Unknown Areas of Activity of Human Ribonuclease Dicer: A Putative Deoxyribonuclease Activity
Abstract
1. Introduction
2. Results
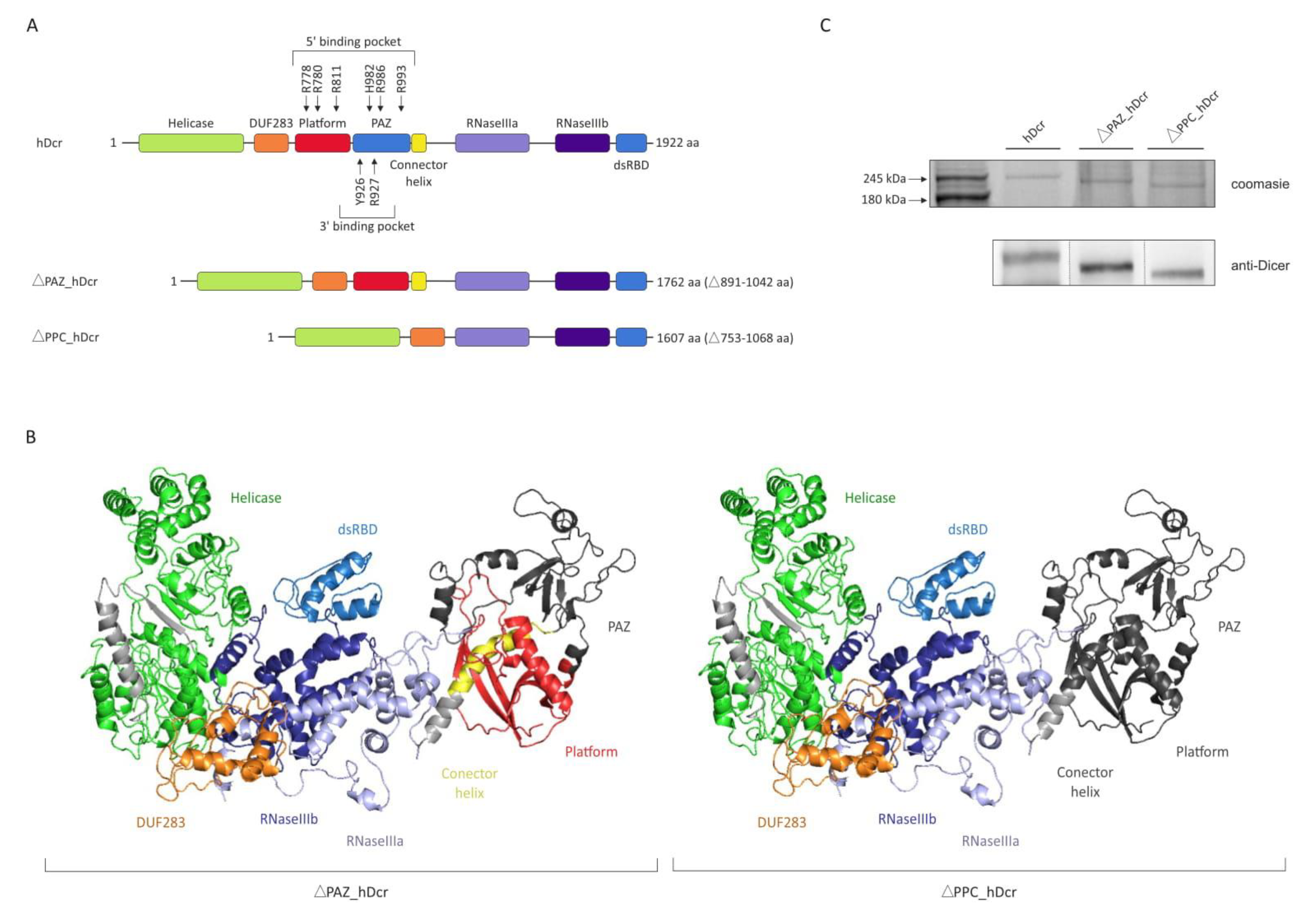
2.1. Production of the hDicer PAZ Domain Deletion Variants
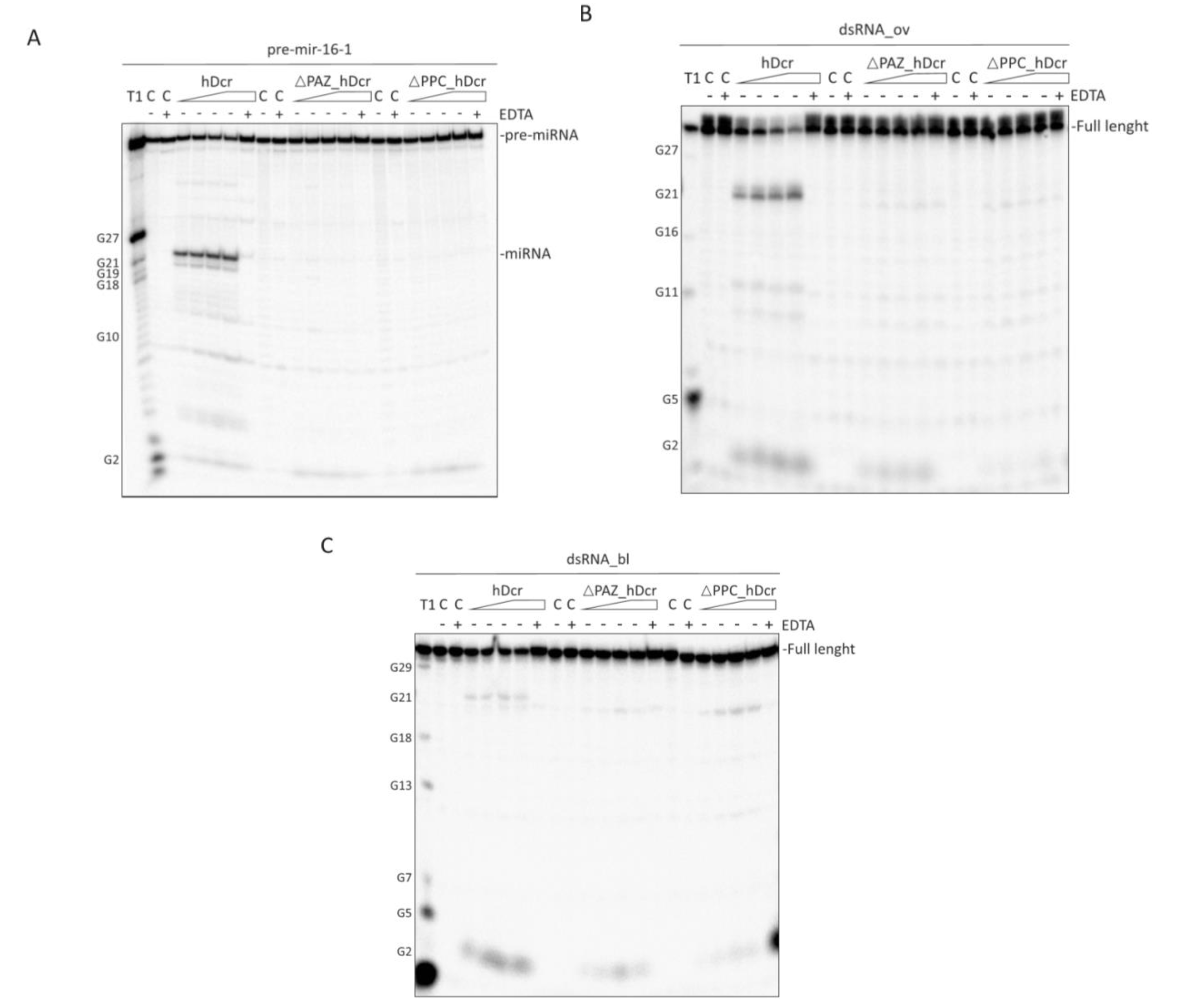
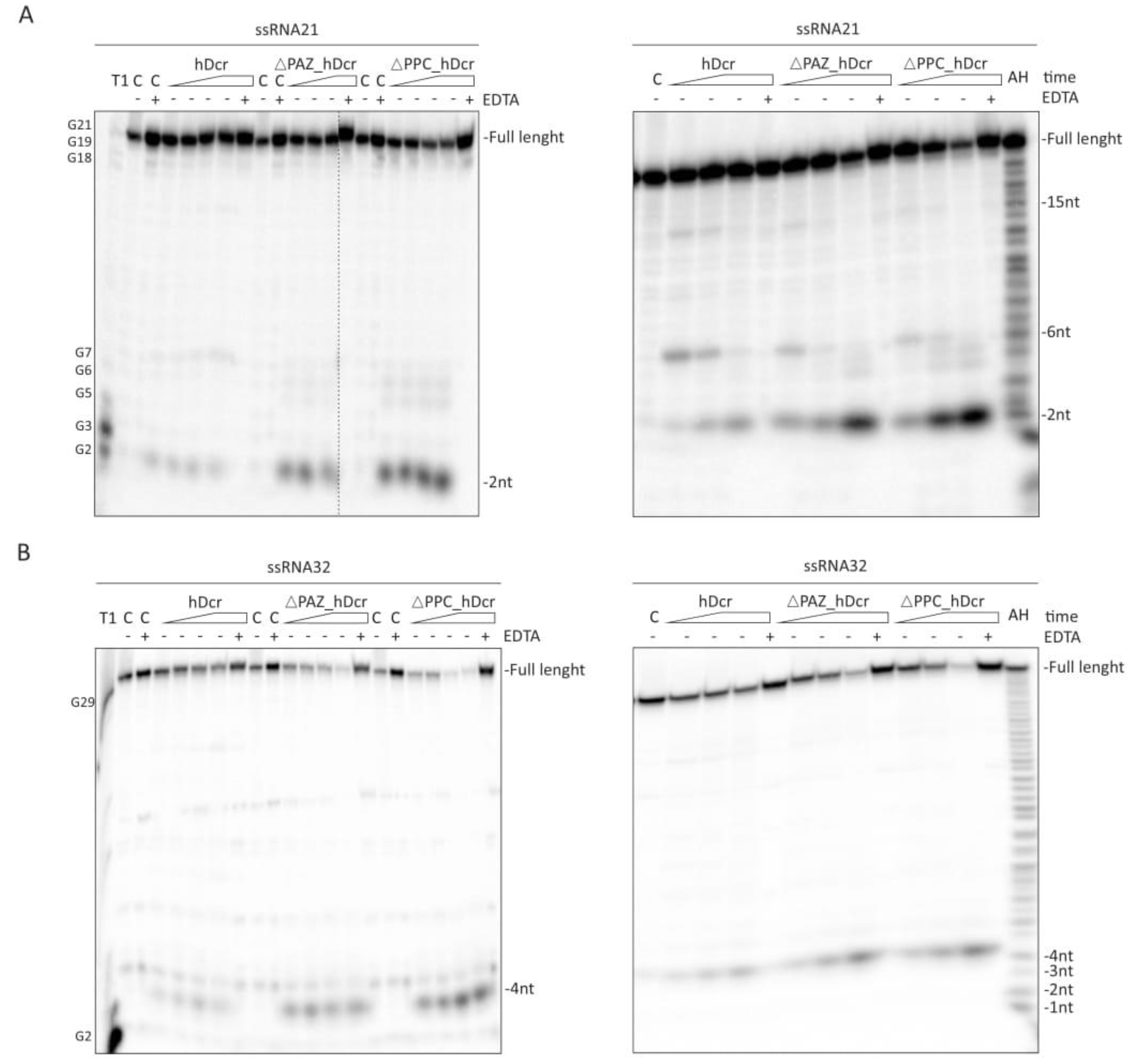
2.2. RNase Activity of Full-Length hDicer and the PAZ Domain Deletion Variants
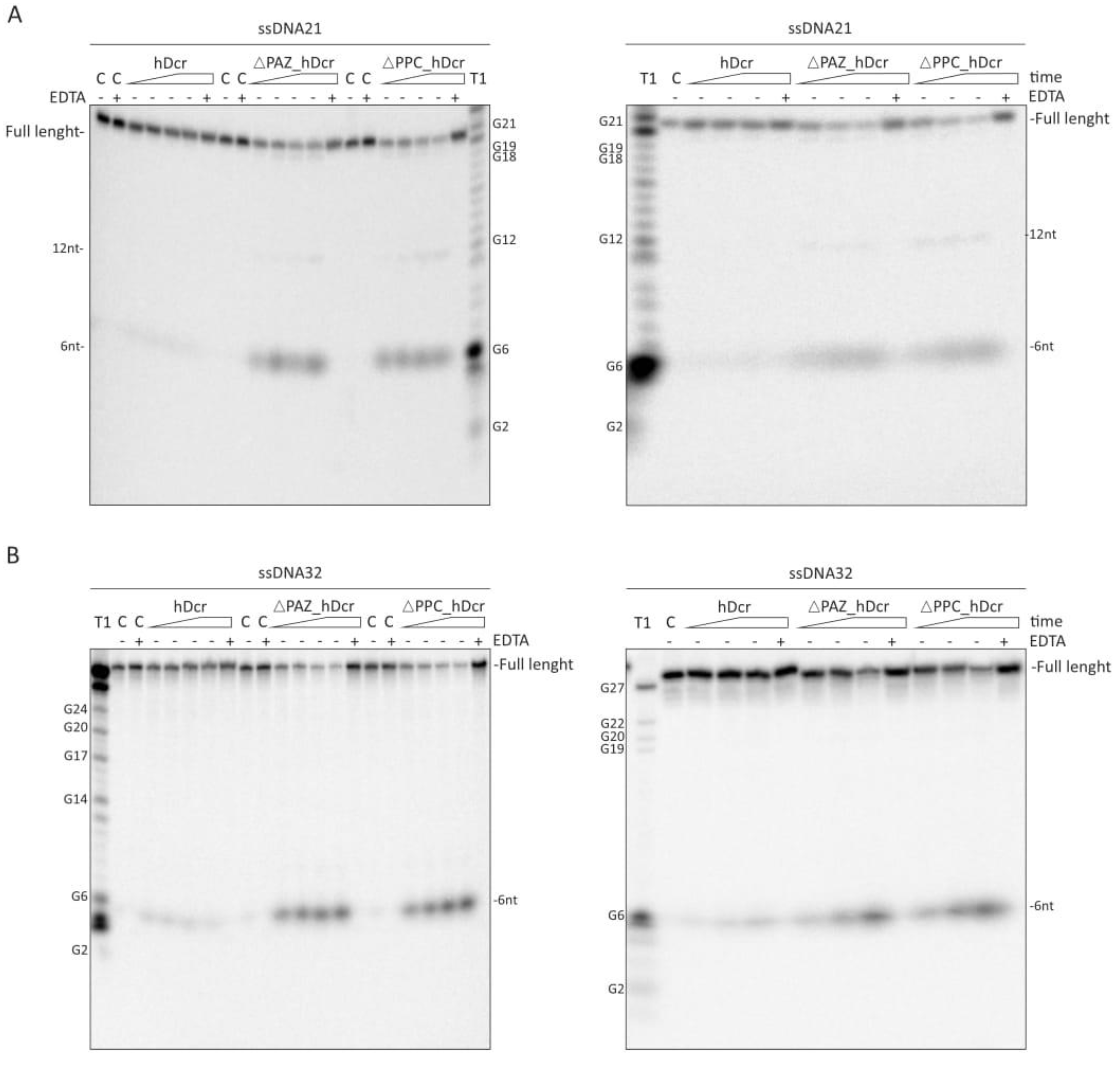
2.3. DNase Activity of Full-Length hDicer and the PAZ Domain Deletion Variants
3. Discussion
4. Materials and Methods
4.1. Oligonucleotides
4.2. The 5ʹ-end Labeling of Oligonucleotides
4.3. Preparation of dsRNA
4.4. Preparation of Expression Plasmids
4.5. Cell Culture and Transfection
4.6. Immunoprecipitation
4.7. Western Blot Analysis
4.8. hDicer Cleavage Assay
4.9. Gel Imaging and Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef]
- Valencia-Sanchez, M.A.; Liu, J.; Hannon, G.J.; Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006, 20, 515–524. [Google Scholar] [CrossRef]
- Grishok, A.; Pasquinelli, A.E.; Conte, D.; Li, N.; Parrish, S.; Ha, I.; Baillie, D.L.; Fire, A.; Ruvkun, G.; Mello, C.C. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 2001, 106, 23–34. [Google Scholar] [CrossRef]
- Volpe, T.A.; Kidner, C.; Hall, I.M.; Teng, G.; Grewal, S.I.; Martienssen, R.A. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 2002, 297, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, K.; Fine, N.A.; Fujisawa, T.; Gorovsky, M.A. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in tetrahymena. Cell 2002, 110, 689–699. [Google Scholar] [CrossRef]
- Baulcombe, D. Viral suppression of systemic silencing. Trends Microbiol. 2002, 10, 306–308. [Google Scholar] [CrossRef]
- Kurzynska-Kokorniak, A.; Jackowiak, P.; Figlerowicz, M.; Figlerowicz, M. Human- and virus-encoded microRNAs as potential targets of antiviral therapy. Mini Rev. Med. Chem. 2009, 9, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012, 4, 143–159. [Google Scholar] [CrossRef]
- Novak, J.; Kruzliak, P.; Bienertova-Vasku, J.; Slaby, O.; Novak, M. MicroRNA-206: A promising theranostic marker. Theranostics 2014, 4, 119–133. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Costinean, S.; Croce, C.M. MicroRNAs, the immune system and rheumatic disease. Nat. Clin. Pract. Rheumatol. 2008, 4, 534–541. [Google Scholar] [CrossRef]
- Mukherjee, K.; Campos, H.; Kolaczkowski, B. Evolution of animal and plant dicers: Early parallel duplications and recurrent adaptation of antiviral RNA binding in plants. Mol. Biol. Evol. 2012, 30, 627–641. [Google Scholar] [CrossRef]
- Macrae, I.J.; Li, F.; Zhou, K.; Cande, W.Z.; Doudna, J.A. Structure of Dicer and mechanistic implications for RNAi. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 73–80. [Google Scholar] [CrossRef]
- Macrae, I.J.; Zhou, K.; Li, F.; Repic, A.; Brooks, A.N.; Cande, W.Z.; Adams, P.D.; Doudna, J.A. Structural basis for double-stranded RNA processing by Dicer. Science 2006, 311, 195–198. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Brondani, V.; Billy, E.; Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002, 21, 5875–5885. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 2004, 118, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.; Fields, C.; Aadland, K.; Wei, T.; Kolaczkowski, O.; Gu, T.; Kolaczkowski, B.; Xie, M. Dicer cleaves 5’-extended microRNA precursors originating from RNA polymerase II transcription start sites. Nucleic Acids Res. 2018, 46, 5737–5752. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Heo, I.; Tian, Y.; Simanshu, D.K.; Chang, H.; Jee, D.; Patel, D.J.; Kim, V.N. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature 2011, 475, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.W.; Guiley, K.Z.; De, N.; Potter, C.S.; Carragher, B.; MacRae, I.J. The molecular architecture of human Dicer. Nat. Struct. Mol. Biol. 2012, 19, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Cheng, H.; Ke, X.; Sun, L.; Zhang, Q.C.; Wang, H.-W. Cryo-EM Structure of Human Dicer and Its Complexes with a Pre-miRNA Substrate. Cell 2018, 173, 1191–1203.e1112. [Google Scholar] [CrossRef]
- MacRae, I.J.; Doudna, J.A. Ribonuclease revisited: Structural insights into ribonuclease III family enzymes. Curr. Opin. Struct. Biol. 2007, 17, 138–145. [Google Scholar] [CrossRef]
- Tian, Y.; Simanshu, D.K.; Ma, J.B.; Park, J.E.; Heo, I.; Kim, V.N.; Patel, D.J. A Phosphate-Binding Pocket within the Platform-PAZ-Connector Helix Cassette of Human Dicer. Mol. Cell 2014, 53, 606–616. [Google Scholar] [CrossRef]
- Mickiewicz, A.; Sarzynska, J.; Milostan, M.; Kurzynska-Kokorniak, A.; Rybarczyk, A.; Lukasiak, P.; Kulinski, T.; Figlerowicz, M.; Blazewicz, J. Modeling of the catalytic core of Arabidopsis thaliana Dicer-like 4 protein and its complex with double-stranded RNA. Comput. Biol. Chem. 2017, 66, 44–56. [Google Scholar] [CrossRef]
- Dincbas-Renqvist, V.; Pepin, G.; Rakonjac, M.; Plante, I.; Ouellet, D.L.; Hermansson, A.; Goulet, I.; Doucet, J.; Samuelsson, B.; Radmark, O.; et al. Human Dicer C-terminus functions as a 5-lipoxygenase binding domain. Biochim. Biophys. Acta 2009, 1789, 99–108. [Google Scholar] [CrossRef]
- Ma, E.; Zhou, K.; Kidwell, M.A.; Doudna, J.A. Coordinated activities of human dicer domains in regulatory RNA processing. J. Mol. Biol. 2012, 422, 466–476. [Google Scholar] [CrossRef]
- Nakagawa, A.; Shi, Y.; Kage-Nakadai, E.; Mitani, S.; Xue, D. Caspase-dependent conversion of Dicer ribonuclease into a death-promoting deoxyribonuclease. Science 2010, 328, 327–334. [Google Scholar] [CrossRef]
- Ge, X.; Zhao, X.; Nakagawa, A.; Gong, X.; Skeen-Gaar, R.R.; Shi, Y.; Gong, H.; Wang, X.; Xue, D. A novel mechanism underlies caspase-dependent conversion of the dicer ribonuclease into a deoxyribonuclease during apoptosis. Cell Res. 2014, 24, 218–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bogerd, H.P.; Whisnant, A.W.; Kennedy, E.M.; Flores, O.; Cullen, B.R. Derivation and characterization of Dicer- and microRNA-deficient human cells. RNA 2014, 20, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.; Behlen, L.; Reynolds, A.; Wolfson, A.; Marshall, W.S.; Karpilow, J.; Khvorova, A. The contributions of dsRNA structure to Dicer specificity and efficiency. RNA 2005, 11, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Koralewska, N.; Hoffmann, W.; Pokornowska, M.; Milewski, M.; Lipinska, A.; Bienkowska-Szewczyk, K.; Figlerowicz, M.; Kurzynska-Kokorniak, A. How short RNAs impact the human ribonuclease Dicer activity: Putative regulatory feedback-loops and other RNA-mediated mechanisms controlling microRNA processing. Acta Biochim. Pol. 2016, 63, 773–783. [Google Scholar] [CrossRef]
- Kurzynska-Kokorniak, A.; Koralewska, N.; Tyczewska, A.; Twardowski, T.; Figlerowicz, M. A New Short Oligonucleotide-Based Strategy for the Precursor-Specific Regulation of microRNA Processing by Dicer. PLoS ONE 2013, 8, e77703. [Google Scholar] [CrossRef]
- Tyczewska, A.; Kurzynska-Kokorniak, A.; Koralewska, N.; Szopa, A.; Kietrys, A.M.; Wrzesinski, J.; Twardowski, T.; Figlerowicz, M. Selection of RNA oligonucleotides that can modulate human dicer activity in vitro. Nucleic Acid Ther. 2011, 21, 333–346. [Google Scholar] [CrossRef]
- Pokornowska, M.; Milewski, M.C.; Ciechanowska, K.; Szczepanska, A.; Wojnicka, M.; Radogostowicz, Z.; Figlerowicz, M.; Kurzynska-Kokorniak, A. The RNA-RNA base pairing potential of human Dicer and Ago2 proteins. Cell Mol. Life Sci. 2019. [Google Scholar] [CrossRef]
- Kurzynska-Kokorniak, A.; Koralewska, N.; Pokornowska, M.; Urbanowicz, A.; Tworak, A.; Mickiewicz, A.; Figlerowicz, M. The many faces of Dicer: The complexity of the mechanisms regulating Dicer gene expression and enzyme activities. Nucleic Acids Res. 2015, 43, 4365–4380. [Google Scholar] [CrossRef]
- Grelier, G.; Voirin, N.; Ay, A.S.; Cox, D.G.; Chabaud, S.; Treilleux, I.; Leon-Goddard, S.; Rimokh, R.; Mikaelian, I.; Venoux, C.; et al. Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br. J. Cancer 2009, 101, 673–683. [Google Scholar] [CrossRef]
- Hinkal, G.W.; Grelier, G.; Puisieux, A.; Moyret-Lalle, C. Complexity in the regulation of Dicer expression: Dicer variant proteins are differentially expressed in epithelial and mesenchymal breast cancer cells and decreased during EMT. Br. J. Cancer 2011, 104, 387–388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Potenza, N.; Papa, U.; Scaruffi, P.; Mosca, N.; Tonini, G.P.; Russo, A. A novel splice variant of the human dicer gene is expressed in neuroblastoma cells. FEBS Lett. 2010, 584, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Cantini, L.P.; Andino, L.M.; Attaway, C.C.; Butler, B.; Dumitriu, A.; Blackshaw, A.; Jakymiw, A. Identification and characterization of Dicer1e, a Dicer1 protein variant, in oral cancer cells. Mol. Cancer 2014, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Nucleases: Diversity of structure, function and mechanism. Q. Rev. Biophys 2011, 44, 1–93. [Google Scholar] [CrossRef]
- Plante, I.; Ple, H.; Landry, P.; Gunaratne, P.H.; Provost, P. Modulation of microRNA Activity by Semi-microRNAs. Front. Genet. 2012, 3, 99. [Google Scholar] [CrossRef]
- Kurzynska-Kokorniak, A.; Pokornowska, M.; Koralewska, N.; Hoffmann, W.; Bienkowska-Szewczyk, K.; Figlerowicz, M. Revealing a new activity of the human Dicer DUF283 domain in vitro. Sci. Rep. 2016, 6, 23989. [Google Scholar] [CrossRef]
- Doyle, M.; Badertscher, L.; Jaskiewicz, L.; Guttinger, S.; Jurado, S.; Hugenschmidt, T.; Kutay, U.; Filipowicz, W. The double-stranded RNA binding domain of human Dicer functions as a nuclear localization signal. RNA 2013, 19, 1238–1252. [Google Scholar] [CrossRef]
- Wostenberg, C.; Lary, J.W.; Sahu, D.; Acevedo, R.; Quarles, K.A.; Cole, J.L.; Showalter, S.A. The role of human Dicer-dsRBD in processing small regulatory RNAs. PLoS ONE 2012, 7, e51829. [Google Scholar] [CrossRef]
- Heckman, K.L.; Pease, L.R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2007, 2, 924–932. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Name | Sequence (5ʹ→3ʹ) |
|---|---|
| hDicer-F (1-20 nt) | CCTTGTTTAAACTTTAAGAGGAGGGCCACCATGAAAAGCCCTGCTTTGCA |
| hDicer-R (5749-5766 nt) | ACTTCCACCGCCTCCAGAACCTCCGCCACCGCTATTGGGAACCTGAGG |
| ΔPAZ-F (3127-3150 nt) | TGACTCCAGCACTTTGATTCCAGCATCACTGTGGAGAAAA |
| ΔPAZ-R (2649-2670 nt) | CAGTGATGCTGGAATCAAAGTGCTGGAGTCATTAACA |
| ΔPPC-F (3205-3223 nt) | TACCCAAAAGCAGAGCTAAGAGCCCAGACTG |
| ΔPPC-R (2238-2256 nt) | GGCTCTTAGCTCTGCTTTTGGGTAGCACTGC |
| DNA21 | TCGAAGTATTCCGCGTACGTG |
| DNA32 | ACCAGAACATGCAATGCAACTACAATGCACAT |
| pre-mir-16-1 | UAGCAGCACGUAAAUAUUGGCGUUAAGAUUCUAAAAUUAUCUCCAGUAUUAACUGUGCUGCUGAA |
| RNA21 | UCGAAGUAUUCCGCGUACGUG |
| RNA32_sense | GUGCAUUGUAGUUGCAUUGCAUGUUCUGGUCA |
| RNA32_bl | UGACCAGAACAUGCAAUGCAACUACAAUGCAC |
| RNA32_ov* | ACCAGAACAUGCAAUGCAACUACAAUGCACAU |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnicka, M.; Szczepanska, A.; Kurzynska-Kokorniak, A. Unknown Areas of Activity of Human Ribonuclease Dicer: A Putative Deoxyribonuclease Activity. Molecules 2020, 25, 1414. https://doi.org/10.3390/molecules25061414
Wojnicka M, Szczepanska A, Kurzynska-Kokorniak A. Unknown Areas of Activity of Human Ribonuclease Dicer: A Putative Deoxyribonuclease Activity. Molecules. 2020; 25(6):1414. https://doi.org/10.3390/molecules25061414
Chicago/Turabian StyleWojnicka, Marta, Agnieszka Szczepanska, and Anna Kurzynska-Kokorniak. 2020. "Unknown Areas of Activity of Human Ribonuclease Dicer: A Putative Deoxyribonuclease Activity" Molecules 25, no. 6: 1414. https://doi.org/10.3390/molecules25061414
APA StyleWojnicka, M., Szczepanska, A., & Kurzynska-Kokorniak, A. (2020). Unknown Areas of Activity of Human Ribonuclease Dicer: A Putative Deoxyribonuclease Activity. Molecules, 25(6), 1414. https://doi.org/10.3390/molecules25061414






