Influence of a Commercial Biological Fungicide containing Trichoderma harzianum Rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil
Abstract
1. Introduction
2. Results
2.1. Clomazone
2.2. Fluazifop-P-butyl
2.3. Metribuzin
2.4. Pendimethalin
2.5. Propyzamide
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Soil Samples Preparation
4.3. GC-µECD Analysis of Herbicide Residues
4.4. Confirmatory GC-MS Analysis of Pesticides and Possible Metabolites
4.5. Method Validation
4.6. Statistical Analysis of Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Podbielska, M.; Szpyrka, E.; Matyaszek, A.; Słowik-Borowiec, M.; Rupar, J.; Kurdziel, A. Occurrence and estimation of pesticide residues in edible minor crops in southeastern Poland in 2013–2014. Environ. Monit Assess. 2016, 188, 386. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic contaminants of high concern: Existence in water resources and their adverse effects. Sci Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture (Basel) 2018, 8, 13. [Google Scholar] [CrossRef]
- Matyjaszczyk, E. Products containing microorganisms as a tool in integrated pest management and the rules of their market placement in the European Union. Pest. Manag Sci. 2015, 71, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, S. Factors affecting the use of microbial biopesticides in plant protection. Prog Plant. Prot. 2012, 52, 957–962. [Google Scholar]
- Tahri Joutey, N.; Bahafid, W.; Sayel, H.; El Ghachtouli, N. Biodegradation: Involved Microorganisms and Genetically Engineered Microorganisms. Biodegradation; Life of Science: London, UK, 2013; Chapter 11, Intech Open; pp. 289–320. ISBN 978-953-51-1154-2. Available online: https://www.intechopen.com/books/biodegradation-life-of-science/biodegradation-involved-microorganisms-and-genetically-engineered-microorganisms (accessed on 8 January 2020). [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial Degradation of Pesticide Residues and an Emphasis on the Degradation of Cypermethrin and 3-phenoxy Benzoic Acid: A Review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Wołejko, E.; Łozowicka, B.; Kaczyński, P.; Jankowska, M.; Piekut, J. The influence of effective microorganisms (EM) and yeast on the degradation of strobilurins and carboxamides in leafy vegetables monitored by LC-MS/MS and health risk assessment. Environ. Monit Assess. 2016, 188, 64. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef]
- Dinesh, R.; Prateeksha, M. A review on interactions of Trichoderma with Plant and Pathogens. Res. J. Agric. For. Sci. 2015, 3, 20–23. Available online: http://www.isca.in/AGRI_FORESTRY/Archive/v3/i2/5.ISCA-RJAFS-2014-062.pdf (accessed on 10 January 2020).
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-based novel formulations: Potential applications for management of Next-Gen agricultural challenges. J. Chem. Technol. Biotechnol. 2018, 93, 2056–2063. [Google Scholar] [CrossRef]
- Patil, K.C.; Matsumura, F.; Boush, G.M. Degradation of endrin, aldrin, and DDT by soil microorganisms. J. App Microbiol. 1970, 19, 879–881. [Google Scholar] [CrossRef]
- Katayama, A.; Matsumura, F. Degradation of Organochlorine Pesticides, Particularly Endosulfan, by Trichoderma Harzianum. Environ. Toxicol. Chem. 1993, 12, 1059–1065. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Anthonisamy, A.; Arunkumar, S.; Sivakumari, V. Biodegradation of Methyl Parathion and Endosulfan Using Pseudomonas Aeruginosa and Trichoderma Viridae. J. Environ. Sci. Eng. 2011, 53, 115–122. [Google Scholar] [PubMed]
- Jayaraman, P.; Naveen Kumar, T.; Maheswaran, P.; Sagadevan, E.; Arumugam, P. In vitro Studies on Biodegradation of Chlorpyrifos by Trichoderma viride and T. harzianum. J. Pure Appl. Microbiol. 2012, 6, 1465–1474. [Google Scholar]
- Harish, R.; Supreeth, M.; Chauhan, J.B. Biodegradation of organophosphate pesticide by soil fungi. Adv. Bio Tech. 2013, 12, 4–8. [Google Scholar]
- Baarschers, W.H.; Heitland, H.S. Biodegradation of fenitrothion and fenitrooxon by the fungus Trichoderma viride. J. Agric. Food Chem. 1986, 34, 707–709. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, M.; Manokaran, R.; Singh, D.V.; Srivastava, M. Use of Trichoderma spp. in biodegradation of carbendazim. Indian J. Agric. Sci. 2016, 86, 891–894. [Google Scholar]
- EU Database. EU Pesticides Database; Directorate-General for Health and Food Safety European Commission: Brussels, Belgium, 2020; Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN (accessed on 9 August 2019).
- BPDB 2019. Bio-Pesticides DataBase; University of Hertfordshire: Hatfield, UK, 2019; Available online: https://sitem.herts.ac.uk/aeru/bpdb/index.htm (accessed on 9 August 2019).
- Koppert BV. TRIANUM-G label; Koppert BV: Berkel en Rodenrijs, The Netherlands, 2017. Available online: https://www.gov.pl/documents/912055/913531/Trianum-G.pdf/12509ee2-50e0-df1b-9c07-d535ac82ca63?version=1.0 (accessed on 11 July 2019).
- Ministry of Agriculture and Rural Development. Labels of Plant Protection Products; Ministry of Agriculture and Rural Development: Warsaw, Poland, 2018. Available online: https://www.gov.pl/web/rolnictwo/etykiety-srodkow-ochrony-roslin (accessed on 12 November 2018).
- PubChem. U.S. National Library of Medicine National Center for Biotechnology Information. 2019. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 11 July 2019).
- PPDB 2019. Pesticide Properties Database; University of Hertfordshire: Hatfield, UK, 2019; Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 27 July 2019).
- Wołejko, E.; Kaczyński, P.; Łozowicka, B.; Wydro, U.; Borusiewicz, A.; Hrynko, I.; Konecki, R.; Snarska, K.; Dec, D.; Malinowski, P. Dissipation of S-metolachlor in plant and soil and effect on enzymatic activities. Environ. Monit Assess. 2017, 189, 355. [Google Scholar] [CrossRef]
- EFSA. Conclusion regarding the peer review of the pesticide risk assessment of the active substance metribuzin. European Food Safety Authority (EFSA). EFSA J. 2006, 88, 1–74. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Brown, C.D. Factors influencing degradation of pesticides in soil. J. Agric. Food Chem. 2007, 55, 4487–4492. [Google Scholar] [CrossRef]
- Van Scoy, A.R.; Tjeerdema, R.S. Environmental fate and toxicology of clomazone. Rev. Environ. Contam Toxicol. 2014, 229, 35–49. [Google Scholar] [CrossRef]
- Zanella, R.; Primel, E.G.; Gonçalves, F.F.; Martins, M.L.; Adaime, M.B.; Marchesan, E.; Machado, S.L.O. Study of the degradation of the herbicide clomazone in distilled and in irrigated rice field waters using HPLC-DAD and GC-MS. J. Braz. Chem. Soc. 2008, 19, 987–995. [Google Scholar] [CrossRef]
- Tomco, P.L.; Holstege, D.M.; Zou, W.; Tjeerdema, R.S. Microbial degradation of clomazone under simulated California rice field conditions. J. Agric. Food Chem. 2010, 58, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Achermann, S.; Helbling, D.E.; Johnson, D.R.; Fenner, K. Relative contribution of ammonia oxidizing bacteria and other members of nitrifying activated sludge communities to micropollutant biotransformation. Water Res. 2017, 109, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.R.; Leão, E.U.; Santos, G.R.; Sarmento-Brum, R.B.C.; Gonçalves, C.G.; Cardon, C.H.; Silva, D.B. Impact of herbicides on strains of Trichoderma spp. Planta Daninha. 2013, 31, 419–426. [Google Scholar] [CrossRef][Green Version]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; López-Bucio, J.; Herrera-Estrella, A. Trichoderma-Induced Acidification Is an Early Trigger for Changes in Arabidopsis Root Growth and Determines Fungal Phytostimulation. Front. Plant. Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Durkin, P.R. Scoping/Screening Level Risk Assessment on Fluazifop-P-butyl; Syracuse Environmental Research Associates Inc.: New York, NY, USA, 2013; p. 293. Available online: https://www.fs.fed.us/foresthealth/pesticide/pdfs/Fluazifop-P-butyl.pdf (accessed on 7 July 2019).
- Wahla, A.Q.; Iqbal, S.; Anwar, S.; Firdous, S.; Mueller, J.A. Optimizing the metribuzin degrading potential of a novel bacterial consortium based on Taguchi design of experiment. J. Hazard. Mater. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Nowara, A.M.; Radwan, M.A. Impact of pesticides on Trichoderma harzianum and on its possible antagonistic activity against Fusarium oxysporum under In vitro conditions. Asian J. Agric. Biol. 2017, 5, 291–302. [Google Scholar]
- Bordjiba, O.; Steiman, R.; Kadri, M.; Semadi, A.; Guiraud, P. Removal of herbicides from liquid media by fungi isolated from a contaminated soil. J. Environ. Qual. 2001, 30, 418–426. [Google Scholar] [CrossRef]
- Kočárek, M.; Artikov, H.; Voříšek, K.; Borůvka, L. Pendimethalin degradation in soil and its interaction with soil microorganisms. Soil Water Res. 2016, 11, 213–219. [Google Scholar] [CrossRef]
- Barrere, C.; Bastide, J.; Coste, C.M. Relations entre la vitesse de dégradation du propyzamide et les propriétés physicochimiques des sols. Weed Res. 2006, 28, 93–99. [Google Scholar] [CrossRef]
- Matsumura, F.; Boush, G.M. Degradation of insecticides by a soil fungi Trichoderma viride. J. Econ. Entomol. 1968, 61, 610–612. [Google Scholar] [CrossRef] [PubMed]
- PN-ISO 11465:1999. Wersja Polska. Jakość Gleby—Oznaczanie Zawartości Suchej Masy Gleby i Wody w Glebie w Przeliczeniu na suchą Masę Gleby—Metoda Wagowa; The Polish Committee for Standardization: Warsaw, Poland, 1999; pp. 1–7. [Google Scholar]
- PN-EN 15662:2018. Foods of Plant Origin—Multimethod for the Determination of Pesticide Residues Using GC- and LC-based Analysis Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE—Modular QuEChERS-method; Polski Komitet Normalizacyjny: Warszawa, Poland, 2018; p. 82. [Google Scholar]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef] [PubMed]
- Ðurović-Pejčev, R.D.; Bursić, V.P.; Zeremski, T.M. Comparison of QuEChERS with Traditional Sample Preparation Methods in the Determination of Multiclass Pesticides in Soil. J. AOAC Int. 2018, 102, 46–51. [Google Scholar] [CrossRef]
- Pszczolińska, K.; Michel, M. The QuEChERS Approach for the determination of pesticide residues in soil samples: An Overview. J. AOAC Int. 2016, 99, 1403–1414. [Google Scholar] [CrossRef]
- SANTE 2017. Document SANTE/11813/2017. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; Manufacturers: Brussels, Belgium, 2017; p. 46. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 5 April 2019).
Sample Availability: Samples of the soil are not available from the authors. |
| Active Substance | Partition Coefficient Octanol-Water log P | Chemical Structure | Chemical Group/HRAC 1 Group—Mode of Action | Soil Degradation/Mobility/Laboratory Half-Life/Field Half-Life | Possible Metabolites in Soil |
|---|---|---|---|---|---|
| Clomazone | 2.58 |  | Isoxazolidinone/F3—carotenoid biosynthesis inhibitor | Non-persistent/moderately mobile /6.3–145.7 days/9.3–195 days | (N-((2-chlorobenzyl))-3-hydroxy-2.2-dimethylpropanamide) |
| Fluazifop-P-butyl | 4.5 | 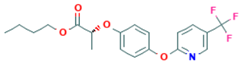 | Aryloxyphenoxypropionate/A1—acetyl CoA carboxylase (ACCase) inhibitor | Non-persistent/slightly mobile/0.3–3.3 days/2.1–38.0 days | no |
| Metribuzin | 1.75 |  | Triazinone/C1—photosystem II inhibitor | Non-persistent/mobile/4.73–12.5 days/19 days | Diketo-metribuzin; desaminodiketo-metribuzin; desamino-metribuzin |
| Pendimethalin | 5.4 | 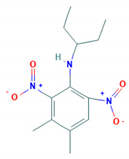 | Dinitroaniline/K1—mitosis inhibitor | Persistent/non-mobile/97–270 days/39.8–187 days | 2-methyl-3.5-dinitro-4-(pentan-3ylamino)benzoic acid |
| Propyzamide | 3.27 | 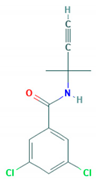 | Benzamide/K1—mitosis inhibitor | Moderately persistent/ slightly mobile/13.9–271.3 days/233 | 2-(3.5-dichlorophenyl)-4.4-dimethyl-5-methylene-oxazoline; N-(1.1-dimethylacetonyl)-3.5-dichlorobenzamide |
| Active Substance | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Equation (R 1) | t½ 2 (day) | Differences in t½ 2 (day) | Differences in t½ 2 (%) | Equation (R 1) | t½ 2 (day) | Differences in t½ 2 (day) | Differences in t½ 2 (%) | |
| Clomazone | y = 25.0012e−0.0078x (0.8889) | 88.8 | 6.3 | 7.1 | y = 18.4837e−0.0095x (0.9374) | 72.9 | 18.3 | 25.1 |
| Clomazone + T. harzianum Rifai T-22 | y = 23.6252e−0.0084x (0.9725) | 82.5 | y = 18.7542e−0.0127x (0.9546) | 54.6 | ||||
| Fluazifop-P-butyl | y = 5.0777e−0.0660x (0.7800) | 10.5 | 0.3 | 2.9 | y = 2.8792e−0.0573x (0.7594) | 12.1 | 1.2 | 9.9 |
| Fluazifop-P-butyl + T. harzianum Rifai T-22 | y = 4.4082e−0.0680x (0.7743) | 10.2 | y = 3.7225e−0.0637x (0.8226) | 10.9 | ||||
| Metribuzin | y = 681.8803e−0.0139x (0.9797) | 49.9 | 9.1 | 18.2 | y = 529.6056e−0.0190x (0.9045) | 36.5 | 5.3 | 14.5 |
| Metribuzin + T. harzianum Rifai T-22 | y = 715.2762e−0.0170x (0.9436) | 40.8 | y = 540.8551e−0.0222x (0.9097) | 31.2 | ||||
| Pendimethalin | y = 3534.1050e−0.0154x (0.9010) | 45.0 | 12.3 | 27.3 | y = 2675.0044e−0.0148x (0.9272) | 46.8 | 0.3 | 0.6 |
| Pendimethalin + T. harzianum Rifai T-22 | y = 3040.6923e−0.0121x (0.9019) | 57.3 | y = 2497.1433e−0.0147x (0.8960) | 47.1 | ||||
| Propyzamide | y = 1398.8502e−0.0204x (0.8942) | 34.0 | 4.3 | 12.6 | y = 763.2167e−0.0128x (0.9664) | 54.1 | 12.1 | 22.4 |
| Propyzamide + T. harzianum Rifai T-22 | y = 1426.6253e−0.0181x (0.8746) | 38.3 | y = 783.1032e−0.0165x (0.9625) | 42.0 | ||||
| Parameter | Soil 1 | Soil 2 |
|---|---|---|
| Type | Horticultural soil recommended for vegetable production | Universal peat substrate mixed with perlite |
| Fraction | 0–5 mm | 0–30 mm |
| pH | 4.6 ± 0.1 1 | 5.3 ± 0.1 |
| Humus content | 69.9 ± 0.5% | 55.6 ± 0.4% |
| Total carbon | 46.4 ± 0.4% | 36.6 ± 0.3% |
| Organic carbon | 40.5 ± 0.4% | 32.2 ± 0.4% |
| Total nitrogen | 1.6 ± 0.1% | 1.4 ± 0.1% |
| Assimilable phosphorus | 104.5 ± 2.2 mg P2O5/100g | 14.9 ± 0.6 mg P2O5/100g |
| Other elements, as mg/kg | Li 0.13 ± 0.01, Be 0.03 ± 0.01, V 1.71 ± 0.30, Cr 1.32 ± 0.30, Mn 25.07 ± 1.80, Co 0.20 ± 0.02, Ni 1.09 ± 0.09, Cu 9.47 ± 1.10, Zn 12.16 ± 2.60, As 1.37 ± 0.10, Se 0.52 ± 0.08, Sr 33.83 ± 4.20, Mo 11.57 ± 1.90, Cd 0.15 ± 0.04, Sb 0.06 ± 0.02, Ba 29.62 ± 2.90, La 0.59 ± 0.12, Ce 1.42 ± 0.30, Eu 0.03 ± 0.01, Gd 0.14 ± 0.02, Tl 0.02 ± 0.01, Pb 5.06 ± 0.80, Bi 0.08 ± 0.01, Na 173.50 ± 8.90, Mg 805.79 ± 19.60, Al 699.63 ± 42.0, K 1080.42 ± 25.0, Ca 14390.90 ± 256.0, Fe 2808.49 ± 86.0 | Li 0.48 ± 0.09, Be 0.07 ± 0.02, V 3.26 ± 0.15, Cr 1.95 ± 0.17, Mn 30.74 ± 1.16, Co 0.93 ± 0.06, Ni 4.30 ±0.13, Cu 3.78 ± 0.41, Zn 6.75 ± 0.90, As 2.66 ± 0.11, Se 0.51 ± 0.02, Sr 85.10 ± 2.10, Mo 0.62 ± 0.02, Cd 0.14 ± 0.03, Sb 0.12 ± 0.02, Ba 20.47 ± 1.10, La 1.49 ± 0.08, Ce 2.97 ± 0.50, Eu 0.06 ± 0.02, Gd 0.30 ± 0.08, Tl 0.02 ± 0.01, Pb 4.62 ± 0.70, Bi 0.10 ± 0.03, Na 299.17 ± 9.20, Mg 637.42 ± 14.10, Al 1410.11 ± 65.0, K 431.25 ± 11.20, Ca 22094.49 ± 131.0, Fe 3290.40 ± 47.0 |
| Plant Protection Product | Active Substance | Active Substance Content in Product | Recommended Dose | Recommended Water Volume | Application Method |
|---|---|---|---|---|---|
| Command 480 EC | clomazone | 480 g/L | 0.2 L/ha | 200–300 L/ha | spray |
| Aurelit 70 WG | metribuzin | 700 g/kg | 0.5 kg/ha | 200–300 L/ha | |
| Stomp Aqua 455 CS | pendimethalin | 455 g/L | 25 mL/100 m2 | 2–4 L/100 m2 | |
| Fusilade Forte 150 EC | fluazifop-P-butyl | 150 g/L | 0.63–1.7 L/ha | 100–400 L/ha | |
| Kerb 50 WP | propyzamide | 500 g/kg | 1–1.5 kg/ha | 300–400 L | |
| Trianum-G | T. harzianum Rifai T-22 | 10 g/kg | 750 g/1 m3 of soil | - | mixing with soil |
| Active Substance | Linearity (R2) 1 | Average Recovery (±RSD 2) (%) | Matrix Effects (±RSD 2) (%) | ||
|---|---|---|---|---|---|
| Soil 1 | Soil 2 | Soil 1 | Soil 2 | ||
| Clomazone | 0.995 | 81 (6) | 73 (2) | 5 | −1 |
| Fluazifop-P-butyl | 0.990 | 84 (3) | 80 (16) | −42 | −28 |
| Metribuzin | 0.997 | 120 (7) | 119 (10) | 10 | 11 |
| Pendimethalin | 0.999 | 114 (5) | 119 (7) | 5 | 2 |
| Propyzamide | 0.999 | 120 (5) | 107 (9) | 4 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpyrka, E.; Podbielska, M.; Zwolak, A.; Piechowicz, B.; Siebielec, G.; Słowik-Borowiec, M. Influence of a Commercial Biological Fungicide containing Trichoderma harzianum Rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil. Molecules 2020, 25, 1391. https://doi.org/10.3390/molecules25061391
Szpyrka E, Podbielska M, Zwolak A, Piechowicz B, Siebielec G, Słowik-Borowiec M. Influence of a Commercial Biological Fungicide containing Trichoderma harzianum Rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil. Molecules. 2020; 25(6):1391. https://doi.org/10.3390/molecules25061391
Chicago/Turabian StyleSzpyrka, Ewa, Magdalena Podbielska, Aneta Zwolak, Bartosz Piechowicz, Grzegorz Siebielec, and Magdalena Słowik-Borowiec. 2020. "Influence of a Commercial Biological Fungicide containing Trichoderma harzianum Rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil" Molecules 25, no. 6: 1391. https://doi.org/10.3390/molecules25061391
APA StyleSzpyrka, E., Podbielska, M., Zwolak, A., Piechowicz, B., Siebielec, G., & Słowik-Borowiec, M. (2020). Influence of a Commercial Biological Fungicide containing Trichoderma harzianum Rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil. Molecules, 25(6), 1391. https://doi.org/10.3390/molecules25061391








