.4 ± 1.5 ab | 0.40 ± 0.03 a | 1.10 ± 0.02 ab | 64 a |
| Sample | Tp1 (°C) | Δh1 (J·g−1) | Tp2 (°C) | Δh2 (J·g−1) | Water Content (%) |
|---|---|---|---|---|---|
| Vitamin A (VA) | ~0 | 43.2 | - | - | - |
| Vitamin E (VE) | - | - | - | - | - |
| MD:CAP:TW | - | - | 82.1 | 155.6 | 6.4 |
| MD:CAP:TW:VA | ~0 | 0.4 | 81.4 | 157.3 | 6.5 |
| MD:CAP:TW:VE | - | - | 80.4 | 158.6 | 6.5 |
| MD:SC:TW | - | - | 77.2 | 161.2 | 6.6 |
| MD:SC:TW:VA | ~0 | 0.2 | 77.7 | 133.6 | 5.5 |
| MD:SC:TW:VE | - | - | 76.5 | 163.9 | 6.7 |
| Samples | Wall Polymer Blends | Vitamin | Emulsifier |
|---|---|---|---|
| MD:CAP:TW:VA | MD:CAP (70:30 % w/w) | VA (1% w/w) | TW (1% w/w) |
| MD:CAP:TW:VE | VE (1% w/w) | ||
| MD:SC:TW:VA | MD:SC (70:30 % w/w) | VA (1% w/w) | |
| MD:SC:TW:VE | VE (1% w/w) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujica-Álvarez, J.; Gil-Castell, O.; Barra, P.A.; Ribes-Greus, A.; Bustos, R.; Faccini, M.; Matiacevich, S. Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry. Molecules 2020, 25, 1357. https://doi.org/10.3390/molecules25061357
Mujica-Álvarez J, Gil-Castell O, Barra PA, Ribes-Greus A, Bustos R, Faccini M, Matiacevich S. Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry. Molecules. 2020; 25(6):1357. https://doi.org/10.3390/molecules25061357
Chicago/Turabian StyleMujica-Álvarez, Javiera, O. Gil-Castell, Pabla A. Barra, A. Ribes-Greus, Rubén Bustos, Mirko Faccini, and Silvia Matiacevich. 2020. "Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry" Molecules 25, no. 6: 1357. https://doi.org/10.3390/molecules25061357
APA StyleMujica-Álvarez, J., Gil-Castell, O., Barra, P. A., Ribes-Greus, A., Bustos, R., Faccini, M., & Matiacevich, S. (2020). Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry. Molecules, 25(6), 1357. https://doi.org/10.3390/molecules25061357








