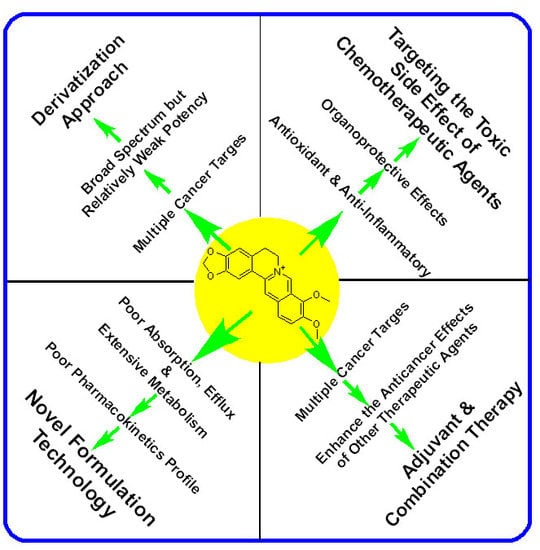
Recent Advances in Berberine Inspired Anticancer Approaches: From Drug Combination to Novel Formulation Technology and Derivatization
Abstract
:1. Introduction
2. The Anticancer Effect of Berberine: Direct Cytotoxicity in Cancer Cells
3. Berberine Ameliorates the Toxicity of Anticancer Drugs in Normal Cells
4. Berberine Enhances the Cytotoxicity of Other Therapeutic Agents in Cancer Cells
5. Enhancement of Berberine’s Anticancer Effect via Formulation Technology
6. Enhancement of Berberine’s Anticancer Effect via Derivatization
6.1. The Berberine-O-Derivatives
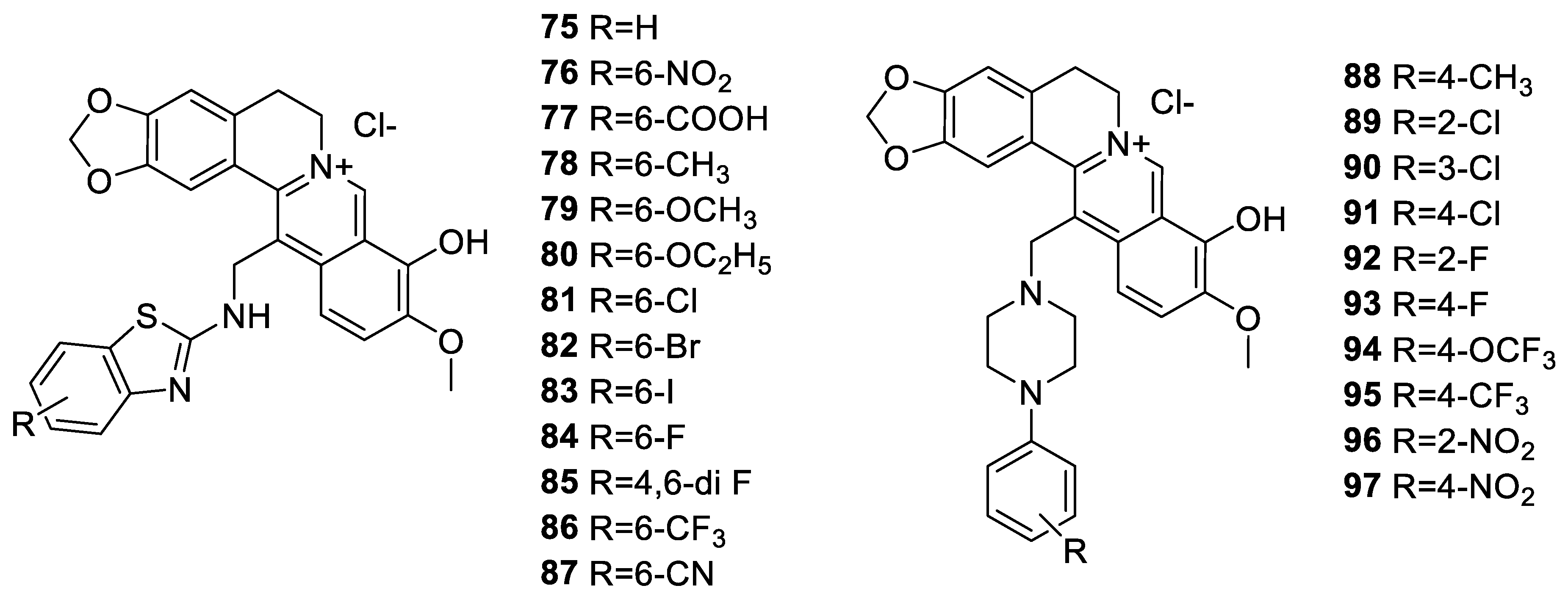
6.2. 9-N-Berberine Derivatives
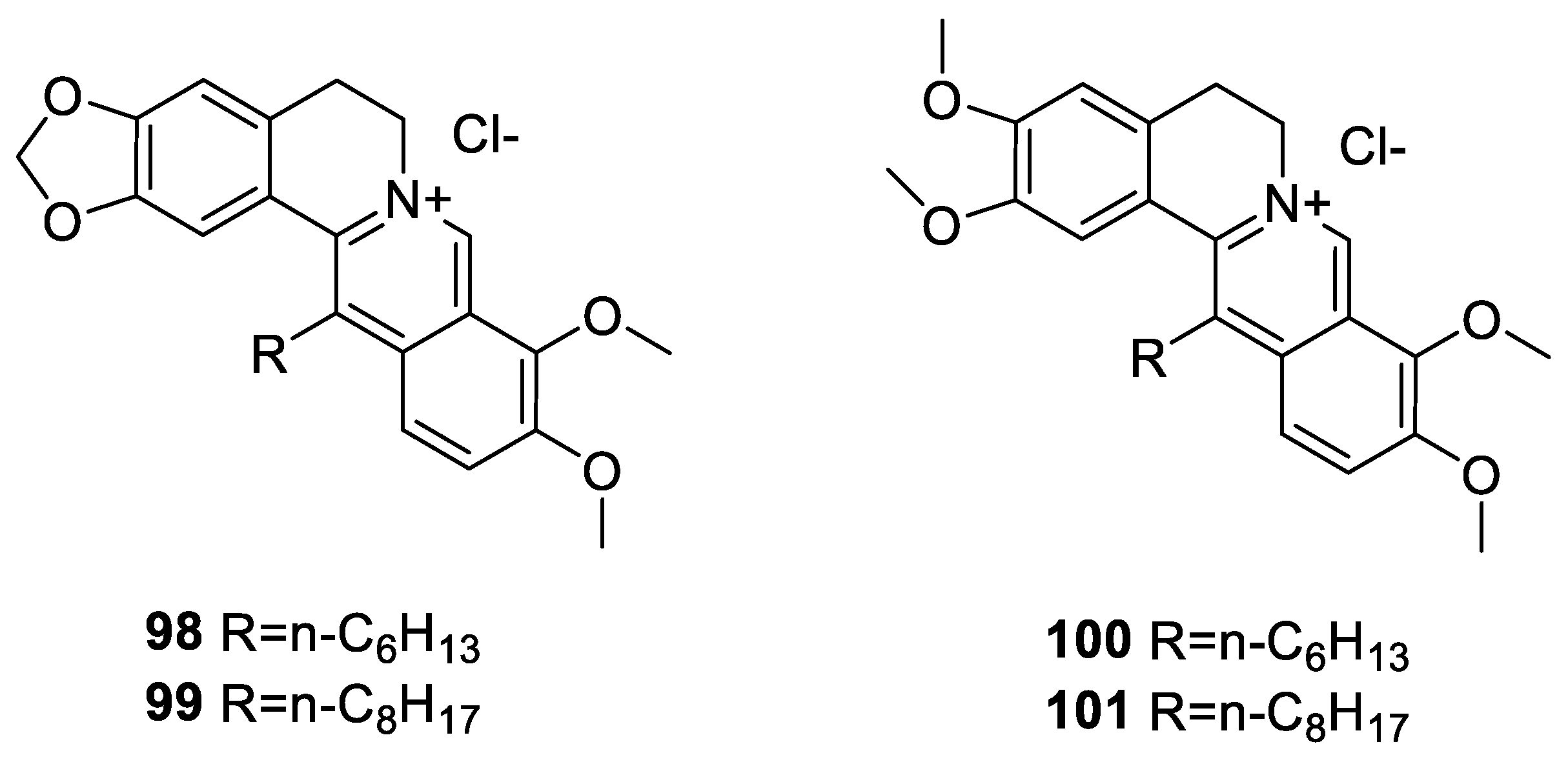
6.3. C-13 Berberine Derivatives
6.4. Cyclizing Berberine A35
7. General Summary and Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Potdar, D.; Hirwani, R.R.; Dhulap, S. Phyto-chemical and pharmacological applications of Berberis Aristate. Fitoterapia 2012, 83, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Duggal, S.; Kaur, N.; Singh, J. Berberine: Alkaloid with wide spectrum of pharmacological activities. J. Nat. Prod. 2010, 3, 64–75. [Google Scholar]
- Habtemariam, S. The hidden treasure in Europe’s garden plants: Case examples Berberis darwinni and Bergenia cordifolia. Med. Arom. Plants 2013, 2, 4. [Google Scholar]
- Habtemariam, S. The therapeutic potential of Berberis darwinii stem-bark: Quantification of berberine and in vitro evidence for Alzheimer’s disease therapy. Nat. Prod. Commun. 2011, 6, 1089–1090. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Bajpai, V.; Srivastava, M.; Arya, K.R.; Kumar, B. Rapid screening and distribution of bioactive compounds in different parts of Berberis petiolaris using direct analysis in real time mass spectrometry. J. Pharm. Anal. 2015, 5, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Suau, R.; Rico, R.; López-Romero, J.M.; Nájera, F.; Cuevas, A. Isoquinoline alkaloids from Berberis Vulgaris subsp. Aus. Phytochem. 1998, 49, 2545–2549. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Tang, C.; Zhang, Y.; Fan, G. Integration of HPLC-based fingerprint and quantitative analyses for differentiating botanical species and geographical growing origins of Rhizoma coptidis. Pharm. Biol. 2016, 54, 3264–3271. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.N.; Roman, M.C. Determination of Hydrastine and Berberine in Goldenseal Raw Materials, Extracts, and Dietary Supplements by High-Performance Liquid Chromatography with UV: Collaborative Study. J. AOAC Int. 2008, 91, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Weber, H.A.; Zart, M.K.; Hodges, A.E.; Molloy, H.M.; O’Brien, B.M.; Moody, L.A.; Clark, A.P.; Harris, R.K.; Overstreet, J.D.; Smith, C.S. Chemical comparison of goldenseal (Hydrastiscanadensis L.) root powder from three commercial suppliers. J. Agric. Food Chem. 2003, 51, 7352–7358. [Google Scholar] [CrossRef]
- Kametani, T.; Noguchi, I.J. Studies on the syntheses of heterocyclic compounds. CCCII. Alternative total syntheses of (±)-nandinine, (±)-canadine, and berberine iodide. Chem. Soc. C 1969, 15, 2036–2038. [Google Scholar] [CrossRef]
- Gatland, A.E.; Pilgrim, B.S.; Procopiou, P.A.; Donohoe, T.J. Short and efficient syntheses of protoberberine alkaloids using palladium-catalyzed enolate arylation. Angew Chem. Int. Ed. Engl. 2014, 53, 14555–14558. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Jadhav, A.S.; Anand, R.V. A room-temperature protocol to access isoquinolines through Ag(I) catalysed annulation of o-(1-alkynyl)arylaldehydes and ketones with NH4OAc: Elaboration to berberine and palmatine. Org. Biomol. Chem. 2015, 13, 3732–3741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tong, R.A. General, concise strategy that enables collective total syntheses of over 50 protoberberine and five aporhoeadane alkaloids within four to eight steps. Chem. Eur. J. 2016, 22, 7084–7089. [Google Scholar] [CrossRef] [PubMed]
- Jamshaid, F.; Dai, J.; Yang, L.X. New development of novel berberine derivatives against bacteria. Mini. Rev. Med. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine and inflammatory bowel disease: A concise review. Pharmacol. Res. 2016, 113 (Pt A), 592–599. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Tabeshpour, J.; Imenshahidi, M.; Hosseinzadeh, H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran. J. Basic. Med. Sci. 2017, 20, 557–568. [Google Scholar]
- Shinjyo, N.; Parkinson, J.; Bell, J.; Katsuno, T.; Bligh, A. Berberine for prevention of dementia associated with diabetes and its comorbidities: A systematic review. J. Integr. Med. 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Habtemariam, S. Going back to the good old days: The merit of crude plant drug mixtures in the 21st century. Int. J. Complement. Alt. Med. 2017, 6, 00182. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.M.; Zhang, Q.Y.; Wang, J.L.; Chen, J.L.; Zhang, Y.L.; Tong, X.L. Poor permeability and absorption affect the activity of four alkaloids from Coptis. Mol. Med. Rep. 2015, 12, 7160–7168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.T.; Yu, Y.Q.; Yan, X.X.; Wang, W.J.; Tian, X.T.; Wang, L.; Zhu, W.L.; Gong, L.K.; Pan, G.Y. Different structures of berberine and five other protoberberine alkaloids that affect P-glycoprotein-mediated efflux capacity. Acta Pharmacol. Sin. 2019, 40, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhu, Z.; Liu, Y.; Wei, L.; Li, B.; Mao, F.; Zhang, J.; Wang, Y.; Liu, Y. MDM2 inhibition-mediated autophagy contributes to the pro-apoptotic effect of berberine in p53-null leukemic cells. Life Sci. 2020, 242, 117228. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Hu, X.; Xu, Y.; Yang, J.; Zong, L.; Wang, C.; Zhu, J.; Li, Z.; Lu, D. Berberine inhibits proliferation and migration of colorectal cancer cells by downregulation of GRP78. Anticancer Drugs 2020, 31, 141–149. [Google Scholar] [CrossRef]
- Tak, J.; Sabarwal, A.; Shyanti, R.K.; Singh, R.P. Berberine enhances posttranslational protein stability of p21/cip1 in breast cancer cells via down-regulation of Akt. Mol. Cell Biochem. 2019, 458, 49–59. [Google Scholar] [CrossRef]
- Belanova, A.; Beseda, D.; Chmykhalo, V.; Stepanova, A.; Belousova, M.; Khrenkova, V.; Gavalas, N.; Zolotukhin, P. Berberine effects on NF-κB, HIF1A and NFE2L2/AP-1 pathways in HeLa Cells. Anticancer Agents Med. Chem. 2019, 19, 487–501. [Google Scholar] [CrossRef]
- Jin, F.; Xie, T.; Huang, X.; Zhao, X. Berberine inhibits angiogenesis in glioblastoma xenografts by targeting the VEGFR2/ERK pathway. Pharm. Biol. 2018, 56, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Agnarelli, A.; Natali, M.; Garcia-Gil, M.; Pesi, R.; Tozzi, M.G.; Ippolito, C.; Bernardini, N.; Vignali, R.; Batistoni, R.; Bianucci, A.M.; et al. Cell-specific pattern of berberine pleiotropic effects on different human cell lines. Sci. Rep. 2018, 8, 10599. [Google Scholar]
- Wang, Y.; Zhang, S. Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2. Biomed. Pharmacother. 2018, 103, 1287–1293. [Google Scholar] [CrossRef]
- Puthdee, N.; Seubwai, W.; Vaeteewoottacharn, K.; Boonmars, T.; Cha’on, U.; Phoomak, C.; Wongkham, S. Berberine induces cell cycle arrest in cholangiocarcinoma cell lines via inhibition of NF-κB and STAT3 pathways. Biol. Pharm. Bull. 2017, 40, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Karnam, K.C.; Ellutla, M.; Bodduluru, L.N.; Kasala, E.R.; Uppulapu, S.K.; Kalyankumarraju, M.; Lahkar, M. Preventive effect of berberine against DMBA-induced breast cancer in female Sprague Dawley rats. Biomed. Pharmacother. 2017, 92, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Raghav, D.; Ashraf, S.M.; Mohan, L.; Rathinasamy, K. Berberine induces toxicity in HeLa cells through perturbation of microtubule polymerization by binding to tubulin at a unique site. Biochemistry 2017, 56, 2594–2611. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jin, X.; Cao, B.; Wang, W. Berberine affects osteosarcoma via downregulating the caspase-1/IL-1β signaling axis. Oncol. Rep. 2017, 37, 729–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadiankia, N.; Moghaddam, H.K.; Mishan, M.A.; Bahrami, A.R.; Naderi-Meshkin, H.; Bidkhori, H.R.; Moghaddam, M.; Mirfeyzi, S.J.A. Berberine suppresses migration of MCF-7 breast cancer cells through down-regulation of chemokine receptors. Iran. J. Basic. Med. Sci. 2016, 19, 125–231. [Google Scholar] [PubMed]
- Seo, Y.S.; Yim, M.J.; Kim, B.H.; Kang, K.R.; Lee, S.Y.; Oh, J.S.; You, J.S.; Kim, S.G.; Yu, S.J.; Lee, G.J.; et al. Berberine-induced anticancer activities in FaDu head and neck squamous cell carcinoma cells. Oncol. Rep. 2015, 34, 3025–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Peng, Y.; Liu, Y.; Yang, J.; Ding, N.; Tan, W. Berberine, a natural compound, suppresses Hedgehog signaling pathway activity and cancer growth. BMC Cancer 2015, 15, 595. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, O.; Kan, M.; Zhang, M.; Shao, D.; Pan, Y.; Zheng, H.; Zhang, X.; Chen, L.; Liu, S. Berberine induces apoptosis by suppressing the arachidonic acid metabolic pathway in hepatocellular carcinoma. Mol. Med. Rep. 2015, 12, 4572–4577. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, E.; Fouladdel, S.; Movahhed, T.K.; Atashpour, S.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer T47D and MCF7 cell lines. Iran. J. Basic. Med. Sci. 2015, 18, 334–342. [Google Scholar]
- Yi, T.; Zhuang, L.; Song, G.; Zhang, B.; Li, G.; Hu, T. Akt signalling is associated with the berberine-induced apoptosis of human gastric cancer cells. Nutr. Cancer 2015, 67, 523–531. [Google Scholar] [CrossRef]
- Kim, J.S.; Oh, D.; Yim, M.J.; Park, J.J.; Kang, K.R.; Cho, I.A.; Moon, S.M.; Oh, J.S.; You, J.S.; Kim, C.S.; et al. Berberine induces FasL-related apoptosis through p38 activation in KB human oral cancer cells. Oncol. Rep. 2015, 33, 1775–1782. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.H.; Tang, W.C.; Sia, P.; Huang, C.C.; Yang, P.M.; Wu, M.H.; Lai, I.L.; Lee, K.H. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance. Int. J. Med. Sci. 2015, 12, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, P.; Zhang, C.; Li, N. Berberine exhibits antitumor effects in human ovarian cancer cells when combined with cisplatin, Berberine showed a strong synergistic anticancer effect against ovarian cancer (SKOV3 cells) cells. Anticancer Agents Med. Chem. 2015, 15, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sung, J.H.; Kim, E.J.; Chung, N. Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Braz. J. Med. Biol. Res. 2015, 48, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.H.; Zheng, H.F.; Wang, W.L.; Wang, Y.; Zhong, L.F.; Wu, J.L.; Li, Q.X. Berberine targets epidermal growth factor receptor signaling to suppress prostate cancer proliferation in vitro. Mol. Med. Rep. 2015, 11, 2125–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhao, S.J.; Shi, H.L.; Qiu, S.P.; Xie, J.Q.; Wu, H.; Zhang, B.B.; Wang, Z.T.; Yuan, J.Y.; Wu, X.J. Berberine hydrochloride IL-8 dependently inhibits invasion and IL-8-independently promotes cell apoptosis in MDA-MB-231 cells. Oncol. Rep. 2014, 32, 2777–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Ma, N.; Li, H.X.; Tian, L.; Ba, Y.F.; Hao, B. Berberine induces apoptosis and DNA damage in MG-63 human osteosarcoma cells. Mol. Med. Rep. 2014, 10, 1734–1738. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Hu, H.; Liu, Y.; Feng, T.; Meng, W.; Jiang, L.; Sun, Y.; Yao, Y. Berberine induces apoptosis in human multiple myeloma cell line U266 through hypomethylation of p53 promoter. Cell Biol. Int. 2014, 38, 563–570. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Liu, A.; Liu, S.; Zhang, L.; Wu, B.; Hu, Q. Berberine induces apoptosis in p53-null leukemia cells by down-regulating XIAP at the post-transcriptional level. Cell Physiol. Biochem. 2013, 32, 1213–1224. [Google Scholar] [CrossRef]
- Fu, L.; Chen, W.; Guo, W.; Wang, J.; Tian, Y.; Shi, D.; Zhang, X.; Qiu, H.; Xiao, X.; Kang, T.; et al. Berberine Targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and Cytochrome-c/Caspase signaling to suppress human cancer cell growth. PLoS ONE 2013, 8, e69240. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Tang, K.; Liu, Q.; Zhu, R.; Cao, Z. Calmodulin as a potential target by which berberine induces cell cycle arrest in human hepatoma Bel7402 cells. Chem. Biol. Drug Des. 2013, 81, 775–783. [Google Scholar] [CrossRef]
- Kuo, H.P.; Chuang, T.C.; Tsai, S.C.; Tseng, H.H.; Hsu, S.C.; Chen, Y.C.; Kuo, C.L.; Kuo, Y.H.; Liu, J.Y.; Kao, M.C. Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J. Agric. Food Chem. 2012, 60, 9649–9658. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, J.B.; Bae, J.; Park, S.Y.; Jee, H.G.; Lee, K.E.; Youn, Y.K. Berberine inhibited the growth of thyroid cancer cell lines 8505C and TPC1. Yonsei Med. J. 2012, 53, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, J.B.; Lee, S.J.; Bae, J. Berberine-induced growth inhibition of epithelial ovarian carcinoma cell lines. J. Obstet. Gynaecol. Res. 2012, 38, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Hamsa, T.P.; Kuttan, G. Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem. Toxicol. 2012, 35, 57–70. [Google Scholar] [CrossRef]
- Pazhang, Y.; Ahmadian, S.; Javadifar, N.; Shafiezadeh, M. COX-2 and survivin reduction may play a role in berberine-induced apoptosis in human ductal breast epithelial tumor cell line. Tumour Biol. 2012, 33, 207–214. [Google Scholar] [CrossRef]
- Xu, L.N.; Lu, B.N.; Hu, M.M.; Xu, Y.W.; Han, X.; Qi, Y.; Peng, J.Y. Mechanisms involved in the cytotoxic effects of berberine on human colon cancer HCT-8 cells. Biocell 2012, 36, 113–120. [Google Scholar]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 Cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar] [CrossRef]
- Wang, G.Y.; Lv, Q.H.; Dong, Q.; Xu, R.Z.; Dong, Q.H. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J. Asian Nat. Prod. Res. 2009, 11, 219–228. [Google Scholar] [CrossRef]
- Choi, M.S.; Yuk, D.Y.; Oh, J.H.; Jung, H.Y.; Han, S.B.; Moon, D.C.; Hong, J.T. Berberine inhibits human neuroblastoma cell growth through induction of p53-dependent apoptosis. Anticancer Res. 2008, 28, 3777–3784. [Google Scholar]
- Lin, J.P.; Yang, J.S.; Wu, C.C.; Lin, S.S.; Hsieh, W.T.; Lin, M.L.; Yu, F.S.; Yu, C.S.; Chen, G.W.; Chang, Y.H.; et al. Berberine induced down-regulation of matrix metalloproteinase-1, -2 and -9 in human gastric cancer cells (SNU-5) in vitro. In Vivo 2008, 22, 223–230. [Google Scholar]
- Lin, C.C.; Yang, J.S.; Chen, J.T.; Fan, S.; Yu, F.S.; Yang, J.L.; Lu, C.C.; Kao, M.C.; Huang, A.C.; Lu, H.F.; et al. Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Anticancer Res. 2007, 27, 3371–3378. [Google Scholar] [PubMed]
- Lin, J.P.; Yang, J.S.; Chang, N.W.; Chiu, T.H.; Su, C.C.; Lu, K.W.; Ho, Y.T.; Yeh, C.C.; Yang, M.D.; Lin, H.J.; et al. GADD153 mediates berberine-induced apoptosis in human cervical cancer Ca ski cells. Anticancer Res. 2007, 27, 3379–3386. [Google Scholar] [PubMed]
- Hsu, W.H.; Hsieh, Y.S.; Kuo, H.C.; Teng, C.Y.; Huang, H.I.; Wang, C.J.; Yang, S.F.; Liou, Y.S.; Kuo, W.H. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch. Toxicol. 2007, 81, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.S.; Yang, J.S.; Lin, H.J.; Yu, C.S.; Tan, T.W.; Lin, Y.T.; Lin, C.C.; Lu, H.F.; Chung, J.G. Berberine inhibits WEHI-3 leukemia cells in vivo. In Vivo 2007, 21, 407–412. [Google Scholar]
- Letasiová, S.; Jantová, S.; Cipák, L.; Múcková, M. Berberine-antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006, 239, 254–262. [Google Scholar] [CrossRef]
- Mitry, M.A.; Laurent, D.; Keith, B.L.; Sira, E.; Eisenberg, C.A.; Eisenberg, L.M.; Joshi, S.; Gupte, S.; Edwards, J.G. Accelerated cardiomyocyte senescence contributes to late-onset doxorubicin-induced cardiotoxicity. Am. J. Physiol. Cell Physiol. 2020, in press. [Google Scholar] [CrossRef]
- Hao, G.; Yu, Y.; Gu, B.; Xing, Y.; Xue, M. Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica 2015, 45, 1024–1029. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine Ameliorates Doxorubicin-Induced Cardiotoxicity via a SIRT1/p66Shc-Mediated Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 2150394. [Google Scholar] [CrossRef]
- Xiong, C.; Wu, Y.Z.; Zhang, Y.; Wu, Z.X.; Chen, X.Y.; Jiang, P.; Guo, H.C.; Xie, K.R.; Wang, K.X.; Su, S.W. Protective effect of berberine on acute cardiomyopathy associated with doxorubicin treatment. Oncol. Lett. 2018, 15, 5721–5729. [Google Scholar] [CrossRef] [Green Version]
- Coelho, A.R.; Martins, T.R.; Couto, R.; Deus, C.; Pereira, C.V.; Simões, R.F.; Rizvanov, A.A.; Silva, F.; Cunha-Oliveira, T.; Oliveira, P.J.; et al. Berberine-induced cardioprotection and Sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2904–2923. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, Y.; Zhu, Z.; Liu, H.; Guo, H.; Xiong, C.; Xie, K.; Zhang, X.; Su, S. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol. Med. Rep. 2016, 13, 3953–6390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Yu, X.; Wang, Y.; Wang, F.; Li, H.; Wang, Y.; Lu, D.; Qi, R.; Wang, H. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS ONE 2012, 7, e47351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Tong, N.; Liao, X.; Wang, E.; Li, Z.; Luo, Y.; Zuo, H. Berberine attenuates doxorubicin-induced cardiotoxicity in mice. J. Int. Med. Res. 2011, 39, 1720–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhang, J.; Tong, N.; Chen, Y.; Luo, Y. Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol. Pharm. Bull. 2012, 35, 796–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domitrović, R.; Cvijanović, O.; Pernjak-Pugel, E.; Skoda, M.; Mikelić, L.; Crnčević-Orlić, Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013, 62, 397–406. [Google Scholar] [CrossRef]
- Rezaee, R.; Monemi, A.; SadeghiBonjar, M.A.; Hashemzaei, M. Berberine Alleviates Paclitaxel-Induced Neuropathy. J. Pharmacopuncture 2019, 22, 90–94. [Google Scholar]
- Mittal, A.; Tabasum, S.; Singh, R.P. Berberine in combination with doxorubicin suppresses growth of murine melanoma B16F10 cells in culture and xenograft. Phytomedicine 2014, 21, 340–347. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, F.; Zhao, Y.; Shao, D.; Zheng, X.; Chen, Y.; He, K.; Li, J.; Chen, L. Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast Cancer. J. Cancer. 2017, 8, 1679–1689. [Google Scholar] [CrossRef] [Green Version]
- Tong, N.; Zhang, J.; Chen, Y.; Li, Z.; Luo, Y.; Zuo, H.; Zhao, X. Berberine sensitizes multiple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol. Lett. 2012, 3, 1263–1267. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Li, L.L.; Xiao, G.F.; Luo, Q.Z.; Liu, Q.Z.; Yao, K.T.; Xiao, G.H. Berberine Increases Doxorubicin Sensitivity by Suppressing STAT3 in Lung Cancer. Am. J. Chin. Med. 2015, 43, 1487–1502. [Google Scholar] [CrossRef]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, H.Y.; Xie, X.M.; Zhang, W.J.; Zhu, H.L.; Jiang, F.Z. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev. Biol. Anim. 2016, 52, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qin, R.; Fang, Y.; Li, H. Berberine Sensitizes Human Ovarian Cancer Cells to Cisplatin Through miR-93/PTEN/Akt Signaling Pathway. Cell Physiol. Biochem. 2015, 36, 956–965. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Y.; Shen, H.; Xu, W.; Li, H. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis. Acta Biochim. Biophys. Sin. 2013, 45, 756–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, N.A.; Liu, S.S.; Leung, T.H.; Tam, K.F.; Liao, X.Y.; Cheung, A.N.; Chan, K.K.; Ngan, H.Y. Loss of programmed cell death 4 (Pdcd4) associates with the progression of ovarian cancer. Mol. Cancer 2009, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Marverti, G.; Ligabue, A.; Lombardi, P.; Ferrari, S.; Monti, M.G.; Frassineti, C.; Costi, M.P. Modulation of the expression of folate cycle enzymes and polyamine metabolism by berberine in cisplatin-sensitive and -resistant human ovarian cancer cells. Int. J. Oncol. 2013, 43, 1269–1280. [Google Scholar] [CrossRef]
- Youn, M.J.; So, H.S.; Cho, H.J.; Kim, H.J.; Kim, Y.; Lee, J.H.; Sohn, J.S.; Kim, Y.K.; Chung, S.Y.; Park, R. Berberine, a natural product, combined with cisplatin enhanced apoptosis through a mitochondria/caspase-mediated pathway in HeLa cells. Biol. Pharm. Bull. 2008, 31, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, A.; Iapichino, A.; Cura, F.; Scapoli, L.; Carinci, F.; Mandrone, M.; Martinelli, M. Pre-treatment with berberine enhances effect of 5-fluorouracil and cisplatin in HEP2 laryngeal cancer cell line. J. Biol. Regul. Homeost. Agents 2018, 32, 167–177. [Google Scholar]
- Zhao, Y.; Jing, Z.; Li, Y.; Mao, W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol. Rep. 2016, 36, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Ji, Y.J. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. Control. Release 2019, 318, 38–49. [Google Scholar] [CrossRef]
- Guo, N.; Yan, A.; Gao, X.; Chen, Y.; He, X.; Hu, Z.; Mi, M.; Tang, X.; Gou, X. Berberine sensitizes rapamycin-mediated human hepatoma cell death in vitro. Mol. Med. Rep. 2014, 10, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Ahan, S.H.; Kim, T.Y. Enhancement of Arsenic Trioxide (As2O3)-Mediated Apoptosis using berberine in human neuroblastoma SH-SY5Y cells. J. Korean Neurosurg. Soc. 2007, 42, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, Y.; Chen, Q.; Chen, B.; Zhan, X.; Qin, X.; Huang, J.; Lv, X. Berberine promotes antiproliferative effects of epirubicin in T24 bladder cancer cells by enhancing apoptosis and cell cycle arrest. Int. J. Clin. Pharmacol. Ther. 2017, 55, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, Y.; Lu, Y.Y.; Lau, E.; Zhao, M.; Zhou, Q.M.; Su, S.B. Berberine and evodiamine act synergistically against human breast cancer MCF-7 cells by inducing cell cycle arrest and apoptosis. Anticancer Res. 2017, 37, 6141–6151. [Google Scholar]
- Yu, M.; Tong, X.; Qi, B.; Qu, H.; Dong, S.; Yu, B.; Zhang, N.; Tang, N.; Wang, L.; Zhang, C. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NF-κB. Mol. Med. Rep. 2014, 9, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhu, M.; Yang, L.; Yang, T.; Zhang, Y. Synergistic effect of TPD7 and berberine against leukemia Jurkat cell growth through regulating ephrin-B2 signaling. Phytother. Res. 2017, 31, 1392–1399. [Google Scholar] [CrossRef]
- Wen, C.; Wu, L.; Fu, L.; Zhang, X.; Zhou, H. Berberine enhances the anti-tumor activity of tamoxifen in drug-sensitive MCF-7 and drug-resistant MCF-7/TAM cells. Mol. Med. Rep. 2016, 14, 2250–2256. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Bao, J.; Jia, X.; Liang, Y.; Wang, X.; Chen, M.; Su, H.; Li, P.; Wan, J.B.; et al. Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Sci. Rep. 2016, 6, 26064. [Google Scholar] [CrossRef] [Green Version]
- Balakrishna, A.; Kumar, M.H. Evaluation of synergetic anticancer activity of berberine and curcumin on different models of A549, Hep-G2, MCF-7, Jurkat, and K562 Cell Lines. Biomed. Res. Int. 2015, 2015, 354614. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, D.; Chowdhury, K.D.; Chatterjee, S.; Sarkar, A.; Paul, S.; Sur, P.K.; Sadhukhan, G.C. Modulation of adenylate cyclase signaling in association with MKK3/6 stabilization under combination of SAC and berberine to reduce HepG2 cell survivability. Apoptosis 2017, 22, 1362–1379. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.X.; Liu, C.M.; Gao, A.H.; Zhou, Y.B.; Li, J. Berberine combined with 2-deoxy-d-glucose synergistically enhances cancer cell proliferation inhibition via energy depletion and unfolded protein response disruption. Biochim. Biophys. Acta 2013, 1830, 5175–5183. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Hudy, D.; Poterala-Hejmo, A.; Hejmo, T.; Buldak, R.J.; Dziedzic, A. Effects of Resveratrol, Berberine and Their Combinations on Reactive Oxygen Species, Survival and Apoptosis in Human Squamous Carcinoma (SCC-25) Cells. Anticancer Agents Med. Chem. 2019, 19, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Hashemi-Niasari, F.; Rabbani-Chadegani, A.; Razmi, M.; Fallah, S. Synergy of theophylline reduces necrotic effect of berberine, induces cell cycle arrest and PARP, HMGB1, Bcl-2 family mediated apoptosis in MDA-MB-231 breast cancer cells. Biomed. Pharmacother. 2018, 106, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.Z.; de Moraes, F.R.; Arni, R.K.; Rahal, P.; Calmon, M.F. Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed. Pharmacother. 2019, 123, 109794. [Google Scholar] [CrossRef] [PubMed]
- Grebinyk, A.; Prylutska, S.; Buchelnikov, A.; Tverdokhleb, N.; Grebinyk, S.; Evstigneev, M.; Matyshevska, O.; Cherepanov, V.; Cherepanov, Y.; Yashchuk, V.; et al. C60 Fullerene as an Effective Nanoplatform of Alkaloid Berberine Delivery into Leukemic Cells. Pharmaceutics 2019, 11, E586. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Elbayoumi, T. Anionic and cationic vitamin E-TPGS mixed polymeric phospholipid micellar vehicles. Methods Mol Biol. 2019, 2000, 31–41. [Google Scholar]
- Fang, L.; Fan, H.; Guo, C.; Cui, L.; Zhang, P.; Mu, H.; Xu, H.; Zhao, F.; Chen, D. Novel Mitochondrial Targeting Multifunctional Surface Charge-Reversal Polymeric Nanoparticles for Cancer Treatment. J. Biomed. Nanotechnol. 2019, 15, 2151–2163. [Google Scholar] [CrossRef]
- Suganthi, S.; Sivaraj, R.; Selvakumar, P.M.; Enoch, I.V.M.V. Supramolecular complex binding to G-quadruplex DNA: Berberine encapsulated by a planar side arm-tethered β-cyclodextrin. J. Biomol. Struct. Dyn. 2019, 37, 3305–3313. [Google Scholar] [CrossRef]
- Popiołek, I.; Niziołek, A.; Kamiński, K.; Kwolek, U.; Nowakowska, M.; Szczubiałka, K. Cellular delivery and enhanced anticancer activity of berberine complexed with a cationic derivative of γ-cyclodextrin. Bioorg. Med. Chem. 2019, 27, 1414–1420. [Google Scholar] [CrossRef]
- Khan, I.; Joshi, G.; Nakhate, K.T.; Ajazuddin Kumar, R.; Gupta, U. Nano-co-delivery of berberine and anticancer drug using PLGA nanoparticles: Exploration of better anticancer activity and in vivo Kinetics. Pharm. Res. 2019, 36, 149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, F.; Shao, D.; Zhang, Z.; Cui, L.; Zhang, J.; Dawulieti, J.; Meng, Z.; Zhang, M.; Chen, L. Gram-scale production of carrier-free fluorescent berberine microrods for selective liver cancer therapy. Biofactors 2018, 44, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Li, X.; Huang, X.; Jiang, W.; Liu, L.; Hou, C.; Yang, Y.; Zhang, L.; Zhang, X.; Ye, L.; et al. Synergistic anticancer effects of nanocarrier loaded with berberine and miR-122. Biosci Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Yahuafai, J.; Asai, T.; Oku, N.; Siripong, P. Anticancer efficacy of the combination of berberine and PEGylated liposomal doxorubicin in meth A sarcoma-bearing mice. Biol. Pharm. Bull. 2018, 41, 1103–1106. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, S.Y.; Cho, H.J. Berberine and zinc oxide-based nanoparticles for the chemo-photothermal therapy of lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2018, 501, 765–770. [Google Scholar] [CrossRef]
- Bhanumathi, R.; Manivannan, M.; Thangaraj, R.; Kannan, S. Drug-carrying Capacity and anticancer effect of the folic acid- and berberine-loaded silver nanomaterial to regulate the AKT-ERK pathway in breast cancer. ACS Omega. 2018, 3, 8317–8328. [Google Scholar] [CrossRef] [Green Version]
- Sreeja, S.; Krishnan Nair, C.K. Tumor control by hypoxia-specific chemotargeting of iron-oxide nanoparticle -Berberine complexes in a mouse model. Life Sci. 2018, 195, 71–80. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.S.; Chang, Z.M.; Li, L.; Zhang, Y.; Lu, M.M.; Zheng, X.; Li, M.; Shao, D.; Li, J.; et al. Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinoma. Chem. Biol. Drug Des. 2017, 89, 464–469. [Google Scholar] [CrossRef]
- Gupta, L.; Sharma, A.K.; Gothwal, A.; Khan, M.S.; Khinchi, M.P.; Qayum, A.; Singh, S.K.; Gupta, U. Dendrimer encapsulated and conjugated delivery of berberine: A novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Bułdak, R.J.; Skonieczna, M.; Cholewa, K. Silver nanoparticles exhibit the dose-dependent anti-proliferative effect against human squamous carcinoma cells attenuated in the presence of berberine. Molecules 2016, 21, 365. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Ruan, P.; Meng, Z.; Zhou, J. The structure-dependent electric release and enhanced oxidation of drug in graphene oxide-based nanocarrier loaded with anticancer herbal drug berberine. J. Pharm. Sci. 2015, 104, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS Pharm. Sci. Tech. 2014, 15, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Kuo, J.Y.; Hsu, C.C.; Tsai, W.C.; Li, W.C.; Yu, M.C.; Wen, H.W. Optimizing manufacture of liposomal berberine with evaluation of its antihepatoma effects in a murine xenograft model. Int. J. Pharm. 2013, 441, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, K.X.; Cao, L.; Xie, Z.F.; Gu, M.; Lü, W.; Nan, F.J. Berberine derivatives with a long alkyl chain branched by hydroxyl group and methoxycarbonyl group at 9-position show improved anti-proliferation activity and membrane permeability in A549 cells. Acta Pharmacol. Sin. 2020. [Google Scholar] [CrossRef] [PubMed]
- Milata, V.; Svedova, A.; Barbierikova, Z.; Holubkova, E.; Cipakova, I.; Cholujova, D.; Jakubikova, J.; Panik, M.; Jantova, S.; Brezova, V.; et al. Synthesis and anticancer activity of novel 9-O-substituted berberine derivatives. Int. J. Mol. Sci. 2019, 20, 2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; He, F.; Peng, D.; Zou, M.; Peng, J.; Liu, P.; Liu, Y.; Liu, Z. Synthesis and anticancer activity of 9-O-pyrazole alkyl substituted berberine derivatives. Anticancer Agents Med. Chem. 2018, 18, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yan, T.H.; Yan, L.; Li, Q.; Wang, R.L.; Hu, Z.L.; Jiang, Y.Y.; Sun, Q.Y.; Cao, Y.B. Design, synthesis, and anticancer activity of novel berberine derivatives prepared via CuAAC “click” chemistry as potential anticancer agents. Drug Des. Devel. Ther. 2014, 8, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Pang, W.Q.; Zeng, Q.X.; Deng, Z.S.; Fan, T.Y.; Jiang, J.D.; Deng, H.B.; Song, D.Q. Synthesis and biological evaluation of new berberine derivatives as cancer immunotherapy agents through targeting IDO1. Eur. J. Med. Chem. 2018, 143, 1858–1868. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.; Xu, S.; Lin, A.; Yao, H.; Xie, W.; Zhu, Z.; Xu, J. Design, synthesis and biological evaluation of novel nitric oxide-donating protoberberine derivatives as antitumor agents. Eur. J. Med. Chem. 2017, 132, 173–183. [Google Scholar] [CrossRef]
- Naimi, E.; Zhou, A.; Khalili, P.; Wiebe, L.I.; Balzarini, J.; Clercq, E.; Knaus, E.E. Synthesis of 3′- and 5′-nitrooxy pyrimidine nucleoside nitrate esters: “nitric oxide donor” agents for evaluation as anticancer and antiviral agents. J. Med. Chem. 2003, 46, 995–1004. [Google Scholar] [CrossRef]
- Tesei, A.; Zoli, W.; Fabbri, F.; Leonetti, C.; Rosetti, M.; Bolla, M.; Amadori, D.; Silvestrini, R. NCX 4040, an NO-donating acetylsalicylic acid derivative: Efficacy and mechanisms of action in cancer cells. Nitric Oxide 2008, 19, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Kang, F.H.; Huang, Z.J.; Xue, X.W.; Lai, Y.S.; Peng, S.X.; Tian, J.; Zhang, Y. Synthesis of CDDO-amino acid-nitric oxide donor trihybrids as potential antitumor agents against both drug-sensitive and drug-resistant colon cancer. J. Med. Chem. 2015, 58, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.T.; Wang, G.Y.; Lin, Y.; Zhang, Y.J.; Pei, L.L.; Yao, H.; Hu, M.; Qiu, Y.; Huang, Z.; Zhang, Y.; et al. Novel anticancer oridonin derivatives possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: Design, synthesis, biological evaluation and nitric oxide release studies. Bioorg. Med. Chem. Lett. 2016, 26, 2795–2800. [Google Scholar] [CrossRef]
- Fang, L.; Feng, M.C.; Chen, F.H.; Liu, X.; Shen, H.; Zhao, J.; Guo, S. Oleanolic acid-NO donor-platinum(II) trihybrid molecules: Targeting cytotoxicity on hepatoma cells with combined action mode and good safety. Bioorg. Med. Chem. 2016, 24, 4611–4619. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.X.; Su, H.F.; Lv, P.; Ma, Y.; Wang, S.K.; Miao, H.; Liu, H.Y.; Tan, J.H.; Qu, T.M.; Guo, L.Q.; et al. A newly identified berberine derivative induces cancer cell senescence by stabilizing endogenous G-quadruplexes and sparking a DNA damage response at the telomere region. Oncotarget 2015, 6, 35625–35635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhowmik, D.; Fiorillo, G.; Lombardi, P.; Kumar, G.S. Recognition of human telomeric G-quadruplex DNA by berberine analogs: Effect of substitution at the 9 and 13 positions of the isoquinoline moiety. J. Mol. Recognit. 2015, 28, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Jaisankar, P.; Kumar, G.S. Binding of novel 9-O-N-aryl/arylalkyl amino carbonyl methyl berberine analogs to poly(U)-poly(A)·poly(U) triplex and comparison to the duplex poly(A)-poly(U). Mol. Biol. Rep. 2014, 41, 5473–5483. [Google Scholar] [CrossRef]
- Li, Z.Q.; Liao, T.C.; Dong, C.; Yang, J.W.; Chen, X.J.; Liu, L.; Luo, Y.; Liang, Y.Y.; Chen, W.H.; Zhou, C.Q. Specifically targeting mixed-type dimeric G-quadruplexes using berberine dimers. Org. Biomol. Chem. 2017, 15, 10221–10229. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Zu, Y.; Liu, T.; Zhang, B.; He, W. Generation of reactive oxygen species by a novel berberine-bile acid analog mediates apoptosis in hepatocarcinoma SMMC-7721 cells. Biochem. Biophys. Res. Commun. 2013, 433, 432–437. [Google Scholar] [CrossRef]
- Lo, C.Y.; Hsu, L.C.; Chen, M.S.; Lin, Y.J.; Chen, L.G.; Kuo, C.D.; Wu, J.Y. Synthesis and anticancer activity of a novel series of 9-O-substituted berberine derivatives: A lipophilic substitute role. Bioorg. Med. Chem. Lett. 2013, 23, 305–309. [Google Scholar] [CrossRef]
- Leyva-Peralta, M.A.; Robles-Zepeda, R.E.; Razo-Hernández, R.S.; Berber, L.P.Á.; Lara, K.O.; Ruiz-Bustos, E.; Gálvez-Ruíz, J.C. Berberine as source of antiproliferative hybrid compounds: In vitro antiproliferative activity and quantitative structure-activity relationship. Anticancer Agents Med. Chem. 2019, 19, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Wang, J.; Chen, H.; Tang, J.; Bian, A.W.; Liu, T.; Yu, L.F.; Yi, Z.; Yang, F. Synthesis and anticancer activity of novel 9,13-disubstituted berberine derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 126821. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, E.; Fiorillo, G.; Lombardi, P.; Salvatore, C.; Geroni, C.; Piacenza, F.; Provinciali, M. Antitumor activity of NAX060: A novel semisynthetic berberine derivative in breast cancer cells. Biofactors 2018, 44, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, E.; Arcamone, A.G.; Buzzetti, F.; Lombardi, P.; Salvatore, C.; Provinciali, M. Antitumor effect of novel berberine derivatives in breast cancer cells. Biofactors 2013, 39, 672–679. [Google Scholar] [CrossRef]
- Pierpaoli, E.; Damiani, E.; Orlando, F.; Lucarini, G.; Bartozzi, B.; Lombardi, P.; Salvatore, C.; Geroni, C.; Donati, A.; Provinciali, M. Antiangiogenic and antitumor activities of berberine derivative NAX014 compound in a transgenic murine model of HER2/neu-positive mammary carcinoma. Carcinogenesis 2015, 36, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Guamán Ortiz, L.M.; Tillhon, M.; Parks, M.; Dutto, I.; Prosperi, E.; Savio, M.; Arcamone, A.G.; Buzzetti, F.; Lombardi, P. Multiple effects of berberine derivatives on colon cancer cells. Biomed. Res. Int. 2014, 2014, 924585. [Google Scholar] [CrossRef] [Green Version]
- Albring, K.F.; Weidemüller, J.; Mittag, S.; Weiske, J.; Friedrich, K.; Geroni, M.C.; Lombardi, P.; Huber, O. Berberine acts as a natural inhibitor of Wnt/β-catenin signaling-identification of more active 13-arylalkyl derivatives. Biofactors 2013, 39, 652–662. [Google Scholar] [CrossRef]
- Vieira, S.; Castelli, S.; Falconi, M.; Takarada, J.; Fiorillo, G.; Buzzetti, F.; Lombardi, P.; Desideri, A. Role of 13-(di)phenylalkyl berberine derivatives in the modulation of the activity of human topoisomerase IB. Int. J. Biol. Macromol. 2015, 77, 68–75. [Google Scholar] [CrossRef]
- Francéschin, M.; Rossetti, L.; D’Ambrosio, A.; Schirripa, S.; Bianco, A.; Ortaggi, G.; Savino, M.; Schultes, C.; Neidle, S. Natural and synthetic G-quadruplex interactive berberine derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 1707–1711. [Google Scholar] [CrossRef]
- Jin, H.; Ko, Y.S.; Park, S.W.; Chang, K.C.; Kim, H.J. 13-Ethylberberine Induces Apoptosis through the Mitochondria-Related Apoptotic Pathway in Radiotherapy-Resistant Breast Cancer Cells. Molecules 2019, 24, 2448. [Google Scholar] [CrossRef] [Green Version]
- Mistry, B.M.; Shin, H.S.; Keum, Y.S.; Pandurangan, M.; Kim, D.H.; Moon, S.H.; Kadam, A.A.; Shinde, S.K.; Patel, R.V. Synthesis and evaluation of antioxidant and cytotoxicity of the N-Mannich base of berberine bearing benzothiazole moieties. Anticancer Agents Med. Chem. 2017, 17, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Mistry, B.; Patel, R.V.; Keum, Y.S.; Kim, D.H. Synthesis of N-Mannich bases of berberine linking piperazine moieties revealing anticancer and antioxidant effects. Saudi J. Biol. Sci. 2017, 24, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, J.; Ma, F.; Yao, S.; Li, N.; Wang, J.; Wang, Y.; Wang, X.; Yao, Q. Synthesis and cytotoxicity evaluation of 13-n-alkyl berberine and palmatine analogues as anticancer agents. Molecules 2012, 17, 11294–11302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, H.; Wang, J.; Wang, M.; Shao, R. Cyclizing-berberine A35 induces G2/M arrest and apoptosis by activating YAP phosphorylation (Ser127). J. Exp. Clin. Cancer Res. 2018, 37, 98. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, G.; Bi, C.; Li, Y.; Liu, J.; Ye, C.; He, H.; Li, L.; Song, D.; Shao, R. The dual topoisomerase inhibitor A35 preferentially and specially targets topoisomerase 2α by enhancing pre-strand and post-strand cleavage and inhibiting DNA relegation. Oncotarget 2015, 6, 37871–37894. [Google Scholar] [CrossRef] [Green Version]







| Cell Type or Animal Model | Cellular Target | Main Endpoint Outcome | Reference |
|---|---|---|---|
| P-53-null leukemic (Jurkat and U937) cell lines | Decreased the mRNA expression of MDM2; enhance MDM2 self-ubiquitination; induce autophagy which could be inhibited by inhibitors 3-methyladenine. | Induction of autophagy in p53-null leukemic cells. | Liu et al. [23] |
| Human colorectal (SW480) cancer cells | Induce apoptosis; inhibit cell surface expression of GRP78; modulate the expression of apoptosis regulators (Bax, Bcl-2 and c-Myc); effect reversed by overexpression of GRP78. | Inhibition of proliferation and cell migration. | Gong et al. [24] |
| Breast cancer(MCF-7 and MDA-MB-231) cells | Induce cytotoxicity and G1 phase arrest; upregulate p21/cip1 and p27/kip1 and their nuclear localization by increasing their post-translational stability; effects mediated via inhibition of Akt. | Induction of cell death. | Tak et al. [25] |
| HeLa cells | Downregulate NF-κB (30 µM) and affect various pathways (HIF1A/NFE2L2/AP-1) at 100 µM. | Inhibition of cell growth. | Belanova et al. [26] |
| Glioblastoma (U87 and U251) cells in vitro and their xenografts in mice | Induce cytotoxicity and inhibit endothelial cell (HUVEC) migration; antitumour effect (survival rate and tumour size) in vivo; inhibit the phosphorylation of VEGFR2 and ERK. | Inhibition of angiogenesis. | Jin et al. [27] |
| Glioblastoma U343 and pancreatic carcinoma MIA PaCa-2 cells vs. Human dermal fibroblasts as non-cancerous cells | Decrease citrate synthase and caspase-3 activity and autophagy; induce cell cycle arrest at G2 and senescence without autophagy in U343 cells; induce G1 arrest, senescence and autophagy in MIA PaCa-2 cells. | Induction of cell-dependent cell cycle arrest and autophagy. | Agnarelli et al. [28] |
| Endometrial cancer (AN3 CA and HEC-1-A) cells in vitro; HEC-1-A xenograft in nude mice. | Suppress COX-2 (protein) PGE2 levels; effect dependent on upregulation of miR-101 via AP-1 modulation. | Inhibition of cell growth, migration, invasion and metastasis both in vitro and in vivo. | Wang and Zhang [29] |
| Cholangiocarcinoma (KKU-213 and -214) cell lines | Induce G1 phase arrest; reduce cyclin D1, and cyclin E; reduce the expression and activation of STAT3 and NF-κB; suppress ERK 1/2. | Cell cycle arrest and growth inhibition. | Puthdee et al. [30] |
| Preventive effect against DMBA-induced breast cancer in female rats. | Suppress lipid peroxidation (MDA level), pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), antioxidants (SOD and CAT, GSH and vitamin C) and NF-κB. | Inhibition of ductal carcinoma and invasiveness. | Karnam et al. [31] |
| Cervical cancer HeLa cells | Inhibit cell proliferation (IC50 of 18 μM), tubulin and microtubule assembly; induce G2/M arrest; bind to tubulin at a single site with a Kd of 11 μM; inhibit the assembly of tubulin into microtubules and disrupt microtubules polymerization in the presence of glutamate and paclitaxel; form a stable complex with tubulin and bind at a novel site 24 Å from the colchicine site on the β-tubulin. | Inhibition of cancer cell proliferation by targeting mitotic microtubules. | Raghav et al. [32] |
| Human Saos-2 and MG-63 osteosarcoma cells | Reduce the expression of caspase-1 and IL-1β. | Improvement of inflammatory microenvironment in addition to cytotoxicity. | Jin et al. [33] |
| MCF-7 breast cancer cells | Suppress chemokine receptors (mRNA) expression (at 10–80 μg/mL) | Suppression of cell migration in wound healing assay. | Ahmadiankia et al. 2016 [34] |
| FaDu head and neck squamous cell carcinoma cells | Upregulate apoptotic ligands (FasL and TRAIL); activate caspase-8, -7 and -3, PARP; upregulate pro-apoptotic factors, (Bax, Bad, Apaf-1, and the active form of caspase-9); downregulate anti-apoptotic factors (Bcl-2 and Bcl-xL); increase the expression of p53; downregulate VEGF, MMP-2 and MMP-9; suppress the phosphorylation (p) of ERK1/2 and p38. | Induction of apoptosis and inhibition of cell migration. | Seo et al. [35] |
| In vitro NIH-3T3 and C3H/10T1/2 mouse embryo fibroblast cells, HEK-293T human epithelial kidney cells, and LS174T colon cancer cells; allografting medulloblastoma into nude mice | Inhibit the hedgehog pathway and associated Smoothened; inhibits medulloblastoma cells (isolated from medulloblastoma in patch+/−; p53−/− mice) growth in hedgehog dependent manor. | Inhibition of cancer cell growth both in vitro and in vivo. | Wang et al. [36] |
| Hepatocellular carcinoma (H22, HepG2 and Bel-7404) cells; H22 transplanted tumour model in mice | Reduce cell viability and induce apoptosis; suppress tumour growth in vivo; reduce cytosolic PLA2 and COX-2 protein levels; elevate the content ratio of arachidonic acid to PGE2. | Induction of apoptosis and tumour growth inhibition both in vitro and in vivo. | Li et al. [37] |
| T47D and MCF7 cell lines. | Induce cytotoxicity (IC50 of 25 µM in both cell lines compared to doxorubicin as 250 nM and 500 nM in T47D and MCF-7 respectively); induce G2/M arrest in the T47D cells, but G0/G1 arrest in the MCF-7 cells; doxorubicin induced G2/M arrest in both cell lines. | Induction of cell cycle arrest and cytotoxicity. | Barzegar et al. [38] |
| BGC-823 gastric cancer cells; xenograft in nude mice injected with human gastric cancer cells | Increase the expression level of cleaved PARP and caspase-3; impair Δψm; inhibit the Akt/mTOR/p70S6/S6 pathway; inhibit Akt activation. | Induction of apoptosis in vitro and tumour growth inhibition in vivo. | Yi et al. [39] |
| KB oral cancer cells | Increase the expression of the death receptor ligand, FasL; activate pro-apoptotic factors (caspase-8, -9 and -3 and PARP, Bax, Bad and Apaf-1); suppress anti-apoptotic factors (Bcl-2 and Bcl-xL); pan-caspase inhibitor (VAD-FMK) inhibit the activation of caspase-3 and PARP by berberine. | Induce apoptosis through both extrinsic death receptor- and intrinsic mitochondrial-dependent signalling pathways. | Kim et al. [40] |
| Prostate cancer (LNCaP, DU-145, and PC-3) cells | Suppress a panel of mesenchymal genes (high BMP7, NODAL and Snail) expression that regulate the developmental epithelial-to-mesenchymal transition. | Inhibition of migration and invasiveness of highly metastatic cancer cells. | Liu et al. [41] |
| Human ovarian (SKOV3) cancer cells | Downregulate anti-apoptotic genes (BCL-2 and survivin); up-regulate pro-apoptotic gene (Bax). | Inhibition of cell proliferation and induction of apoptosis. | Jin et al. [42] |
| Pancreatic cancer (PANC-1 and MIA-PaCa2) cell lines | Induce G1-phase arrest through mechanisms related to ROS production and caspase 3/7 activation. | Induction of cell cycle arrest and apoptosis. | Park et al. [43] |
| Human prostate (LnCaP and PC-3) cancer cell lines | Induce G1 phase arrest; inhibit the expression of PSA and the activation of EGFR. | Inhibition of cell growth and induction of apoptosis. | Huang et al. [44] |
| MDA-MB-231 breast cancer cells | Inhibit IL-8 secretion by suppressing PI3K, JAK2, NF-κB and AP-1; downregulate gene expression of MMP-2, EGF, E-cadherin, bFGF and fibronectin; induce G2/M arrest and cell apoptosis in an IL-8-independent manner; activate p38 MAPK and JNK while suppressing JAK2, p85 PI3K, Akt and NF-κB. | Inhibition of cell proliferation and cell invasion induced by IL-8. | Li et al. [45] |
| MG-63 human osteosarcoma cells | Induce DNA damage and apoptosis. | Induction of apoptosis. | Zhu et al. [46] |
| Human multiple myeloma cell line U266 | Suppress the expression of DNA methyltransferases (DNMT1 and DNMT3B) which triggers hypomethylation of TP53 by changing the DNA methylation level and the alteration of p53 dependent signal pathway. | Induction of apoptosis. | Qing et al. [47] |
| p53-Null leukaemia cells | Induce apoptotic cell death via inhibition of XIAP protein; inhibit MDM2 expression. | Induction of apoptosis. | Liu et al. [48] |
| Human non-small-cell lung cancer (NSCLC) cells | Inhibit AP-2α and AP-2β expression and their binding on hTERT promoters; inhibit hTERT expression; suppress NF-κB mobilisation and binding to COX-2 promoter; inhibit COX-2 expression; downregulate HIF-1α and VEGF expression; inhibit Akt and ERK phosphorylation; induce cytochrome-c release from mitochondria; promote caspase and PARP activity; modulate Bax and Bcl-2 expression. | Inhibition of cell proliferation, migration, and colony formation, and induction of apoptosis. | Fu et al. [49] |
| Human hepatoma Bel7402 cells. | Induce G1 cell cycle arrest; effect enhanced by calmodulin inhibitors; decrease the phosphorylation of calmodulin kinase II; block MEK1 activation and p27 protein degradation. | Cell cycle arrest and inhibition of cell growth | Ma et al. [50] |
| MDA-MB-231 breast cancer cells | Downregulate MMP2 (activities) and MMP9 (expression); inhibit Akt, NF-κB and AP-1; supress Akt expression via modulating its mRNA expression and protein degradation. | Potential for inhibition of cancer metastasis | Kuo et al. [51] |
| Thyroid cancer 8505C and TPC1cell lines. | Induce cell cycle arrest at the G0/G1 phase (TPC1 cells); upregulate p-27; induce cell death (IC50 of 10 µM). | Inhibition of growth and induction of apoptosis | Park et al. [52] |
| Human epithelial ovarian carcinoma (OVCAR-3 and SKOV-3) cell lines | Induce cytotoxicity and G2/M phase (OVCAR-3 cells) and S phase (SKOV-3 cells) arrest; upregulate p27. | Inhibition of cell proliferation | Park et al. [53] |
| Angiogenesis using B16F-10 melanoma cells and capillary formation in C57BL/6 mice; angiogenesis model of endothelial cells from rat aortic ring | Inhibition in tumour-directed capillary formation and in various proangiogenic factors (VEGF, IL-1β, IL-6, TNF-α, and GM-CSF); increase the serum levels of antitumor factors (IL-2 and TIMP); suppress NF-ĸB, c-Fos, CREB, and ATF-2; inhibit the expression (mRNA) levels of proangiogenic factors (COX-2, iNOS, and HIF). | Inhibition of angiogenesis both in vitro and in vivo; inhibition of endothelial cell motility, migration, tube formation, and vessel sprouting. | Hamsa and Kuttan [54] |
| Human ductal breast epithelial tumour (T47D) cell line | Decrease COX-2, iNOS and survivin proteins. | Inhibition of cell viability and induction of apoptosis. | Pazhang et al. [55] |
| Human colon cancer (HCT-8) cell line | Induce cell cycle arrest at S phase; upregulate p-regulated mRNA and/or protein expressions of Fas, FasL, TNF-α and caspase-3; down-regulate pro-caspase-3; decrease Bcl-2 and increase of Bax (mRNA and protein) expressions. | Inhibition of cell growth and induce apoptosis. | Xu et al. [56] |
| Non-small cell human lung (A549 as a wild-type p53, and H1299 as p53-deficient) cancer cells in vitro and H1299 tumour xenograft growth athymic nude mice. | p53-Dependent cell death; disrupt Δψm, reduce the levels of Bcl-2, Bcl-xl while increasing in Bax, Bak; activate caspase-3. | Inhibition of cell proliferation and induction of apoptotic cell death in vitro and inhibition of tumour growth in vivo. | Katiyar et al. [57] |
| Human hepatocellular carcinoma (HepG2) cells in vitro and in vivo | Increase the expression level of Fas and P53, cause depolarization of mitochondrial membrane and decrease Δψm; activate caspase-3, -8, and -9. | Reduce cell growth and induce apoptosis; reduce tumour growth rates in mice. | Wang et al. [58] |
| Human neuroblastoma SK-N-SH and SK-N-MC cells | More cytotoxic to p53-expressing SK-N-SH (IC50 = 37 μM) than p53-deficient SK-N-MC cells (IC50 ≥ 100 μM); induce cell cycle arrest at G0/G1 phase; decrease G0/G1 phase-associated CDK (cyclin D1, cyclin E, Cdk2, and Cdk4) expression; increase apoptotic gene expression and activate caspase-3 in susceptible cells. | Induction of apoptotic cell death in cancer but not in normal cortical neuronal cells. | Choi et al. [59] |
| Human gastric SNU-5 cancer cells | Downregulate MMP-1 -2, and -9 (no effect on the level of MMP-7); inhibit gene expression for MMP-1, -2 and -9 (no effect on MMP-7); induce ROS production. | Reduction of cell viability. | Lin et al. [60] |
| Human oral squamous cell carcinoma (HSC-3) cells | Induce mainly G0/G1-phase arrest; increase intracellular levels of ROS and Ca2+; reduce Δψm. | Inhibition of cell growth and induction of apoptosis. | Lin et al. [61] |
| Human cervical cancer Ca Ski cells | Increase the ratio of p53 and Bax/Bcl-2 proteins; increase the levels of ROS and Ca2+; disrupt Δψm; promote caspase-3 activity; induce the expression of transcription factor GADD153. | Induction of apoptosis | Lin et al. [62] |
| Human colonic carcinoma SW620 cells | Activate caspases (-3 and -8), cleavage of PARP and the release of cytochrome-c; downregulate the expression of anti-apoptosis factor (c-IAP1, Bcl-2, and Bcl-XL); increase the phosphorylation of JNK and p38 MAPK; induce ROS generation; increase the cellular levels of c-Jun and FasL. | Induction of apoptosis | Hsu et al. [63] |
| Murine leukaemia WEHI-3 cells in vitro; and in vivo WEHI-3 cancer cells injected in mice | Induce cytotoxicity in cancer cells in vitro; promote differentiation of the B-cells precursors in vivo; reduce Mac-3 and CD11b markers (inhibit differentiation of precursors of macrophages and granulocytes); no effect on the CD14- and CD19-augmented (promotion of B-cells precursors differentiation). | Induction of cytotoxicity in vitro and reduction of spleen weight in cancer bearing animals in vivo. | Yu et al. [64] |
| Melanoma B16 cells and U937 cells | B16 cells (IC100 < 1 μg/mL) much more sensitive than U937 cells (IC100 < 100 μg/mL) U937 cells; apoptotic cell death in U937 and necrosis in NB16 cells | Cytotoxic to cancer cells | Letasiová et al. [65] |
| Preparation | Characteristics | Assay Model & Main Outcome | Reference |
|---|---|---|---|
| Nanosized carbon nanoparticle-C60 fullerene (C60) | Water dispersions of noncovalent C60-Ber nanocomplexes in the 1:2, 1:1, and 2:1 molar ratios. | Promote Ber intracellular uptake; higher antiproliferative potential towards CCRF-CEM cells free - Berberine < 1:2 < 1:1 < 2:1 molar ratio preparations; activate caspase 3/7; cell cycle arrest at sub-G1 phase; induce apoptosis. | Grebinyk et al. [106] |
| Anionic and cationic vitamin E-TPGS mixed polymeric phospholipid micellar vehicles | Lipid-based nanocarriers, amphiphilic mixed micelles composed of polymeric phospholipid conjugates and PEG-succinate ester of tocopherol. | Human prostate cancer cell lines (PC3 and LNPaC)—enhance apoptosis induction with 30-fold potential improvement of pharmacokinetics. | Yao and Elbayoumi [107] |
| Novel mitochondria targeting surface charge-reversal polymeric nanoparticles | Vitamin B6-oligomeric hyaluronic acid (OHA)-dithiodipropionic acid-berberine preparation; berberine conjugated with OHA and OHA further conjugated to B6. Micelles of 172.9 nm formed by formulating conjugates with Cur-loaded nanoparticles. | Induce cytotoxicity in vitro against PANC-1 cells and tumour growth in nude mice bearing PANC-1 cells xenograft; subcellular drug distribution shows mitochondria as target. | Fang et al. [108] |
| Planar side arm-tethered β-cyclodextrin encapsulation | Fluorenyl derivative of β-cyclodextrin used to encapsulate berberine. | Strongly binds with duplex and G-quadruplex DNAs although its association with the cavity of β-cyclodextrin diminishes the binding strength. | Suganthi et al. [109] |
| Cationic γ-cyclodextrin derivative | A cationic derivative of γ-cyclodextrin synthesised through modification with propylenediamine; mucoadhesive with resistance to digestion by ∝-amylase. | Localised in lysosomes with cytotoxicity twice higher than berberine in murine melanoma (B16-F10) and 4T1 cells. | Popiołek et al. [110] |
| PLGA nanoparticles | PLGA-doxorubicin conjugate used for encapsulation of berberine. | Anti-proliferative against MDA-MB-231 and T47D breast cancer cell lines were observed with IC50 of 1.94 ± 0.22 and 1.02 ± 0.36 μM; alter mitochondrial permeability and arrest cell cycle at sub G1 phase; 14-fold increase in half-life of berberine in rats. | Khan et al. [111] |
| Self-carried berberine microrods | Carrier prepared by mixing trimethylamine with berberine hydrochloride in DMSO to form about 20–100 μm length and 5–20 μm width irregular size product. | Hepatocellular carcinoma (HepG2, SMMC-7721, Hep3B, H22 cells) and normal cell lines (HL-7702 cells, HUVEC cells, C2C12 cells, and H9C2 cells) used for cytotoxicity assay; With about 40 µg/mL IC50 value, about twice more selective than berberine in cancer cells. | Zheng et al. [112] |
| Polyethyleneimine (PEI)-cholesterol (PC) berberine nanocarrier complexed with miR-122 | Berberine incorporated to PC with further electrostatic complex with miR-122; good drug loading (8.4%) and release (63.0) capacity of nanoparticles of about 146 nm. | Decrease OSCC cells invasion and migration in transwell assay when compared with single drug treatments. | Lei et al. [113] |
| Berberine with PEGylated Liposomal Doxorubicin (PEG-lip-DOX) | Berberine combined with polyethylene glycolated liposomal doxorubicin. | Inhibit the vascular endothelial growth factor (VEGF) expression in human umbilical vein endothelial cells (HUVECs); inhibit (via i.v.) tumour growth in Meth A sarcoma-transplanted mice; effect stronger than berberine or PEG-lip-DOX alone. | Yahuafai et al. [114] |
| Zinc oxide-based nanoparticles | Berberine and zinc oxide (ZnO) combined through facile blending at the ratio of 39:61 to form 200–300 nm size nanoparticles. | Enhance antiproliferative activity in A549 (human lung adenocarcinoma) cells; no obvious severe hepatotoxicity, renal toxicity, and haemotoxicity in rats by i.v. | Kim et al. [115] |
| Folic acid- and berberine-loaded silver nanomaterial (FA-PEG@BBR-AgNPs) | Encapsulating berberine on citrate-capped silver nanoparticles (AgNPs) through electrostatic interactions (berberine-AgNPs) followed by conjugation with polyethylene glycol-functionalized folic acid through hydrogen bonding interactions. | Enhance apoptosis in MDA-MB-231 breast cancer cells; induce ROS; modulate PI3K, AKT, Ras, Raf, ERK, VEGF, HIF1α, Bcl-2, Bax, cytochrome-c, caspase-9, and caspase-3; inhibit tumour growth in vivo when administered intravenously into MDA-MB-231 tumour-bearing athymic nude mice. | Bhanumathi et al. [116] |
| Hypoxia-specific chemo-targeting iron-oxide nanoparticle–Berberine complexes | Hypoxic cell-sensitizer sanazole (SAN) -directed targeting of cytotoxic drug berberine and iron-oxide nanoparticle complexes. | Reduce tumour volume in mice bearing solid tumour in hind limb; increase DNA damage; suppress the levels of transcription of HIF-1α, VEGF, Akt and Bcl2; increase Bax and caspases expressions. | Sreeja and Krishnan [117] |
| Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy | Janus magnetic mesoporous silica nanoparticles (Fe3O4-mSiO2 nanoparticles): Fe3O4 head for magnetic targeting and a mesoporous SiO2 body for pH-dependent berberine delivery. | Magnetic field-induced endocytosis and pH-responsive drug release leading to improved cytotoxicity against hepatocellular HepG2 carcinoma cells. | Wang et al. [118] |
| Dendrimer encapsulated and conjugated delivery of berberine | Dendrimer (G4 PAMAM) encapsulated and conjugated berberine formulations of 100–200 nm size; entrapment efficiency of 29.9% or percentage conjugation of 37.49%. | Higher drug payload in conjugation method; sustained and efficient release pattern in vitro; higher anticancer effect in vitro against MCF-7 and MDA-MB-468 breast cancer cells; no haemolytic effect ex vivo; improved pharmacokinetic in rats with about 2-fold improvement in half-life (t1/2). | Gupta et al. [119] |
| Silver nanoparticles | Nanosize silver particles with berberine chloride. | Human tongue squamous carcinoma SCC-25–IC50 of 5.19 μg/mL; cell cycle arrest at G0/G1 phase; increase of Bax/Bcl-2 ratio gene expression. | Dziedzic et al. [120] |
| Graphene oxide-based berberine nanocarrier | Electric-sensitive drug release and redox sensitive graphene oxide nanocomposite loading berberine. | - | Yu et al. [121] |
| Solid lipid nanoparticle encapsulation. | Solid lipid nanoparticle (SLN) with particle size of 81 nm and zeta potential of 28.67 ± 0.71 mV. | More cell proliferation inhibitory effect on MCF-7, HepG 2, and A549 cancer cells than berberine; induce cell cycle arrest, and apoptosis. | Wang et al. [122] |
| Liposomal berberine | Polyethenyl glycol (PEG) with maximum encapsulation efficiency berberine as 14%. | 2.5-times more active in inhibiting the growth of HepG2 cells than berberine (IC50 of 1.67 μg/mL vs. 4.23 μg/mL); induce apoptosis through the caspase/mitochondria-dependent pathway; lower rate of elimination in both plasma and tissues; improved antitumour effect in vivo when tested in tumour xenograft mice bearing HepG2-induced tumour. | Lin et al. [123] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, S. Recent Advances in Berberine Inspired Anticancer Approaches: From Drug Combination to Novel Formulation Technology and Derivatization. Molecules 2020, 25, 1426. https://doi.org/10.3390/molecules25061426
Habtemariam S. Recent Advances in Berberine Inspired Anticancer Approaches: From Drug Combination to Novel Formulation Technology and Derivatization. Molecules. 2020; 25(6):1426. https://doi.org/10.3390/molecules25061426
Chicago/Turabian StyleHabtemariam, Solomon. 2020. "Recent Advances in Berberine Inspired Anticancer Approaches: From Drug Combination to Novel Formulation Technology and Derivatization" Molecules 25, no. 6: 1426. https://doi.org/10.3390/molecules25061426
APA StyleHabtemariam, S. (2020). Recent Advances in Berberine Inspired Anticancer Approaches: From Drug Combination to Novel Formulation Technology and Derivatization. Molecules, 25(6), 1426. https://doi.org/10.3390/molecules25061426