Influence of Bacillus subtilis and Trichoderma harzianum on Penthiopyrad Degradation under Laboratory and Field Studies
Abstract
1. Introduction
2. Results
2.1. Studies on Metabolic Activity of B. subtilis Cells
2.2. Determination of MIC of Penthiopyrad for B. subtilis and a Reference Fungus S. Cerevisiae
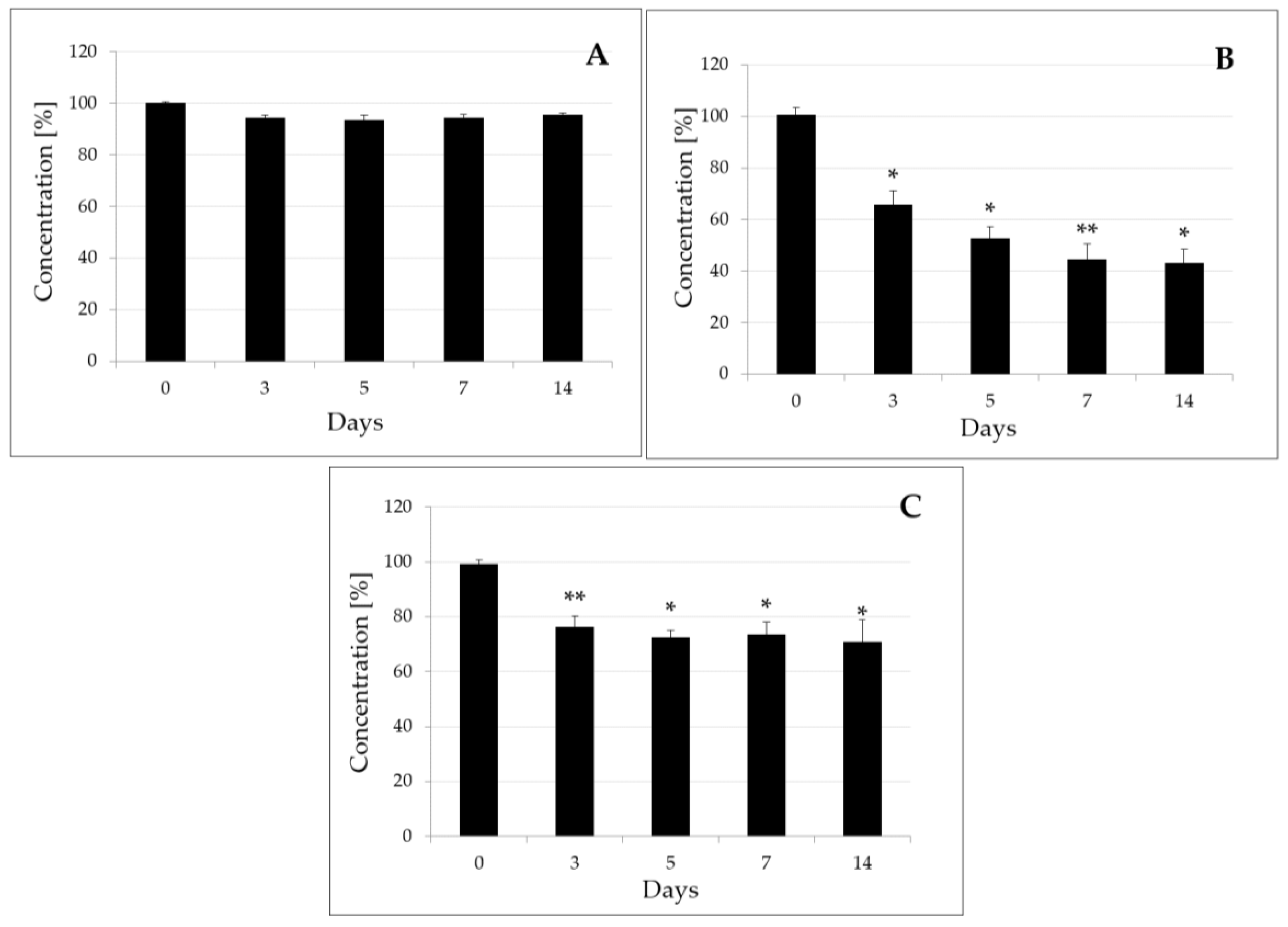
2.3. Degradation of Penthiopyrad by B. subtilis, T. harzianum, and a Mixed Culture of B. subtilis and T. harzianum in Laboratory Conditions
2.4. Field Experiments
2.5. Penthiopyrad Dissipation Kinetics
2.6. Mycotoxins in Apple Samples
2.7. Estimation of Consumer Exposure
2.8. Method Validation
3. Discussion
4. Materials and Methods
4.1. Laboratory Trials
4.1.1. Determination of Concentration Inhibitory Effect on B. subtilis and a Reference Fungus S. Cerevisiae
4.1.2. Degradation of Penthiopyrad by B. subtilis, T. harzianum, and a Mixed Culture of Bacteria and Fungi in Laboratory Conditions
4.1.3. Evaluation of Bacteria B. subtilis Metabolic Activity
4.2. Field Trials
4.2.1. Sampling
4.2.2. Determination of Penthiopyrad
Samples for Laboratory Experiments
Samples for Field Experiments
GC-MS Analysis of Penthiopyrad
4.3. Determination of Mycotoxins in Apple Samples
4.3.1. Patulin
4.3.2. Trichothecenes and Zearalenone
4.4. Method Validation
4.5. Statistical Analysis
4.6. Estimation of Chronic and Acute Exposure
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Bryk, H.; Kruczyńska, D. Possibilities in growing and protection of apple trees against diseases in organic orchards. J. Agric. En. Res. 2011, 56, 40–44. [Google Scholar]
- Koch, A.; Felsenstein, F.; Stammler, G. No evidence of QoI resistance in apple powdery mildew (Podosphaera leucotricha). J. Phytopathol. 2015, 163, 178–184. [Google Scholar] [CrossRef]
- Abad-Fuentes, A.; Ceballos-Alcantarilla, E.; Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Esteve-Turrillas, F.A. Determination of succinate-dehydrogenase-inhibitor fungicide residues in fruits and vegetables by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4207–4211. [Google Scholar] [CrossRef] [PubMed]
- The Pesticide Properties Database (PPDB). Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 20 August 2019).
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Plant Protection Products Search. Available online: http://agrofy.pl/wyszukiwarka (accessed on 20 August 2019).
- Alavanja, M.C.R.; Ross, M.K.; Bonner, M.R. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA. Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef]
- Lwin, T.Z.; Than, A.A.; Min, A.Z.; Robson, M.G.; Siriwong, W. Effects of pesticide exposure on reproductivity of male groundnut farmers in Kyauk Kan village, nyaung-U, Mandalay region, Myanmar. Risk Manag. Healthc. Policy 2018, 11, 235–241. [Google Scholar] [CrossRef]
- Kalliora, C.; Mamoulakis, C.; Vasilopoulos, E.; Stamatiades, G.A.; Kalafati, L.; Barouni, R.; Karakousi, T.; Abdollahi, M.; Tsatsakis, A. Association of pesticide exposure with human congenital abnormalities. Toxicol. Appl. Pharmacol. 2018, 346, 58–75. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Y.; Liu, L.; Yan, H. Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Mcgorrin, R.J. One Hundred Years of Progress in Food Analysis. J. Agric. Food Chem. 2009, 57, 8076–8088. [Google Scholar] [CrossRef]
- Hernández, F.; Cervera, M.I.; Portolés, T.; Beltrán, J.; Pitarch, E. The role of GC-MS/MS with triple quadrupole in pesticide residue analysis in food and the environment. Anal. Methods 2013, 5, 5875–5894. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Computational-based study of QuEChERS extraction of cyclohexanedione herbicide residues in soil by chemometric modeling. Molecules 2018, 23, 2009. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sandín-España, P.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides from natural products: Current development, legislative framework, and future trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Mark Tatchell, G.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, Z.; Cyplik, P.; Juzwa, W.; Czarny, J.; Staninska, J.; Piotrowska-Cyplik, A. Antibacterial effect of the Trichoderma viride fungi on soil microbiome during PAH’s biodegradation. Int. Biodeterior. Biodegrad. 2015, 104, 170–177. [Google Scholar] [CrossRef]
- Regulation 2013. Regulation of 8 March 2013 on plant protection products. Dz.U. 2013 pos. 455. Available online: http://www.ilo.org/dyn/natlex/natlex4.detail?p_lang=en&p_isn=99717 (accessed on 19 August 2019).
- Harwood, C.R.; Cranenburgh, R. Bacillus protein secretion: An unfolding story. Trends Microbiol. 2008, 16, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, X.; Shi, Y.; Su, Z.C. Bacterial Degradation of Chlorpyrifos by Bacillus Cereus. In Proceedings of the Advanced Materials Research, Shanghai, China, 21–23 October 2011; pp. 676–680. [Google Scholar]
- Purnomo, A.S. Microbe-assisted degradation of aldrin and dieldrin. In Microbe-Induced Degradation of Pesticides; Singh, S.N., Ed.; Springer International Publishing: Zurich, Switzerland, 2017; pp. 1–22. [Google Scholar]
- Sariwati, A.; Purnomo, A.S.; Kamei, I. Abilities of Co-cultures of Brown-Rot Fungus Fomitopsis pinicola and Bacillus subtilis on Biodegradation of DDT. Curr. Microbiol. 2017, 74, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Tahri, N.; Bahafid, W.; Sayel, H.; El Ghachtouli, N. Biodegradation: Involved Microorganisms and Genetically Engineered Microorganisms. In Biodegradation: Life of Science; InTech: Rijeka, Croatia, 2013; pp. 289–320. [Google Scholar]
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Biodegradation of soil-applied pesticides by selected strains of plant growth-promoting rhizobacteria (PGPR) and their effects on bacterial growth. Biodegradation 2012, 23, 297–310. [Google Scholar] [CrossRef]
- Thabit, T.M.A.M.; El-Naggar, M.A.H. Diazinon decomposition by soil bacteria and identification of degradation products by GC-MS. Soil Environ. 2013, 32, 96–102. [Google Scholar]
- Kumar, M.; Lakshmi, C.V.; Khanna, S. Biodegradation and bioremediation of endosulfan contaminated soil. Bioresour. Technol. 2008, 99, 3116–3122. [Google Scholar] [CrossRef]
- Yang, C.; Song, C.; Freudl, R.; Mulchandani, A.; Qiao, C. Twin-arginine translocation of methyl parathion hydrolase in bacillus subtilis. Environ. Sci. Technol. 2010, 44, 7607–7612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Hou, Z.; Wu, X.; Gao, H.; Sun, F.; Pan, H. Biodegradation of triazine herbicide metribuzin by the strain Bacillus sp. N1. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2014, 49, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.W.; Sabit, H.; Tawakkol, W. Biodegradation of Malathionby Pseudomonas Spp. and Bacillus Spp. Isolated Frompolluted Sites in Egypt. Am. J. Agric. Environ. Sci. 2014, 14, 855–862. [Google Scholar]
- Gangola, S.; Sharma, A.; Bhatt, P.; Khati, P.; Chaudhary, P. Presence of esterase and laccase in Bacillus subtilis facilitates biodegradation and detoxification of cypermethrin. Sci. Rep. 2018, 8, 12755. [Google Scholar] [CrossRef]
- Gangireddygari, V.S.R.; Kanderi, D.K.; Golla, R.; Bangeppagari, M.; Gundi, V.A.K.B.; Ntushelo, K.; Bontha, R.R. Biodegradation of quinalphos by a soil bacterium-Bacillus subtilis. Pakistan J. Biol. Sci. 2017, 20, 410–422. [Google Scholar]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Vinale, F.; Ghisalberti, E.L.; Sivasithamparam, K.; Marra, R.; Ritieni, A.; Ferracane, R.; Woo, S.; Lorito, M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett. Appl. Microbiol. 2009, 48, 705–711. [Google Scholar]
- Harish, R.; Supreeth, M.; Chauhan, J.B. Biodegradation of Organophosphate Pesticide by Soil Fungi. Adv. Biotech 2013, 12, 4–8. [Google Scholar]
- Jayaraman, P.; Naveen Kumar, T.; Maheswaran, P.; Sagadevan, E.; Arumugam, P. In vitro studies on biodegradation of chlorpyrifos by Trichoderma viride and T. harzianum. J. Pure Appl. Microbiol. 2012, 6, 1465–1474. [Google Scholar]
- Senthilkumar, S.; Anthonisamy, A.; Arunkumar, S.; Sivakumari, V. Biodegradation of methyl parathion and endosulfan using Pseudomonas aeruginosa and Trichoderma viridae. J. Environ. Sci. Eng. 2011, 53, 115–122. [Google Scholar] [PubMed]
- Sharma, P.; Sharma, M.; Raja, M.; Singh, D.V.; Srivastava, M. Use of Trichoderma spp. in biodegradation of Carbendazim. Indian J. Agric. Sci. 2016, 86, 891–894. [Google Scholar]
- European Commission, Directorate General for Health and Food Safety. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues and Analysis in Food and Feed. 2017. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 23 August 2019).
- Kim, W.G.; Weon, H.Y.; Seok, S.J.; Lee, K.H. In Vitro Antagonistic Characteristics of Bacilli Isolates against Trichoderma spp. and Three Species of Mushrooms. Mycobiology 2008, 36, 266–269. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, X.; Ma, L. Influence of environmental factors on degradation of carbendazim by Bacillus pumilus strain NY97-1. Int. J. Environ. Pollut. 2009, 38, 309–317. [Google Scholar] [CrossRef]
- Salunkhe, V.P.; Sawant, I.S.; Banerjee, K.; Wadkar, P.N.; Sawant, S.D.; Hingmire, S.A. Kinetics of degradation of carbendazim by B. subtilis strains: Possibility of in situ detoxification. Environ. Monit. Assess. 2014, 186, 8599–8610. [Google Scholar] [CrossRef]
- Panda, J.; Kanjilal, T.; Das, S. Optimized biodegradation of carcinogenic fungicide Carbendazim by Bacillus licheniformis JTC-3 from agro-effluent. Biotechnol. Res. Innov. 2018, 2, 45–57. [Google Scholar] [CrossRef]
- Cycoń, M.; Wójcik, M.; Piotrowska-Seget, Z. Biodegradation kinetics of the benzimidazole fungicide thiophanate-methyl by bacteria isolated from loamy sand soil. Biodegradation 2011, 22, 573–583. [Google Scholar] [CrossRef]
- Youness, M.; Sancelme, M.; Combourieu, B.; Besse-Hoggan, P. Identification of new metabolic pathways in the enantioselective fungicide tebuconazole biodegradation by Bacillus sp. 3B6. J. Hazard. Mater. 2018, 351, 160–168. [Google Scholar] [CrossRef]
- Wang, X.; Hou, X.; Liang, S.; Lu, Z.; Hou, Z.; Zhao, X.; Sun, F.; Zhang, H. Biodegradation of fungicide Tebuconazole by Serratia marcescens strain B1 and its application in bioremediation of contaminated soil. Int. Biodeterior. Biodegrad. 2018, 127, 185–191. [Google Scholar] [CrossRef]
- Obanda, D.N.; Shupe, T.F.; Catallo, W.J. Resistance of Trichoderma harzianum to the biocide tebuconazol - Proposed biodegradation pathways. Holzforschung 2008, 62, 613–619. [Google Scholar] [CrossRef]
- Fontelis 200 SC Label. Available online: https://www.gov.pl/documents/912055/913531/Fontelis_200_SC_-_ET_18-06-2018.pdf (accessed on 22 August 2019).
- Podbielska, M.; Szpyrka, E.; Piechowicz, B.; Sadło, S.; Sudoł, M. Assessment of boscalid and pyraclostrobin disappearance and behavior following application of effective microorganisms on apples. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard, 12th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Hieke, A.S.C.; Pillai, S.D. Escherichia coli cells exposed to lethal doses of electron beam irradiation retain their ability to propagate bacteriophages and are metabolically active. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zumba Plant Label. Available online: http://www.naturalcrop.com/images/produkty_info/zumbaplant/zumbaplant.pdf (accessed on 22 August 2019).
- Regulation 2007. Regulation of the Minister of Health of October 17, 2007 on the Taking of Food Samples to Determine Levels of Pesticide Residues. Dz.U. 2007 No. 207 pos. 1502. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20072071502 (accessed on 19 March 2020). (In Polish)
- PN-EN 15662:2018. Foods of Plant Origin–Multimethod for the Determination of Pesticide Residues Using GC- and LC-based Analysis Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE. Modular QuEChERS-method; The Polish Committee for Standardization: Warsaw, Poland, 2018; pp. 1–82. [Google Scholar]
- Malmierca, M.G.; Mccormick, S.P.; Cardoza, R.E.; Alexander, N.J.; Monte, E.; Gutiérrez, S. Production of trichodiene by Trichoderma harzianum alters the perception of this biocontrol strain by plants and antagonized fungi. Environ. Microbiol. 2015, 17, 2628–2646. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/applications/pesticides/tools (accessed on 23 August 2019).
- EU Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN (accessed on 23 August 2019).
Sample Availability: Samples of the apples and broths are not available from authors. |



| Days | Penthiopyrad + B. subtilis ± SD [µg/mL] | Penthiopyrad Control ± SD [µg/mL] | Penthiopyrad + T. harzianum ± SD [µg/mL] | Penthiopyrad Control ± SD [µg/mL] | Pentiopirad + B. subtilis + T. harzianum ± SD [µg/mL] | Penthiopyrad Control ± SD [µg/mL] |
|---|---|---|---|---|---|---|
| 0 | 114.8 ± 5.7 | 114.6 ± 5.1 | 106.7 ± 5.4 | 106.0 ± 2.5 | 109.8 ± 4.3 | 110.6 ± 2.4 |
| 3 | 106.3 ± 3.2 | 112.5 ± 4.6 | 69.1 ± 7.6 | 105.0 ± 2.8 | 80.3 ± 2.1 | 105.3 ± 2.7 |
| 5 | 98.4 ± 4.8 | 105.3 ± 2.8 | 54.2 ± 3.8 | 101.8 ± 15.6 | 79.3 ± 7.1 | 109.3 ± 5.7 |
| 7 | 98.9 ± 6.9 | 104.6 ± 8.7 | 45.3 ± 3.2 | 101.4 ± 6.5 | 77.5 ± 8.8 | 105.1 ± 5.8 |
| 14 | 99.5 ± 3.5 | 104.0 ± 4.2 | 42.9 ± 7.6 | 99.4 ± 5.1 | 71.5 ± 3.5 | 100.8 ± 6.0 |
| Sampling Date | Number of Days after Chemical Treatment | Penthiopyrad Concentration [mg/kg] | SD [mg/kg] | Pentiopirad Concentration after Treatment with the Biological Preparation [mg/kg] | SD [mg/kg] |
|---|---|---|---|---|---|
| Gala variety | |||||
| 8/17/2017 | 1 | 0.161 | 0.034 | – | – |
| 8/21/2017 | 5 | 0.150 | 0.044 | – | – |
| 8/24/2017 | 8 | 0.105 | 0.036 | 0.102 | 0.018 |
| 8/28/2017 | 12 | 0.065 | 0.013 | 0.064 | 0.008 |
| 8/31/2017 | 15 | 0.041 | 0.013 | 0.033 | 0.014 |
| 9/6/2017 | 21 | 0.037 | 0.014 | 0.032 | 0.010 |
| Golden delicious variety | |||||
| 9/5/2018 | 1 | 0.421 | 0.046 | – | – |
| 9/9/2018 | 5 | 0.261 | 0.015 | – | – |
| 9/12/2018 | 8 | 0.269 | 0.039 | 0.265 | 0.042 |
| 9/16/2018 | 12 | 0.166 | 0.010 | 0.165 | 0.053 |
| 9/19/2018 | 15 | 0.105 | 0.011 | 0.083 | 0.018 |
| 9/25/2018 | 21 | 0.078 | 0.017 | 0.063 | 0.017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podbielska, M.; Kus-Liśkiewicz, M.; Jagusztyn, B.; Piechowicz, B.; Sadło, S.; Słowik-Borowiec, M.; Twarużek, M.; Szpyrka, E. Influence of Bacillus subtilis and Trichoderma harzianum on Penthiopyrad Degradation under Laboratory and Field Studies. Molecules 2020, 25, 1421. https://doi.org/10.3390/molecules25061421
Podbielska M, Kus-Liśkiewicz M, Jagusztyn B, Piechowicz B, Sadło S, Słowik-Borowiec M, Twarużek M, Szpyrka E. Influence of Bacillus subtilis and Trichoderma harzianum on Penthiopyrad Degradation under Laboratory and Field Studies. Molecules. 2020; 25(6):1421. https://doi.org/10.3390/molecules25061421
Chicago/Turabian StylePodbielska, Magdalena, Małgorzata Kus-Liśkiewicz, Bartosz Jagusztyn, Bartosz Piechowicz, Stanisław Sadło, Magdalena Słowik-Borowiec, Magdalena Twarużek, and Ewa Szpyrka. 2020. "Influence of Bacillus subtilis and Trichoderma harzianum on Penthiopyrad Degradation under Laboratory and Field Studies" Molecules 25, no. 6: 1421. https://doi.org/10.3390/molecules25061421
APA StylePodbielska, M., Kus-Liśkiewicz, M., Jagusztyn, B., Piechowicz, B., Sadło, S., Słowik-Borowiec, M., Twarużek, M., & Szpyrka, E. (2020). Influence of Bacillus subtilis and Trichoderma harzianum on Penthiopyrad Degradation under Laboratory and Field Studies. Molecules, 25(6), 1421. https://doi.org/10.3390/molecules25061421







