Flow Chemistry in Contemporary Chemical Sciences: A Real Variety of Its Applications
Abstract
1. Introduction
2. Milestones in the Development of Various Areas of Flow Chemistry
2.1. Flow Analysis
2.2. Flow Synthesis
2.3. Fundamental Physico-Chemical Measurements under Flow Conditions
2.4. Chemical Processing under Flow Conditions for Non-Analytical and Non-Synthetic Purposes
3. Similarities in Development Trends in Flow Analysis and Flow Chemistry
3.1. Detectors, Reactors, Manifolds
3.2. On-Line Processes Supporting Flow Analysis and Flow Synthesis
3.3. Microfluidics in Flow Analysis and Flow Synthesis
4. Toward the Automation of Chemical Flow Systems
4.1. In-Line Analytical Monitoring in Flow Synthetic Systems
4.2. Automated Flow Analytical and Synthetic Systems
4.3. Robotics, Digital Transmission, and Camera-Enabled Techniques in Flow Chemistry Instrumentation
5. Conclusions and Perspectives
Conflicts of Interest
References
- Driscoll, R.J.; Moon, L.F. Pressure recovery in chemical lasers. AIAA J. 1977, 15, 665–673. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Billone, M.C.; Fischer, A.K.; Tam, S.W.; Clemmer, R.G.; Hollenberg, G.W. Solid tritium breeder materials-Li2O and LiAlO2: A data base review. Fision Technol. 1985, 8, 1970–1984. [Google Scholar] [CrossRef]
- Joseph, H.C.; Origley, E.D. Role of suspended sediment in irrigation return flow chemistry, Southern Alberta. Water Resour. Res. 1986, 22, 643–654. [Google Scholar] [CrossRef]
- Gupta, R.N. Aerothermodynamic analysis of stardust sample return capsule with coupled radiation and ablation. J. Spacecraft Rock. 2000, 37, 507–514. [Google Scholar] [CrossRef]
- Wang, X.D.; Cardwell, T.J.; Cattrall, R.W.; Jenkins, G.E. Pulsed flow chemistry. A new approach to the generation of concentration profiles in flow analysis. Anal. Commun. 1998, 33, 97–101. [Google Scholar] [CrossRef]
- West, J.; Karamata, B.; Lillis, B.; Gleeson, J.P.; Alderman, J.; Collins, J.K.; Lane, W.; Mathewson, A.; Berney, H. Application of magneto-hydrodynamic actuation to continuous flow chemistry. Lab. Chip. 2002, 2, 224–230. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Deeley, J.; Griffiths, C.M.; Ley, S.V.; Saaby, S.; Tranmer, G.K. A flow process for the multi-step synthesis of the alkaloid natural product oxomaritidine: A new paradigm for molecular assembly. Chem. Commun. 2006, 24, 2566–2568. [Google Scholar] [CrossRef]
- Bayer, E.; Jung, E.; Halasz, I.; Sebastian, I. A new support for polypeptide synthesis in columns. Tetrahedron Lett. 1970, 4503–4505. [Google Scholar] [CrossRef]
- Cwiet, M.S. O нoвoй кaтeгopии aдcopбциoнныx явлeний и o пpимeнeнии иx к биo-xимичecкoмy aнaлизy. Proc. Warsaw Soc. Nat. Sci. Biol. Sec. 1903, 14, 20–39. [Google Scholar]
- Tswett, M. Adsorptionsanalyse und chromatographishe Methode. Anwendung auf die Chemie des Chlorophylls. Ber. Dtsch. Botan. Ges. 1906, 24, 385–392. [Google Scholar]
- Reed, L. Notes on capillary separation of substances in solution. Proc. Chem. Soc. 1893, 9, 123–126. [Google Scholar]
- Skeggs, L.T., Jr. An automatic method for colorimetric analysis. Clin. Chem. 1956, 2, 241. [Google Scholar] [CrossRef]
- Skeggs, L.T., Jr. An automatic method for colorimetric analysis. Am. J. Clin. Path. 1957, 28, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Kołacińska, K. Flow injection methods in chemical analysis. Analyst 2016, 141, 2085–2139. [Google Scholar] [CrossRef] [PubMed]
- Furman, W.B. Continuous Flow Analysis. Theory and Practice; Marcel Dekker, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Blaedel, W.J.; Hicks, G.P. Continuous analysis by measurement of the rate of enzyme catalyzed reactions. Anal. Chem. 1962, 34, 388–394. [Google Scholar] [CrossRef]
- Nagy, G.; Fegher, Z.; Pungor, E. Application of silicone rubber-based graphite electrodes for continuous flow measurements: Part II. Voltammetric study of active substances injected into electrolyte streams. Anal. Chim. Acta 1970, 52, 47–54. [Google Scholar] [CrossRef]
- Ruzicka, J.; Hansen, E.H. Flow injection analysis. 1. New concept of fast continuous-flow analysis. Anal. Chim. Acta 1975, 78, 145–157. [Google Scholar]
- Ruzicka, J.; Stewart, J.W.B. Flow injection analysis. 2. Ultrafast determination of phosphorus in plant material by continuous-flow spectrophotometry. Anal. Chim. Acta 1975, 79, 79–91. [Google Scholar]
- Stewart, J.W.B.; Ruzicka, J.; Bergamin Filho, H.; Zagatto, E.A.G. Flow injection analysis. 3. Comparison of conti- nuous-flow spectrophotometry and potentiometry for rapid-determination of total nitrogen content in plant digests. Anal. Chim. Acta 1976, 81, 371–386. [Google Scholar] [CrossRef]
- Stewart, K.K.; Beecher, G.R.; Hare, P.E. Automated high-speed analyses of discrete samples – Use of nonsegmented, continuous-flow systems. Fed. Proc. 1974, 33, 1439. [Google Scholar]
- Stewart, K.K.; Beecher, G.R.; Hare, P.E. Rapid analysis of discrete samples: The use of nonsegmented, continuous flow. Anal. Biochem. 1976, 70, 167–173. [Google Scholar] [CrossRef]
- Trojanowicz, M. Commercially available instrumentation for FIA. In Flow Injection Analysis. Instrumentation and Applications; World Scientific: Singapore, 2000. [Google Scholar]
- Ruzicka, J.; Marshall, G.D. Sequential injection: A new concept for chemical sensors, process analysis and laboratory assays. Anal. Chim. Acta 1990, 237, 329–343. [Google Scholar] [CrossRef]
- Ruzicka, J. Lab-on-valve: Universal microflow analyzer based on sequential and bead injection. Analyst 2000, 125, 1053–1060. [Google Scholar] [CrossRef]
- Terry, S.S.; Jerman, J.H.; Angell, J.B. Gas-chromatogaphic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron. Dev. 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Johnson, B.; Lofas, S.; Lindquist, G. Immobilization of proteins to a carboxymethyldextrin-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 1991, 198, 268–277. [Google Scholar] [CrossRef]
- Verpoorte, E.M.J.; Van Der Schoot, B.H.; Jeanneret, S.; Manz, A.; Widmer, H.M.; De Rooij, N.F. Three-dimensional micro flow manifolds for miniaturized chemical analysis systems. J. Micromech. Microeng. 1994, 4, 246–256. [Google Scholar] [CrossRef]
- Karnatz, F.A.; Whitmore, F.C. Dehydration of diethylcarbinol. J. Am. Chem. Soc. 1932, 54, 3461. [Google Scholar] [CrossRef]
- Walker, R.E. Chemical reaction and diffusion in a catalytic tubular reactor. Phys. Fluids 1961, 4, 1211–1216. [Google Scholar]
- Pines, H.; Csicsery, S.M. Alumina: Catalyst and support. XVI. Aromatization and dehydroisomerization of branched C6-C8 hydrocarbons over “nonacidic” chromia-alumina catalyst. J. Catal. 1962, 1, 313–328. [Google Scholar] [CrossRef]
- Davis, B.R.; Scott, D.S. Rate of isomerization of cyclopropane in a flow reactor. I&EC Fundamentals 1964, 3, 20–23. [Google Scholar]
- Bodi, L.J. A flow synthesis of gallium phosphide and some properties of gallium phosphide powder layers. J. Electrochem. Soc. 1962, 109, 497–501. [Google Scholar] [CrossRef]
- Carroll, D. Continuous-flow salt gradient dialysis for the preparation of polynucleotide-polypeptide complexes. Anal. Biochem. 1971, 44, 496–502. [Google Scholar] [CrossRef]
- Dye, J.L.; Lok, M.T.; Tehan, F.J.; Ceraso, J.M.; Voorhees, K.J. Flow synthesis. A substitute for the high-dilution steps in cryptate synthesis. J. Org. Chem. 1973, 38, 1773–1775. [Google Scholar] [CrossRef]
- Lukas, T.J.; Prystowsky, M.B.; Erickson, B.W. Solid-phase peptide synthesis under continuous-flow conditions. Proc. Natl. Acad. Sci. USA 1981, 78, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.P. Automated continuous flow peptide synthesis. Nature 1986, 319, 429–430. [Google Scholar] [CrossRef]
- Warner, B.D.; Warner, M.E.; Karns, G.A.; Lu, L. Construction and evaluation of an instrument for the automated synthesis of oligodeoxyribonucleotides. DNA 1984, 3, 401–411. [Google Scholar] [CrossRef]
- Vagner, J.; Kocna, P.; Krchnak, V. Continuous-flow synthesis of alpha-gliadin peptides in an ultrasonic field and assay of their inhibition of intestinal sucrase activity. Peptide Res. 1991, 4, 284–288. [Google Scholar]
- Wong Hawkes, S.Y.V.; Chapela, M.J.V.; Montembault, M. Leveraging the advantages offered by microfluidics to enhance the drug discovery process. QSAR Comd. Sci. 2005, 24, 712–721. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Pitts, M.R. Microwave flow chemistry: The next evolutionary step in synthetic chemistry? Chim. Oggi 2006, 24, 41–45. [Google Scholar] [CrossRef]
- Lee, C.-C.; Sui, G.; Elizarov, A.; Shu, C.J.; Shin, Y.-S.; Dooley, A.N.; Huang, J.; Daridon, A.; Wyatt, P.; Stout, D.; et al. Multistep synthesis of a radiolabeled imaging probe using integrated microfluidics. Science 2005, 310, 1793–1796. [Google Scholar] [CrossRef]
- Richards, P. MIT and Novartis in new partnership aimed at transforming pharmaceutical manufacturing. Available online: http://news.mit.edu/2007/novartis-0928 (accessed on 15 March 2020).
- Chemat, F.; Esveld, D.C.; Poux, M.; Di-Martino, J.L. The role of selective heating in the microwave activation of hetero- geneous catmnsis reactions using a continuous microwave reactor. J. Microwave Power Electrom. Energ. 1998, 33, 88–94. [Google Scholar] [CrossRef]
- Nikbin, N.; Watts, P. Solid-supported flow synthesis in microreactors using electroosmotic flow. Org. Proc. Res. Dev. 2004, 8, 942–944. [Google Scholar] [CrossRef]
- Marre, S.; Park, J.; Rempel, J.; Guan, J.; Bawendi, M.G.; Jensen, K.F. Supercritical continuous-microflow synthesis of narrow size distribution quantum dots. Adv. Mater. 2008, 20, 4830–4834. [Google Scholar] [CrossRef]
- Kobayashi, J.; Mori, Y.; Okamoto, K.; Akiyama, R.; Ueno, M.; Kitamori, T.; Kobayashi, S. A microfluidic device for conducting gas-liquid-solid hydrogenation reactions. Science 2004, 304, 1305–1308. [Google Scholar]
- Duraiswamy, S.; Khan, S.A. Droplet-based microfluidic synthesis of anisotropic metal nanocrystals. Small 2005, 5, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H.R.; Krali, J.G.; Jensen, K.F. Multistep continuous-flow microchemical synthesis involving multiple reactions and separations. Angew. Chem. Int. Ed. 2007, 46, 5704–5708. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, A.; O’Brien, M.; Petersen, T.P.; Baxendale, I.R.; Ley, S.V. The continuous-flow synthesis of carboxylic acids using CO2 in a tube-in-tube gas permeable membrane reactor. Angew. Chem. Ind. Ed. 2011, 50, 1190–1193. [Google Scholar] [CrossRef]
- Okafor, O.; Weilhard, A.; Fernandes, J.A.; Karjalainen, E.; Goodridge, R.; Sans, V. Advanced reactor engineering with 3D printing for the continuous-flow synthesis of silver nanoparticles. React. Chem. Eng. 2017, 2, 129–136. [Google Scholar] [CrossRef]
- Coley, C.W.; Thomas, D.A.; Lummiss, J.A.M.; Jaworski, J.N.; Breen, C.P.; Schultz, V.; Hart, T.; Fishman, J.S.; Rogers, L.; Gao, H.; et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 2019, 365, eaax1566. [Google Scholar] [CrossRef]
- Wynkoop, R.; Wilhelm, R. Kinetics in tubular flow reactor – hydrogenation of ethylene over copper- magnesia catalyst. Chem. Eng. Progr. 1950, 46, 300–310. [Google Scholar]
- Chance, B. The kinetics of the enzyme-substrate compound of peroxidase. J. Biol. Chem. 1943, 151, 553–577. [Google Scholar]
- Vreeman, H.J.; van Rooijen, P.J.; Visser, S. Segmented flow analysis as applied to kinetic studies of peptide bond hydrolysis. Anal. Biochem. 1977, 77, 251–264. [Google Scholar] [CrossRef]
- Young, H.H., Jr.; Hammett, L.P. Kinetic measurements in a stirred flow reactor; the alkaline bromination of acetone. J. Am. Chem. Soc. 1950, 72, 280–283. [Google Scholar] [CrossRef]
- Keles, H.; Susanne, F.; Livingstone, H.; Hunter, S.; Wade, C.; Bourdon, R.; Rutter, A. Development of a Robust and Reusable Microreactor Employing Laser Based Mid-IR Chemical Imaging for the Automated Quantification of Reaction Kinetics. Org. Process. Res. Dev. 2017, 21, 1761–1768. [Google Scholar] [CrossRef]
- Seibt, S.; With, S.; Bernet, A.; Schmidt, H.; Förster, S. Hydrogelation kinetics measured in a microfluidic device with in-situ X-ray and fluorescence detection. Langmuir 2018, 34, 5535–5544. [Google Scholar] [CrossRef] [PubMed]
- Schwolow, S.; Braun, F.; Rädle, M.; Kockman, N.; Röder, T. Fast and efficient acquisition of kinetic data in microreactors using in-line Raman analysis. Org. Proc. Res. Dev. 2015, 19, 1286–1292. [Google Scholar] [CrossRef]
- Butkolvsjaya, N.I.; Setser, D.W. Infrared chemiluminescence study of the reaction of hydroxyl radicals with formamide and the secondary unimolar reaction of chemically activated carbamic acid. J. Phys. Chem. A 2018, 122, 3735–3746. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, H.; Yan, S.; Song, W. Kinetics studies and mechanistic considerations on the reactions of superoxide radical ions with dissolved organic matter. Water Res. 2019, 149, 56–64. [Google Scholar] [CrossRef]
- Chance, B. Rapid and sensitive spectrophotometry. I. The accelerated and stopped-flow methods for the measurement of the reaction kinetics and spectra of unstable compounds in the visible region of the spectrum. Rev. Sci. Instr. 1951, 22, 619–627. [Google Scholar] [CrossRef]
- Mukai, K.; Nagai, K.; Ouchi, A.; Izumisawa, K.; Naganka, S. Finding of remarkable synergistic effect on the aroxyl radical-scavenging rate under the coexistence of α-tocopherol and catechnis. Int. J. Chem. Kinet. 2019, 51, 643–656. [Google Scholar] [CrossRef]
- Delgadillo, R.F.; Mueser, T.C.; Zaleta-Rivera, K.; Carnes, K.A.; Gonzalez-Valdez, J.; Parkhurst, L.J. Detailed characterization of the solution kinetics and thermodynamics of biotin, biocytin and HABA binding to avidin and streptavidin. PLOS ONE 2019, 0204194. [Google Scholar] [CrossRef] [PubMed]
- Walklate, J.; Geeves, M.A. Temperature manifold for a stopped-flow machine to allow measurements from −10 to + 40 °C. Anal. Biochem. 2015, 476, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bajuszova, Z.; Naif, H.; Ali, Z.; McGinnis, J.; Islam, M. Cavity enhanced liquid-phase stopped-flow kinetics. Analyst 2018, 143, 493–502. [Google Scholar] [CrossRef]
- Bayley, P.; Anson, M. Stopped-flow circular dichroism: A rapid kinetic study of the binding of a sulphonamide drug to bovine carbonic anhydrase. Biochem. Biophys. Res. Commun. 1975, 62, 717–722. [Google Scholar] [CrossRef]
- Makabe, K.; Nakamura, T.; Dhar, D.; Ikura, T.; Koine, S.; Kuwajima, K. An overlapping region between the two terminal holding units of the outer surface protein A (Ospa) controls its holding behavior. J. Mol. Biol. 2018, 430, 1799–1813. [Google Scholar] [CrossRef]
- Reback, M.L.; Roske, C.W.; Bitterwolf, T.E.; Griffiths, P.R.; Manning, C.J. Stopped-flow ultra-rapid-scanning Fourier transform infrared spectroscopy on the millisecond time scale. Appl. Spectrosc. 2010, 64, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Christianson, M.D.; Tan, E.H.P.; Landis, C.R. Stopped-flow NMR: Determining the kinetics of [rac-(C2H4(1-indenyl)2) ZrMe][MeB(C6F5)3]-catalyzed polymerization of 1-hexene by direct observation. J. Am. Chem. Soc. 2010, 132, 11461–11463. [Google Scholar] [CrossRef]
- Sirs, J.A. Electrometric stopped flow measurements of rapid reactions in solution. Part 1 – Conductivity measurements. Trans. Faraday Soc. 1958, 54, 201–206. [Google Scholar] [CrossRef]
- Chauhan, S.; Hosseinzadeh, P.; Lu, Y.; Blackburn, N.J. Stopped-flow studies of the reduce-tion of the copper centers suggest a bifuricated electron transfer pathway in peptidyl-glycine monooxygenase. Biochemistry 2016, 55, 2008–2021. [Google Scholar] [CrossRef]
- Peacock, D.G.; Richardson, J.F. Chemical Engineering, Vol.3: Chemical and Biochemical Reactors and Process Control; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ravi, R.; Vinu, R.; Gummadi, S.N. Coulson and Richardson’s Chemical Engineering, Vol.3A: Chemical and Biochemical Reactors and Reaction Engineering; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Reynolds, J.H. An immobilized α-galactose continuous flow reactor. Biotechnol. Bioeng. 1974, 16, 135–147. [Google Scholar] [CrossRef]
- Basso, A.; Moreira, R.; Jose, H.J. Effect of operational conditions on photocatalytic ethylene degradation applied to control tomato ripening. J. Photochem. Phtobiol. A: Chemistry 2018, 367, 294–301. [Google Scholar] [CrossRef]
- Maia, M.; Soares, T.R.; Mota, A.I.; Rosende, M.; Magalhaes, L.; Miró, M.; Segundo, M.A. Dynamic flow-through approach to evaluate readily bioaccessible antioxidants in solid food samples. Talanta 2017, 166, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Dickinson, J.E.; Galvin, K.P. The kinetics of fast flotation using the reflux flotation cell. Chem. Eng. Sci. 2010, 196, 463–477. [Google Scholar] [CrossRef]
- Maliutina, K.; Tahmasebi, A.; Yu, J.; Saltykov, S.N. Comparative study on flash purolysis characteristics of microalgal and lignocellulosic biomass in entrained-flow reactor. Energy Conv. Manag. 2017, 151, 426–438. [Google Scholar] [CrossRef]
- Elliott, D.C.; Schmidt, A.J.; Hart, T.R.; Billing, J.M. Conversion of a wet waste feedstock to biocrude by hydrothermal processing in a continuous-flow reactor: Grape pomace. Biomass Conv. Bioref. 2017, 7, 455–465. [Google Scholar] [CrossRef]
- Cheng, F.; Jarvis, J.M.; Yu, J.; Jena, U.; Nirmalakhandan, N.; Schaub, T.M.; Brewer, C.E. Bio-crude oil from hydro-thermal liquefaction of wastewater microalgae in a pilot-scale continuous flow reactor. Bioresource Technol. 2019, 294, 122184. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, Y.; Chirinos, J.; Bengoa, C.; Stuber, F.; Font, J.; Fortuny, A.; Fabregat, A. Nitrate removal in an innovative up-flow stirred packed-bed bioreactor. Chem. Eng. Proc. Process. Intens. 2017, 121, 57–64. [Google Scholar] [CrossRef]
- Lin, Y.-H. Adsorption and biodegradation of 2-chlorophenol by mixed culture using activated carbon as a supporting medium-reactor performance and model verification. Appl. Water Sci. 2017, 7, 3741–3757. [Google Scholar] [CrossRef]
- Meng, F.; Huang, W.; Liu, D.; Zhao, Y.; Huang, W.; Lei, Z.; Zhang, Z. Application of aerobic granules-continuous flow reactor for saline wastewater treatment: Granular stability, lipid production and symbiotic relationship between bacteria and algae. Bioresource Technol. 2020, 295, 122291. [Google Scholar] [CrossRef]
- Cambie, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noël, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Cosma, P.; Rizzi, V.; Race, M. Evaluation of the hydraulic and hydrodynamic parameters influencing photo-catalytic degradation of bio-persistent pollutants in a pilot plant. Chem. Eng. Commun. 2019, 206, 1286–1296. [Google Scholar] [CrossRef]
- Yaday, A.; Verma, N. Carbon bead-supported copper-dispersed carbon nanofibers: An efficient catalyst for wet air oxidation of industrial wastewater in a recycle flow reactor. J. Ind. Eng. Chem. 2018, 67, 448–460. [Google Scholar]
- Hu, Y.; Boyer, T.H. Removal of multiple drinking water contaminants by combined ion exchange resin in a completely mixed flow reactor. J. Wat. Supp: Res. Technol. 2018, 67, 659–672. [Google Scholar] [CrossRef]
- Naddeo, V.; Ricco, D.; Scannapieco, D.; Belgiorono, V. Degradation of antibiotics in wastewater during sonolysis, ozonation, and their simultaneous application: Operating condition effects and process evaluation. Int. J. Photoenergy 2012, 624270. [Google Scholar] [CrossRef]
- Xu, L.; Ma, X.; Niu, J.; Chen, J.; Zhou, C. Removal of trace naproxen from aqueous solution using a laboratory-scale reactive flow-through membrane electrode. J. Hazard. Mater. 2019, 379, 120692. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.N.; Ferreira, M.B.; de Moura Santos, E.C.M.; Leon, J.J.L.; Ganiyu, S.O.; Martinez-Huitle, C.A. Electro- chemical degradation of Azo-dye Acid Violet 7 using BDD anode: Effect of flow reactor configuration on cell hydrodynamics and dye removal efficiency. J. Appl. Electrochem. 2018, 48, 1321–1330. [Google Scholar] [CrossRef]
- Urtiaga, A.; Fernandez-Gonzalez, C.; Gomez-Lavin, S.; Ortiz, I. Kinetics of the electrochemical minera- lization of perfluorooctanoic acid on ultrananocrystalline boron doped conductive diamond electrodes. Chemosphere 2015, 129, 20–26. [Google Scholar] [CrossRef]
- Pikaev, A.K.; Podzorova, E.A.; Bakhtin, O.M. Combined electron beam and ozone treatment of wastewater in the aerosol flow. Radiat. Phys. Chem. 1997, 49, 155–157. [Google Scholar] [CrossRef]
- Trojanowicz, M. Flow chemistry vs. flow analysis. Talanta 2016, 146, 621–640. [Google Scholar] [CrossRef]
- Ruzicka, J. Flow chemistry and flow analysis. J. Flow Chem. 2015, 5, 55. [Google Scholar] [CrossRef]
- Feng, S.; Yuan, D.; Huang, Y.; Lin, K.; Zhu, Y.; Ma, J. A catalytic spectrophotometric method for determination of nanomolar manganese in seawater using reverse flow injection analysis and a long path length liquid waveguide capillary cell. Talanta 2018, 178, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, M.; Luque de Castro, M.D. Integration of reaction (retention) and spectroscopic detection in continuous-flow systems. Analyst 1990, 115, 699–703. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Worsfold, P.J.; Clinch, J.R. Solid-state photometric detectors for flow injection analysis. Trends Anal.Chem. 1988, 7, 301–305. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Szpunar-Łobińska, J. Simultaneous flow-injection determination of aluminium and zinc using LED photometric detection. Anal. Chim. Acta 1990, 230, 125–130. [Google Scholar] [CrossRef]
- Fiedoruk, M.; Mieczkowska, E.; Koncki, R.; Tymecki, Ł. A bimodal optoelectronic flow-through detector for phosphate determination. Talanta 2014, 128, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Strzelak, K.; Koncki, R. Nephelometry and turbidimetry with paired emitter detector diodes and their application for determination of total urinary protein. Anal. Chim. Acta 2013, 788, 68–73. [Google Scholar] [CrossRef]
- Trojanowicz, M. Recent Developments in Electrochemical Flow Detections – A Review. Part I. Flow Analysis and Capillary Electrophoresis. Anal. Chim. Acta 2009, 653, 36–58. [Google Scholar] [CrossRef]
- Bavol, D.; Economou, A.; Zima, J.; Barek, J.; Dejmkova, H. Simultaneous determination of tert-butyl- hydroquinone, propyl gallate, and butylated hydroxyanisole by flow-injection analysis with multiple- pulse amperometric detection. Talanta 2018, 178, 231–236. [Google Scholar] [CrossRef]
- Wilson, D.; del Valle, M.; Alegret, S.; Valderrama, C.; Florido, A. Potentiometric electronic tongue-flow injection analysis system for the monitoring of heavy metal biosorption processes. Talanta 2012, 91, 285–292. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Reder, S.; Lopez de Alda, M.; Gauglitz, G.; Barcelo, D. Simultaneous multi-analyte deter- mination of estrone, isoproturon and atrazine in natural waters by River ANAlyser (RIANA), an optical immunosensor. Biosens. Bioelectron. 2004, 19, 633–640. [Google Scholar] [CrossRef]
- Cantillo, D.; de Frutos, O.; Rincon, J.A.; Mateos, C.; Kappe, C.O. A scalable procedure for light-benzylic brominations in continuous flow. J. Org. Chem. 2014, 79, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Öhrngren, P.; Fardost, A.; Russo, F.; Schanmche, J.-S.; Fagrell, M.; Larhed, M. Evaluation of a nonresonant microwave applicator for continuous-flow chemistry applications. Org. Process. Res. Dev. 2012, 16, 1053–1063. [Google Scholar] [CrossRef]
- Jensen, K.F.; Reizman, B.J.; Newmann, S.G. Tools for chemical synthesis in micro-systems. Lab. Chip 2014, 14, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.C.; Wirth, T. Controlling hazardous chemicals in microreactors: Synthesis with iodine azide. Beilstein J. Org. Chem. 2009, 5, 30. [Google Scholar] [CrossRef]
- Szymborski, T.; Jankowski, P.; Garstecki, P. Teflon microreactors for organic syntheses. Sens. Actuat. B Chemical 2018, 255, 2274–2281. [Google Scholar] [CrossRef]
- Hornung, C.H.; Hallmark, B.; Baumann, M.; Baxendale, I.R.; Ley, S.V.; Hester, P.; Clayton, P.; Mackley, M.R. Multiple microcapillary reactor for organic synthesis. Ind. Eng. Chem. Res. 2010, 49, 4576–4582. [Google Scholar] [CrossRef]
- Sachse, A.; Galarneau, A.; Coq, B.; Fajula, F. Monolithic flow microreactors improve fine chemical synthesis. New J. Chem. 2011, 35, 259–264. [Google Scholar] [CrossRef]
- Gunther, F.A.; Ott, D.E. Rapid automated determination of biphenyl in citrus fruit rind. Analyst 1966, 91, 475–481. [Google Scholar] [CrossRef]
- Sonne, K.; Dasgupta, P.K. Simultaneous photometric flow injection determination of sulfide, polysulfide, sulfite, thiosulfate, and sulfate. Anal. Chem. 1991, 63, 427–432. [Google Scholar] [CrossRef]
- Kika, F.S. Low pressure separations using automated flow and sequential injection analysis coupled to monolithic columns. J. Chromatogr. Sci. 2009, 47, 648–655. [Google Scholar] [CrossRef][Green Version]
- Soldi, L.; Ferstl, W.; Loebbecke, S.; Maggi, R.; Malmassari, C.; Sartori, G.; Yada, S. Use of immobilized organic base catalysts for continuous-flow fine chemical synthesis. J. Catal. 2008, 258, 289–295. [Google Scholar] [CrossRef]
- Tian, K.; Dasgupta, P.K. Simultaneous flow-injection measurement of hydroxide, chloride, hypochlorite and chlorate in chlor-alkali cell effluents. Talanta 2000, 52, 623–630. [Google Scholar] [CrossRef]
- Cerda, A.; Oms, M.T.; Forteza, R.; Cerda, V. Speciation of nitrogen in wastewater by flow injection. Analyst 1996, 121, 13–17. [Google Scholar] [CrossRef]
- Lopez Pasquali, C.E.; Fernandez Hernando, P.; Durand Alegria, J.S. Spectrophotometric simultaneous determination of nitrite, nitrate and ammonium in soils by flow injection analysis. Anal. Chim. Acta 2007, 600, 177–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Miro, M.; Kolev, S.D. A novel hybrid flow platform for on-line simultaneous dynamic fractionation and evaluation of mercury liability in environmental solids. Talanta 2018, 178, 622–628. [Google Scholar] [CrossRef]
- Wieczorek, M.; Kościelniak, P.; Świt, P.; Paluch, J.; Kozak, J. Solenoid micropump-based flow system for generalized calibration strategy. Talanta 2015, 133, 21–26. [Google Scholar] [CrossRef]
- Urbanyi, T.; O’Connell, A. Simultaneous automated determination of hydralazine hydro-chloride, hydrochloro- thiazide, and reserpine in single tablet formulations. Anal. Chem. 1972, 44, 565–570. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Valcarcel, M. Flow injection methods based on multidetection. Trends Anal. Chem. 1986, 5, 71–74. [Google Scholar] [CrossRef]
- Saurina, J. Flow-injection analysis for multi-component determinations of drugs based on chemometric approaches. Trends Anal. Chem. 2010, 29, 1027–1037. [Google Scholar] [CrossRef]
- Miro, M.; Cerda, V.; Estela, J.M. Multisyringe flow injection analysis. Trends Anal. Chem. 2002, 21, 199–210. [Google Scholar] [CrossRef]
- Sharma, Y.; Nikam, A.V.; Kulkarni, A.K. Telescoped sequence of exothermic and endothermic reactions in multistep flow synthesis. Org. Proc. Res. Dev. 2019, 23, 170–176. [Google Scholar] [CrossRef]
- Usutani, H.; Tomida, Y.; Nagaki, A.; Okamoto, H.; Nokami, T.; Yoshida, J. Generation and reactions of o-bromo- phenyllithium without benzyne formation using a microreactor. J. Am. Chem. Soc. 2007, 129, 3046–3047. [Google Scholar] [CrossRef] [PubMed]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.; Jamison, T.F. Continuous flow multi-step organic synthesis. Chem. Sci. 2010, 1, 675–680. [Google Scholar] [CrossRef]
- Wegner, J.J.; Ceylan, S.; Kirschning, A. Flow chemistry – a key enabling technology for (multistep) organic synthesis. Adv. Synth. Catal. 2012, 354, 17–57. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Ley, S.V.; Smith, C.D.; Tranmer, G.K. A flow reactor process for the synthesis of peptides utilizing immobilized reagents, scavengers and catch and release protocols. Chem. Commun. 2006, 4835–4837. [Google Scholar] [CrossRef]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.-C.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef]
- Abolhasani, M.; Jensen, K.F. Oscillatory multiphase flow strategy for chemistry and biology. Lab. Chip 2016, 16, 2775–2784. [Google Scholar] [CrossRef]
- Barlow, I.M.; Harrison, S.P.; Hogg, G.L. Evaluation of the Technicon CHEM-1. Clin. Chem. 1988, 34, 2340–2344. [Google Scholar] [CrossRef]
- Alexander, P.W.; Thalib, A. Nonsegmented rapid-flow analysis with ultraviolet/visible spectrophotometric determi- nation for short sampling times. Anal. Chem. 1983, 55, 497–501. [Google Scholar] [CrossRef]
- Pasquini, C.; de Oliveira, W.A. Monosegmented system for continuous flow analysis. Spectrophotometric determination of chromium(VI), ammonia, and phosphorus. Anal. Chem. 1985, 57, 2575–2579. [Google Scholar] [CrossRef]
- Tian, L.; Sun, X.; Xu, Y.; Zhi, Z. Segmental flow-injection analysis: Device and applications. Anal. Chim. Acta 1990, 238, 183–190. [Google Scholar]
- Boedicker, J.Q.; Li, L.; Kline, T.R.; Ismagilov, R.F. Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug-based microfluidics. Lab. Chip 2008, 8, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Onal, Y.; Lucas, M.; Claus, P. Application of a capillary microreactor for selective hydrogenation of α, β-unsaturated aldehydes in aqueous multiphase catalysis. Chem. Eng. Technol. 2005, 28, 972–978. [Google Scholar] [CrossRef]
- Wheeler, R.C.; Benali, O.; Deal, M.; Farrant, E.; MacDonald, S.J.F.; Warrington, B.H. Mesoscale flow chemistry: A plug-flow approach to reaction optimization. Org. Proc. Res. Dev. 2007, 11, 704–710. [Google Scholar] [CrossRef]
- Kreutz, J.E.; Shukhaev, A.; Du, W.; Druskin, S.; Daugulis, O.; Ismagilov, R.F. Evolution of catalysts directed by genetic algorithms in a plug-based microfluidic device tested with oxidation of methane by oxygen. J. Am. Chem. Soc. 2010, 132, 3128–3132. [Google Scholar] [CrossRef]
- Hochlowski, J.E.; Searle, P.A.; Tu, N.P.; Pan, J.Y.; Spanton, S.G.; Djuric, S.W. An integrated synthesis-purification system to accelerate the generation of compounds in pharmaceutical discovery. J. Flow Chem. 2011, 2, 56–61. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Liu, S. Electroosmosis: A reliable fluid propulsion system for flow injection analysis. Anal. Chem. 1994, 66, 1792–1798. [Google Scholar] [CrossRef]
- Haswell, S.J. Development and operating characteristics of micro flow injection analysis systems based on electro- osmotic flow. Analyst 1997, 122, 1R–10R. [Google Scholar] [CrossRef]
- Fletcher, P.D.; Haswell, S.J.; Pombo-Villar, E.; Warrington, B.H.; Watts, P.; Wong, S.Y.; Zhang, X. Micro reactors: principles and applications in organic synthesis. Tetrahedron 2002, 58, 4735–4757. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P.; Haswell, S.J. The use of electroosmotic flow as a pumping mechanism for semi-preparative scale continuous flow synthesis. Chem. Commun. 2007, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, J.A.; Dasgupta, P.K. Applications of in situ detection with an automated micro batch analyzer. Anal. Chim. Acta 1988, 214, 107–120. [Google Scholar] [CrossRef]
- Honorato, R.S.; Araujo, M.C.U.; Lima, R.A.C.; Zagatto, E.A.G.; Lapa, R.A.S.; Costa Lima, J.L.F. A flow-batch titrator exploiting a one-dimensional optimization algorithm for end point search. Anal. Chem. Acta 1999, 396, 91–97. [Google Scholar] [CrossRef]
- Honorato, R.C.; Carneiro, J.M.T.; Zagatto, E.A.G. Spectrophotometric flow-batch determination of aluminum in plant tissues exploiting a feedback mechanism. Anal. Chim. Acta 2001, 441, 309–315. [Google Scholar] [CrossRef]
- Almeida, L.F.; Vale, M.G.; Dessuy, M.B.; Da Silva, M.M.; Lima, R.S.; Dos Santos, V.B.; Diniz, P.H.G.D.; Araujo, M. A flow-batch analyzer with piston propulsion applied to automatic preparation of calibration solutions for Mn determination in mineral waters by ET AAS. Talanta 2007, 73, 906–912. [Google Scholar] [CrossRef]
- Barreto, I.S.; Lima, M.B.; Andrade, S.I.E.; Araujo, M.C.U.; Almeida, L.F. Using a flow-batch analyzer for photometric determination of Fe(III) in edible and lubricating oils without external pretreatment. Anal. Meth. 2013, 5, 1040–1045. [Google Scholar] [CrossRef]
- Fernandez, C.J.; Domini, C.E.; Grűnhut, M.; Lista, A.G. A soft material for chromium speciation in water samples using a chemiluminescence automatic system. Chemosphere 2018, 196, 361–367. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Dombrowski, A.W. Emerging trends in flow chemistry and applications to the pharma- ceutical industry. J. Med. Chem. 2019, 62, 6422–6468. [Google Scholar] [CrossRef]
- Hafner, A.; Filipponi, P.; Piccioni, L.; Meisenbach, M.; Schenkel, B.; Venturoni, F.; Sedelmeier, J. A simple scale-up strategy for organolithium chemistry in flow mode: From feasibility to kilogram quantities. Org. Proc. Res. Dev. 2016, 20, 1833–1837. [Google Scholar] [CrossRef]
- Hafner, A.; Mancino, V.; Meisenbach, M.; Schenkel, B.; Sedelmeier, J. Dichloromethyllithum: Synthesis and application in continuous flow mode. Org. Lett. 2017, 19, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, D.; Kappe, C.O. Lab-scale production of anhydrous diazomethane using membrane separation technology. Nat. Protoc. 2017, 12, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.E.; Ley, S.V. Engineering chemistry; integrating batch and flow reactions on a single, automated reactor platform. React. Chem. Eng. 2016, 1, 629–635. [Google Scholar] [CrossRef]
- Burguera, J.L.; Burguera, M. Determination of sulphur anions by flow injection with a molecular emission cavity detector. Anal. Chim. Acta 1984, 157, 177–181. [Google Scholar] [CrossRef]
- Kagenov, H.; Jensen, A. Kinetic determination of magnesium and calcium by stopped-flow injection analysis. Anal. Chim. Acta 1983, 145, 125–133. [Google Scholar] [CrossRef]
- Betteridge, D.; Fields, B. Two point kinetic simultaneous determination of cobalt(II) and nickel(II) in aqueous solution using flow injection analysis (FIA). Fresenius Z. Anal. Chem. 1983, 314, 386–390. [Google Scholar] [CrossRef]
- Műller, H.V.; Műller, V.; Hansen, E.H. Simultaneous differential rate determination of iron(II) and iron(III) by flow- injection analysis. Anal. Chim. Acta 1990, 230, 113–123. [Google Scholar] [CrossRef]
- Hu, X.; Takenaka, N.; Kitano, M.; Bandow, H.; Maeda, Y. Determination of trace amounts of urea by using flow injection with chemiluminescence detection. Analyst. 1994, 119, 1829–1833. [Google Scholar] [CrossRef]
- Burguera, J.L.; Townshend, A.; Greenfield, S. Flow injection analysis for monitoring chemiluminescent reactions. Anal. Chim. Acta 1980, 114, 209–214. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Wang, J. Flow-injection chemiluminescence determination of dihydralazine sulfate in serum using luminol and diperiodatocuprate (III) system. Spectrochim. Acta A Mol. Biomol. Spectr. 2010, 75, 77–82. [Google Scholar] [CrossRef]
- Pulgarin, J.A.M.; Bermejo, L.F.G.; Gallego, J.M.L.; Garcia, M.N.S. Simultaneous stopped-flow determination of morphine and naloxone by time-resolved chemiluminescence. Talanta 2008, 74, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dai, C.; Jamison, T.F.; Jensen, K.F. A rapid total synthesis of ciprofloxacin hydrochloride in continuous flow. Angew. Chem. Int. Ed. 2017, 56, 8870–8873. [Google Scholar] [CrossRef] [PubMed]
- Pieber, B.; Glasnov, T.; Kappe, C.O. Flash carboxylation: Fast lithiation-carboxylation sequence at room temperature in continuous flow. RSC Adv. 2014, 4, 13430–13433. [Google Scholar] [CrossRef]
- Nieuwland, P.J.; Koch, K.; van Harskamp, N.; Wehrens, R.; van Hest, J.C.M.; Rutjes, F.J.T. Flash chemistry extensively optimized: High-temperature Swern-Moffatt oxidation in an automated microreactor platform. Chem. Asian J. 2010, 5, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J. Flash chemistry using electrochemical method and microsystems. Chem. Commun. 2005, 4509–4516. [Google Scholar] [CrossRef]
- Yoshida, J.; Takahashi, Y.; Nagaki, A. Flash chemistry: Flow chemistry that cannot be done in batch. Chem. Commun. 2013, 49, 9896–9904. [Google Scholar] [CrossRef]
- Browne, D.L.; Baumann, M.; Harji, B.H.; Baxendale, I.R.; Ley, S.V. A new enabling technology for convenient laboratory scale continuous flow processing at low temperatures. Org. Lett. 2011, 13, 3312–3315. [Google Scholar] [CrossRef]
- Basel, C.L.; Defreese, J.D.; Whittemore, D.O. Interferences in automated Phenol Red method for determination of bromide in water. Anal. Chem. 1982, 54, 2090–2094. [Google Scholar] [CrossRef]
- Bergamin, F.H.; Reis, B.F.; Jacintho, A.O.; Zagatto, E.A.G. Ion exchange in flow injection analysis: Determination of ammonium ions at the μg L−1 level in natural waters with pulsed Nessler reagent. Anal. Chim. Acta 1980, 117, 81–89. [Google Scholar] [CrossRef]
- Olsen, S.; Pessenda, L.C.R.; Ruzicka, J.; Hansen, E.H. Combination of flow injection analysis with flame atomic-absorption spectrophotometry: Determination of trace amounts of heavy metals in polluted seawater. Analyst 1983, 108, 905–917. [Google Scholar] [CrossRef]
- Faizullah, A.T.; Townshend, A. Applications of ion-exchange minicolumns in a flow-injection system for the spectrophotometric determination of anions. Anal. Chem. Acta 1986, 179, 233–244. [Google Scholar] [CrossRef]
- Miro, M.; Gomez, E.; Estela, J.M.; Casa, M.; Cerda, V. Sequential injection 90Sr determination in environmental samples using a wetting-film extraction method. Anal. Chem. 2002, 74, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Chirillo, R.; Caenaro, G.; Pavan, B.; Pin, A. The use of immobilized enzyme reactors in continuous-flow analyzers for the determination of glucose, urea, and uric acid. Clin. Chem. 1979, 25, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Yamini, Y.; Safari, M. Modified magnetic nanoparticles with catechol as a selective sorbent for magnetic solid phase extraction of ultra-trace amounts of heavy metals in water and fruit samples followed by flow injection ICP-OES. Talanta 2018, 143, 503–511. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Koźmiński, P.; Dias, H.; Brett, C.M.A. Batch injection stripping voltammetry (tube-less flow-injection analysis) of trace metals with on-line sample pretreatment. Talanta 2005, 68, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.B.; Miro, M.; Estela, J.M.; Cerda, V. Automated on-line renewable solid-phase extraction-liquid chromatography exploiting multisyringe flow injection-bead injection lab-on-valve analysis. Anal. Chem. 2006, 78, 2832–2840. [Google Scholar] [CrossRef]
- Kołacińska, K.; Chajduk, E.; Dudek, J.; Samczyński, Z.; Łokas, E.; Bojanowska-Czajka, A.; Trojanowicz, M. Automation of sample processing for ICP-MS determination of 90Sr radionuclide at ppq level for nuclear technology and environmental purposes. Talanta 2017, 169, 216–226. [Google Scholar] [CrossRef]
- Fang, Z.-L. Flow-injection Separation and Preconcentration; VCH Verlag: Weinheim, Germany, 1993. [Google Scholar]
- Liu, Y.; Ingle, J.D., Jr. Automated two-column ion exchange system for determination of the speciation of trace metals in natural waters. Anal. Chem. 1989, 51, 525–529. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Hayward, J.J.; Lanners, S.; Ley, S.V.; Smith, C.D. Heterogeneous reaction. In Microreactors in Organic Synthesis and Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Ley, S.V.; Baxendale, I.R.; Bream, R.N.; Jackson, P.S.; Leach, A.G.; Longbottom, D.A.; Nesi, M.; Scott, J.S.; Storer, R.I.; Taylor, S.J. Multi-step organic synthesis using solid-supported reagents and scavengers: A new paradigm in chemical library generation. J. Chem. Soc., Perkin Trans. 2000, 1, 3815–4195. [Google Scholar] [CrossRef]
- Ley, S.V.; Baxendale, I.R. New tools and concepts for modern organic synthesis. Nature Rev. 2002, 1, 573–586. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Schou, S.C.; Sedelmaier, J.; Ley, S.V. Multi-step synthesis by using modular flow reactors: The preparation of yne-ones and their use in heterocyclic synthesis. Chem. Eur. J. 2010, 16, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Guetzoyan, L.; Nikbin, N.; Baxendale, I.R.; Ley, S.V. Flow chemistry synthesis of zolpidem, alpidem and other ABAA agonists and their biological evaluation and through the use of in-line frontal affinity chromatography. Chem. Sci. 2013, 4, 764–769. [Google Scholar] [CrossRef]
- Baadenhuijsen, H.; Seuren-Jacobs, H.E.H. Determination of total CO2 in plasma by automated flow- injection analysis. Clin. Chem. 1979, 25, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Svenson, G.; Anfält, T. Rapid determination of ammonia in whole blood and plasma using flow injection analysis. Clin. Chim. Acta 1982, 119, 7–14. [Google Scholar] [CrossRef]
- Aoki, T.; Munemori, M. Continuous flow determination of free chlorine in water. Anal. Chem. 1989, 55, 209–212. [Google Scholar] [CrossRef]
- Rodrigues, S.S.M.; Oleksiak, Z.; Ribeiro, D.S.M.; Poboży, E.; Trojanowicz, M.; Prior, J.A.; Santos, J.L. Selective determination of sulphide based on photoluminescence quenching of MPA-capped CdTe nanocrystals by exploiting a gas-diffusion multi-pumping flow manifold. Anal. Meth. 2014, 6, 7956–7966. [Google Scholar] [CrossRef]
- Gross, U.; Koos, P.; O’Brien, M.; Polyzos, A.; Ley, S.V. A general continuous flow method for palladium catalysed carbonylation reactions using single and multiple tube-in-tube gas-liquid microreactors. Eur. J. Org. Chem. 2014, 6418–6430. [Google Scholar] [CrossRef]
- Mallia, C.J.; Baxendale, I.R. The use of gases in flow synthesis. Org. Proc. Res. Dev. 2016, 20, 327–360. [Google Scholar] [CrossRef]
- Sawyer, R.; Dixon, E.J. The automatic determination of original gravity of beer. Part II. The determination of alcohol and gravity lost. Analyst 1968, 93, 680–687. [Google Scholar] [CrossRef]
- Maquieira, A.; Casamayor, F.; Puchades, R.; Sagrado, S. Determination of total and free sulphur dioxide in wine with a continuous-flow microdistillation system. Anal. Chim. Acta 1993, 283, 401–407. [Google Scholar] [CrossRef]
- Baumann, M. Integrating reactive distillation with continuous flow processing. React. Chem. Eng. 2019, 4, 368–371. [Google Scholar] [CrossRef]
- Bittorf, L.; Reichmann, F.; Schmalenberg, M.; Soboll, S.; Kockmann, N. Equipment and separation units for flow chemistry applications and process development. Chem. Eng. Technol. 2019, 42, 1985–1995. [Google Scholar] [CrossRef]
- Kabeshov, M.A.; Musio, B.; Murray, P.R.D.; Browne, D.L.; Ley, S.V. Expedient preparation of nazlinine and a small library of indole alkaloids using flow electrochemistry as an enabling technology. Org. Lett. 2014, 16, 4618–4621. [Google Scholar] [CrossRef] [PubMed]
- Hopkin, M.D.; Baxendale, I.R.; Ley, S.V. An expeditious synthesis of imatinib and analogues utilising flow chemi- stry methods. Org. Biomol. Chem. 2013, 11, 1822–1839. [Google Scholar] [CrossRef]
- Cole, K.P.; Groh, J.M.; Johnson, M.D.; Burcham, C.L.; Campbell, B.M. Kilogram-scale prexasertib monolactate monohydrate synthesis under continuous-flow CGMP conditions. Science 2017, 356, 1144–1150. [Google Scholar] [CrossRef]
- Dolan, J.W.; Gant, J.R.; Tanaka, N.; Giese, R.W.; Karger, B.L. Continuous-flow automated HPLC analysis of fat- soluble vitamins in tablets. J. Chromatogr. Sci. 1978, 16, 616–622. [Google Scholar] [CrossRef]
- Snyder, L.R. Continuous-flow analysis: Present and future. Anal. Chim. Acta 1980, 114, 3–18. [Google Scholar] [CrossRef]
- Deadman, B.J.; Battilocchio, C.; Slivinski, E.; Ley, S.V. A prototype device for evaporation in batch and flow chemical processes. Green Chem. 2013, 15, 2050–2055. [Google Scholar] [CrossRef]
- Luque-Garcia, J.L.; Luque de Castro, M.D. Where is microwave-based analytical equipment for solid sample pretreatment. Trends Anal. Chem. 2003, 22, 90–98. [Google Scholar] [CrossRef]
- Burguera, M.; Burguera, J.L.; Alarcon, O.M. Flow-injection and microwave-oven sample decomposition for determination of copper, zinc and iron in whole-blood by atomic-absorption spectrometry. Anal. Chim. Acta 1986, 179, 351–357. [Google Scholar] [CrossRef]
- Balconi, M.L.; Borgarello, M.; Ferraroli, R.; Realini, F. Chemical Oxygen Demand determination in well and river waters by flow-injection analysis using a microwave oven during the oxidation step. Anal. Chim. Acta 1992, 261, 295–299. [Google Scholar] [CrossRef]
- Han, B.; Jiang, X.; Hou, X.; Zheng, C. Miniaturized dielectric barrier discharge carbon atomic emission spectrometry with online microwave-assisted oxidation for determination of Total Organic Carbon. Anal. Chem. 2014, 86, 6214–6219. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Baxendale, I.R. The changing face of organic synthesis. Chimia 2008, 62, 162–168. [Google Scholar] [CrossRef]
- Wegner, J.; Ceylan, S.; Kirschning, A. Ten key issues in modern flow chemistry. Chem. Commun. 2011, 47, 4583–4592. [Google Scholar] [CrossRef]
- Noël, T.; Buchwald, S.L. Cross-coupling in flow. Chem. Soc. Rev. 2011, 40, 5010–5029. [Google Scholar] [CrossRef]
- Hartwig, J.; Ceylan, S.; Kupracz, L.; Coutable, L.; Kirschning, A. Heating under high-frequency inductive conditions: Application to the continuous synthesis of the Neurolepticum Olanzapine (Zyprexa). Angew. Chem. Int. Ed. 2013, 52, 9813–9817. [Google Scholar] [CrossRef]
- He, P.; Haswell, S.J.; Fletcher, P.D.I. Microwave heating of heterogeneously catalysed Suzuki reactions in a micro reactor. Lab. Chip 2004, 4, 38–41. [Google Scholar] [CrossRef]
- Comer, E.; Organ, M.G. A microreactor for microwave-assisted capillary (continuous flow) organic synthesis. J. Am. Chem. Soc. 2005, 127, 8160–8167. [Google Scholar] [CrossRef]
- Ramarao, C.; Ley, S.V.; Smith, S.C.; Shirley, I.M.; DeAlmeida, N. Encapsulation of palladium in polyurea micro- capsules. Chem. Commun. 2002, 1132–1133. [Google Scholar] [CrossRef]
- Smith, C.J.; Iglesias-Siguenza, F.J.; Baxendale, I.R.; Ley, S.V. Flow and batch mode focused microwave synthesis of 5-amino-4-cyanopyrazoles and their further conversion to 4-aminopyrazolopyrimidines. Org. Biomol. Chem. 2007, 5, 2758–2761. [Google Scholar] [CrossRef]
- Ceylan, S.; Friese, C.; Lammel, C.; Mazac, K.; Kieschning, A. Inductive heating for organic synthesis by using functionalized magnetic nanoparticles inside microreactors. Angew. Chem. Int. Ed. 2008, 120, 8950–8953. [Google Scholar] [CrossRef] [PubMed]
- Wegner, J.; Ceylan, S.; Friese, C.; Kirschning, A. Inductively heated oxides inside micro-reactors - Facile oxidations under flow conditions. Eur. J. Org. Chem. 2010, 4372–4375. [Google Scholar]
- Ceylan, S.; Klande, T.; Vogt, C.; Friese, C.; Kirschning, A. Chemical synthesis with inductively heated copper flow reactors. Synlett. 2010, 2009–2013. [Google Scholar]
- Ilcheva, L.; Cammann, K. A simple, selective and sensitive liquid-chromatographic of flow-injection detector for chloroorganic compounds based on ion-selective electrodes. Fresenius Z. Anal. Chem. 1986, 325, 11–14. [Google Scholar] [CrossRef]
- Edwards, R.T.; McKelvie, I.D.; Ferret, P.C.; Hart, B.T.; Bapat, J.B.; Koshy, K. Sensitive flow-injection technique for the determination of dissolved organic carbon in natural and wastewaters. Anal. Chim. Acta 1992, 261, 287–294. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Llorent-Martinez, E.J.; Ortega-Barrales, P.; Fernandez de Cordoba, M.L.; Ruiz-Medina, A. Photo-chemically induced fluorescence determination of tigecycline by a stopped-flow multicom- mutated flow-analysis assembly. Anal. Lett. 2011, 44, 127–136. [Google Scholar] [CrossRef]
- Cambie, D.; Noël, T. Solar photochemistry in flow. Topics Curr. Chem. 2018, 376, 45. [Google Scholar] [CrossRef]
- Takei, G.; Kitamori, T.; Kim, H. Photocatalytic redox-combined synthesis of L-pipecolininc acid with a titania-modified microchannel chip. Catal. Commun. 2005, 6, 357–360. [Google Scholar] [CrossRef]
- Pinho, V.D.; Gutmann, B.; Kappe, C.O. Continuous flow synthesis of β-amino acids from α-amino acids via Ardndt-Eistert homologation. RSC Adv. 2014, 4, 37419–37472. [Google Scholar] [CrossRef]
- Talla, A.; Driessen, B.; Straathof, N.J.W.; Milroy, L.-G.; Brunsveld, L.; Hessel, V.; Noël, T. Metal-free photocatalytic aerobic oxidation of thiols to disulfides in batch and continuous-flow. Adv. Synth. Catal. 2015, 357, 2180–2186. [Google Scholar] [CrossRef]
- Atobe, M.; Tateno, H.; Matsumara, Y. Applications of flow microreactors in electrosynthetic processes. Chem. Rev. 2018, 118, 4541–4572. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, D.; Green, R.A.; Brown, R.C.D. Flow electrolysis cells for the synthetic organic chemistry laboratory. Chem. Rev. 2018, 118, 4573–4591. [Google Scholar] [CrossRef] [PubMed]
- Noël, T.; Cao, Y.; Laudadio, G. The fundamentals behind the use of flow reactors in electrochemistry. Acc. Chem. Res. 2019, 52, 2858–2869. [Google Scholar] [CrossRef] [PubMed]
- Schothorst, R.C.; Van Son, M.; Den Boef, G. The application of strongly reducing agents in flow injection analysis: Part 4. Uranium(III). Anal. Chim. Acta 1984, 162, 1–8. [Google Scholar] [CrossRef]
- Schothorst, R.S.; Den Boef, G. The application of strongly oxidizing agents in flow injection analysis: Part 1. Silver(II). Anal. Chim. Acta 1985, 169, 99–107. [Google Scholar] [CrossRef]
- Nakata, R. Spectrophotometric determination of nitrite produced by flow-electrolysis of nitrate. Fresenius Z. Anal. Chem. 1984, 317, 115–117. [Google Scholar] [CrossRef]
- Bergamin, F.H.; Krug, F.J.; Zagatto, E.A.G.; Arruda, E.C.; Coutinho, C.A. On-line electrolytic dissolution of alloys in flow-injection analysis: Part 1. Principles and applications in the determination of soluble aluminum in steels. Anal. Chim. Acta 1986, 190, 177–184. [Google Scholar] [CrossRef]
- Mishra, S.K.; Dasgupta, P.K. Electrodialytic reagent introduction in flow systems. Anal. Chem. 2010, 82, 3981–3984. [Google Scholar] [CrossRef]
- Belmont, C.; Girault, H.H. Coplanar interdigitated band electrodes for electrosynthesis - Part II: Methoxy- lation of furan. J. Appl. Electrochem. 1994, 24, 719–724. [Google Scholar] [CrossRef]
- Pintauro, P.N.; Johnson, D.K.; Park, K.; Baizer, M.M.; Nobe, K. The paired electrochemical synthesis of sorbitol and gluconic acid in undivided flow cells. I. J. Appl. Electrochem. 1984, 14, 209–220. [Google Scholar] [CrossRef]
- Ruzicka, J.; Hansen, E.H. Integrated microconduits for flow injection analysis. Anal. Chim. Acta 1984, 161, 1–25. [Google Scholar] [CrossRef]
- Ruzicka, J. Flow injection analysis. From test tube to integrated microconduits. Anal. Chem. 1983, 56, 1040A–1053A. [Google Scholar]
- Jacobson, S.C.; Moore, A.W.; Ramsey, J.M. Fused quartz substrates for microchip electrophoresis. Anal. Chem. 1995, 67, 2059–2063. [Google Scholar] [CrossRef]
- Tantra, R.; Manz, A. Integrated potentiometric detector for use in chip-based flow cells. Anal. Chem. 2000, 72, 2875–2878. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Watts, P.; Chin, F.T.; Hong, J.; Musachio, J.L.; Briard, E.; Pike, V.W. Syntheses of 11C- and 18F-labeled carboxylic esters within a hydrodynamically-driven micro-reactor. Lab. Chip 2004, 4, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pumera, M.; Chatrathi, M.P.; Escarpa, A.; Musameh, M.; Collins, G.; Mulchandani, A.; Lin, Y.; Olsen, K. Single-channel microchip for fast screening and detailed identification of nitroaromatic explosives or organophosphate nerve agents. Anal. Chem. 2002, 74, 1187–1191. [Google Scholar] [CrossRef]
- Tokeshi, M.; Minagawa, T.; Uchiyama, K.; Hibara, A.; Sato, K.; Hisamoto, H.; Kitamori, T. Continuous-flow chemical processing on a microchip by combining microunit operations and a multiphase flow network. Anal. Chem. 2002, 74, 1565–1571. [Google Scholar] [CrossRef]
- Choi, J.-W.; Oh, K.W.; Thomas, J.H.; Heineman, W.R.; Halsall, H.B.; Nevin, J.H.; Helmicki, A.J.; Henderson, H.T.; Ahn, C.H. An integrated microfluidic biochemical detection system for protein analysis with magnetic bead-based sampling capabilities. Lab. Chip 2002, 2, 27–30. [Google Scholar] [CrossRef]
- Hong, C.; Chang, P.; Lin, C.; Hong, C. A disposable microfluidic biochip with on-chip molecularly imprinted biosensors for optical detection of anesthetic propofol. Biosens. Bioelectron. 2010, 25, 2058–2064. [Google Scholar] [CrossRef]
- Kiss, A.; Gaspar, A. Fabrication of a microfluidic flame atomic emission spectrometer: A flame-on-a-chip. Anal. Chem. 2018, 90, 5995–6000. [Google Scholar] [CrossRef]
- Neuzil, P.; Campos, C.D.M.; Wong, C.C.; Soon, J.B.W.; Reboud, J.; Manz, A. From chip-in-a-lab to lab-on-a-chip: Towards a single handheld electronic system for multiple application-specific lab-on-a- chip. Lab. Chip 2014, 14, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Weaver, W.; Kittur, H.; Dhar, M.; Di Carlo, D. Research highlights: Microfluidic point-of-care diagnostics. Lab. Chip 2014, 14, 1962–1965. [Google Scholar] [CrossRef]
- Murphy, E.R.; Martinelli, J.R.; Zaborenko, N.; Buchwald, S.L.; Jensen, K.F. Accelerating reactions with microreactors at elevated temperatures and pressures: Profiling amino-carbonylation reactions. Angew. Chem. Int. Ed. 2007, 46, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hassan, A.; Sandre, O.; Cabuil, O.V. Microfluidics in inorganic chemistry. Angew. Chem. Int. Ed. 2010, 49, 6268–6286. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.; Wiles, C. Micro reactors, flow reactors and continuous flow synthesis. J. Chem. Res. 2012, 181–193. [Google Scholar] [CrossRef]
- Marre, S.; Roig, Y.; Aymonier, C. Supercritical microfluidics: Opportunities in flow-through chemistry and materials science. J. Supercrit. Fluids 2012, 66, 251–264. [Google Scholar] [CrossRef]
- Badilescu, S.; Packirisamy, M. Microfluidics-nano-integration for synthesis and sensing. Polymers 2012, 4, 1278–1310. [Google Scholar] [CrossRef]
- Scheler, O.; Postek, W.; Garstecki, P. Recent developments of microfluidics as a tool for biotechnology and microbiology. Curr. Opinion Biotechnol. 2019, 55, 60–67. [Google Scholar] [CrossRef]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab. Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef]
- Focke, M.; Kosse, D.; Müller, C.; Reinecke, H.; Zengelrle, R.; von Stetten, F. Lab-on-a-Foil: Microfluidics on thin and flexible films. Lab. Chip 2010, 10, 1365–1366. [Google Scholar] [CrossRef]
- Taylor, D.; Dyer, D.; Lew, V.; Khine, M. Shrink film patterning by craft cutter: Complete plastic chips with high resolution/high-aspect ratio channel. Lab. Chip 2010, 10, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Luecha, J.; Hsiao, A.; Brodsky, S.; Liu, G.L.; Kokini, J.L. Green microfluidic devices made of corn proteins. Lab. Chip 2011, 11, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Nat. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Fu, J.; Wu, W. Fabrication of paper-based microfluidic analysis devices: A review. RSC Adv. 2015, 5, 78109–78127. [Google Scholar] [CrossRef]
- Akyazi, T.; Basabe-Desmonds, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialisation. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Jayawardane, B.M.; Kolev, S.D.; McKelvie, I.D. Developments of microfluidic paper- based analytical devices (μPADs) for water analysis: A review. Talanta 2018, 177, 176–190. [Google Scholar] [CrossRef]
- Horning, M.P.; Delahunt, C.B.; Singh, S.R.; Garing, S.H.; Nichols, K.P. A paper microfluidic cartridge for automated staining of malaria parasites with an optically transparent microscopy window. Lab. Chip 2014, 14, 2040–2046. [Google Scholar] [CrossRef]
- Peters, J.J.; Almeida, M.I.G.S.; Sraj, L.O.; McKelvie, I.D.; Kolev, S.D. Development of a micro-distillation microfluidic paper-based analytical device as a screening tool for total ammonia monitoring in freshwaters. Anal. Chim. Acta 2019, 1079, 120–128. [Google Scholar] [CrossRef]
- Deiss, F.; Matochko, W.L.; Govindasamy, N.; Lin, E.Y.; Derda, R. Flow-through synthesis on Teflon- patterned paper to produce peptide arrays for cell-based assays. Angew. Chem. Int. Ed. 2014, 53, 6374–6377. [Google Scholar] [CrossRef]
- Nilghaz, A.; Wicaksono, D.H.B.; Gustiono, D.; Majid, F.A.A.; Supriyanto, E.; Kadir, M.R.A. Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique. Lab. Chip 2012, 12, 209–218. [Google Scholar] [CrossRef]
- Theberge, A.B.; Whyte, G.; Frenzel, M.; Fidalgo, L.M.; Wootton, R.C.R.; Huck, T.S. Suzuki-Miyaura coupling reactions in aqueous microdroplets with catalytically active fluorous interfaces. Chem. Commun. 2009, 6225–6227. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Coley, C.W.; Abolhasani, M.; Marzinzik, A.L.; Koch, G.; Spanka, C.; Lehmann, H.; Jensen, K.F. A segmented flow platform for on-demand medicinal chemistry and compound synthesis in oscillating droplets. Chem. Commun. 2017, 53, 6649–6652. [Google Scholar] [CrossRef]
- Du, W.; Sun, M.; Gu, S.; Zhu, Y.; Fang, Q. Automated microfluidic screening assay platform based on DropLab. Anal. Chem. 2010, 82, 9941–9947. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.M.; Phillips, T.W.; Bannock, J.H.; de Mello, J.C. Controlled multistep synthesis in a three-phase droplet reactor. Nature Commun. 2014, 5, 3777. [Google Scholar] [CrossRef]
- Song, H.; Tice, J.D.; Isamgilov, R.F. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Shestooalov, B.; Tice, J.D.; Ismagilov, R.F. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab. Chip 2004, 4, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Wilding, P.; Shoffner, M.A.; Kricka, L.J. PCR in a silicon microstructure. Clin. Chem. 1994, 40, 1815–1818. [Google Scholar]
- Wang, F.; Burns, M.A. Performance of nanoliter-sized droplet-based microfluidic PCR. Biomed. Microdevices 2009, 11, 1071–1080. [Google Scholar] [CrossRef][Green Version]
- Barikbin, Z.; Rahman, M.T.; Parthiban, P.; Rane, A.S.; Jain, V.; Duraiswamy, S.; Lee, S.S.; Khan, S.A. Ionic liquid-based compound droplet microfluidics for “on-drop” separations and sensing. Lab. Chip 2010, 10, 2458–2463. [Google Scholar] [CrossRef]
- Fair, R.B. Digital microfluidics: Is a true lab-on-a-chip possible? Microfluid. Nanofluid. 2007, 3, 245–281. [Google Scholar] [CrossRef]
- Coudron, L.; McDonnell, M.B.; Munro, I.; McCluskey, D.K.; Johnston, I.; Tan, C.K.; Tracey, M.C. Fully integrated digital microfluidics platform for automated immunoassay; A versatile tool for rapid, specific detection of a wide range of pathogens. Biosens. Bioelectron. 2019, 128, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; van der Ecken, S.; Swyer, I.; Li, C.; Jenne, A.; Vincent, F.; Schmidig, D.; Kuehn, T.; Beck, A.; Busse, F.; et al. Rapid chemical reaction monitoring by digital micro- fluidics-NMR: Proof of principle towards an automated synthetic discovery. Angew. Chem. Int. Ed. 2019, 58, 15372–15376. [Google Scholar] [CrossRef] [PubMed]
- Gach, P.C.; Iwai, K.; Kim, P.W.; Hillson, N.J.; Singh, A.K. Droplet microfluidics for synthetic biology. Lab. Chip 2017, 17, 3388–3400. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.; Burns, M.A. Microfluidic assembly blocs. Lab. Chip 2008, 8, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Capel, A.J.; Edmondson, S.; Christie, S.; Goodridge, R.D.; Bibb, R.J.; Thurstans, M. Design and additive manufacture for flow chemistry. Lab. Chip 2013, 13, 4583–4590. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Kim, Y.C.; Wang, M.; Kim, H.-I.; Byun, Y.; Nam, J.-D.; Chou, T.-W.; Ajayan, P.M.; Ci, L.; Suhr, J. Experimental investigation of mechanical properties of UV-curable 3D printing materials. Polym. 2018, 143, 88–94. [Google Scholar] [CrossRef]
- Dragone, V.; Sans, V.; Rosnes, M.H.; Kitson, P.J.; Cronin, L. 3D-printed devices for continuous-flow organic chemistry. Beilstein J. Org. Chem. 2013, 9, 951–959. [Google Scholar]
- Peng, M.; Mittmann, E.; Wenger, L.; Hubbuch, J.; Engqvist, M.K.M.; Niemeyer, C.M.; Rabe, K.S. 3D-printed phenacrylate decarboxylase flow reactors for the chemoenzymatic synthesis of 4-hydroxystilbene. Chem. - A Eur. J. 2019, 25, 15998–16001. [Google Scholar] [CrossRef]
- Bressan, L.P.; Robles-Najar, J.; Adamo, C.B.; Quero, R.F.; Costa, B.M.C.; de Jesus, D.P.; da Silva, J.A. 3D-printed microfludic device for the synthesis of silver and gold nanoparticles. Microchem. J. 2019, 146, 1083–1089. [Google Scholar] [CrossRef]
- Zhang, J.M.; Aguirre-Pablo, A.A.; Li, E.Q.; Buttner, U.; Thoroddsen, S.T. Droplet generation in cross-flow for cost-effective 3D-printed “plug-and-play” microfluidic devices. RSC Adv. 2016, 6, 81120–81129. [Google Scholar] [CrossRef]
- Spilstead, K.; Learey, J.J.; Doeven, E.H.; Barbante, G.J.; Mohr, S.; Barnett, N.W.; Terry, J.M.; Hall, R.M.; Francis, P.S. 3D-printed and CNC milled flow cells for chemiluminescence detection. Talanta 2014, 126, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Michalec, M.; Tymecki, Ł. 3D printed flow-through cuvette insert for UV-Vis spectrophotometric and fluorescence measurements. Talanta 2016, 190, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Calderilla, C.; Maya, F.; Cerda, V.; Leal, L.O. 3D printed device including disk-based solid-phase extraction for the automated speciation of iron using the multisyringe flow injection analysis technique. Talanta 2017, 175, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Mattio, E.; Robert-Peillard, F.; Vassalo, L.; Branger, C.; Margaillan, A.; Brach-Papa, C.; Knoery, J.; Boudenne, J.-L.; Coulomb, B. 3D-printed lab-on-valve for fluorescent determination of cadmium and lead in water. Talanta 2018, 183, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Lockwood, S.Y.; Spence, D.M. Recent advances in analytical chemistry by 3D printing. Anal. Chem. 2017, 89, 57–70. [Google Scholar] [CrossRef]
- Bishop, G.; Satterwhite, J.E.; Bhakta, S.; Kadimisetty, K.; Gillette, K.M.; Chen, E.; Rusling, J.F. 3D-printed fluidic devices for nanoparticle preparation and flow-injection amperometry using integrated Prussian blue nanoparticle-modified electrodes. Anal. Chem. 2015, 87, 5437–5443. [Google Scholar] [CrossRef]
- Gal-Or, E.; Gershoni, Y.; Scotti, G.; Nilsson, S.M.E.; Saarinen, J.; Jokinen, V.; Strachan, C.J.; Gennäs, G.B.A.; Yli-Kauhaluoma, J.T.; Kotiaho, T. Chemical analysis using 3D printed glass microfluidics. Anal. Meth. 2019, 11, 1802–1810. [Google Scholar] [CrossRef]
- Kingstone, H.M.; Kingstone, M.L. Nomenclature in laboratory robotics and automation. Pure Appl. Chem. 1994, 66, 609–630. [Google Scholar] [CrossRef]
- Empel, C.; Koenigs, R.M. Artificial-intelligence-driven organic synthesis – En route towards autonomous synthesis? Angew. Chem. Int. Ed. 2019, 58, 2–5. [Google Scholar] [CrossRef]
- Schindler, R.; Lendl, B. FTIR spectroscopy as detection principle in aqueous flow analysis. Anal. Commun. 1999, 36, 123–126. [Google Scholar] [CrossRef]
- Gallignani, M.; Ayala, C.; del Rodario Brunetto, M.; Burguera, J.L.; Burguera, M. A simple strategy for determining ethanol in all types of alcoholic beverages based on its on-line liquid-liquid extraction with chloroform, using a flow injection system and Fourier transform infrared spectrometric detection in the mid-IR. Talanta 2005, 68, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Lendl, B.; Frank, J.; Schindler, R.; Muller, A.; Beck, M.; Faist, J. Mid-infrared quantum cascade lasers for flow injection analysis. Anal. Chem. 2000, 72, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Thygese, L.G.; Jorgensen, K.; Møller, B.L.; Engelsen, S.B. Raman spectroscopoic analysis of cynaogenic glucosides in plants: Development of a flow injection surface-enhanced Raman scatter (SERS) method for determination of cyanide. Appl. Spectrosc. 2004, 58, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Louden, D.; Handley, A.; Taylor, S.; Lenz, E.; Miller, S.; Wilson, I.D.; Sage, A. Flow injection spectroscopic analysis of model drugs using on-line UV-diode array, FT-infrared and 1H-nuclear magnetic resonance spectroscopy and time-of-flight mass spectrometry. Anal. 2000, 125, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Godejohann, M.; Martin, F.-P.; Collino, S.; Bürkle, A.; Moreno-Villanueva, M.; Bernhardt, J.; Toussaint, O.; Grubeck-Loebenstein, B.; Gonos, E.S.; et al. High-resolution quantitative metabolome analysis of urine by automated flow injection NMR. Anal. Chem. 2013, 85, 5801–5809. [Google Scholar] [CrossRef] [PubMed]
- Mol, H.G.J.; van Dam, R.C.J. Rapid detection of pesticides not amenable to multi-residue methods by flow injection-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 6817–6825. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Liao, H.-W.; Tseng, Y.J.; Tsai, I.-L.; Kuo, C. A matrix-induced ion suppression method to normalize concentration in urinary metabolomics studies flow injection analysis electrospray ionization mass spectrometry. Anal. Chim. Acta 2015, 864, 21–29. [Google Scholar] [CrossRef]
- Krishnadasan, S.; Brown, R.J.C.; deMello, A.J.; deMello, J.C. Intelligent routes to the controlled synthesis of nanoparticles. Lab Chip 2007, 7, 1434–1441. [Google Scholar] [CrossRef]
- Von Bomhard, S.; Schramm, J.; Bleul, R.; Thiermann, R.; Höbel, P.; Krtschil, U.; Löb, P.; Maskos, M. Modular manufacturing platform for continuous synthesis and analysis of versatile nanomaterials. Chem. Eng. Technol. 2019, 42, 2085–2094. [Google Scholar] [CrossRef]
- Suga, S.; Okajima, M.; Fujiwara, K.; Yoshida, J. “Cation flow” method: A new approach to conventional and combinatorial organic syntheses using electrochemical microflow systems. J. Am. Chem. Soc. 2001, 123, 7941–7942. [Google Scholar] [CrossRef]
- Carter, C.F.; Lange, H.; Ley, S.V.; Baxendale, I.R.; Wittkamp, B.; Goode, J.G.; Gaunt, N.L. ReactIR flow cell: A new analytical tool for continuous flow chemical processing. Org. Proc. Res. Dev. 2010, 14, 393–403. [Google Scholar] [CrossRef]
- Smith, C.J.; Nikbin, N.; Ley, S.V.; Lange, H.; Baxendale, I.R. A fully automated, multistep flow synthesis of 5-amino-4-cynao-1,2,3-triazoles. Org. Biomol. Chem. 2011, 9, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.F.; Dearing, T.I.; Martin, S.; Marquardt, B.J. Integration of continuous flow reactors and online Raman spectroscopy for process optimization. J. Pharm. Innov. 2012, 7, 69–75. [Google Scholar] [CrossRef]
- Browne, D.L.; Wright, S.; Deadman, B.; Dunnage, S.; Baxendale, I.R.; Turner, R.M.; Ley, S.V. Continuous flow reaction monitoring using an on-line miniature mass spectrometer. Rapid Commun. Mass Spectrom. 2012, 26, 1999–2010. [Google Scholar] [CrossRef]
- Perera, D.; Tucker, J.W.; Brahmbhatt, S.; Helal, C.J.; Chong, A.; Farrell, W.; Richardson, P.; Sach, N.W. A platform for automated nanomole-scale reaction screening and micromole-scale synthesis in flow. Science 2018, 359, 429–434. [Google Scholar] [CrossRef]
- Sutherland, J.D.; Tu, N.P.; Nemcek, T.A.; Searle, P.A.; Hochlowski, J.E.; Djuric, S.W.; Pan, J.Y. An automated synthesis- purification-sample-management platform for the accelerated generation of pharmaceutical candidates. J. Lab. Autom. 2014, 19, 176–182. [Google Scholar] [CrossRef]
- Welch, C.J.; Gong, X.; Cuff, J.; Dolman, S.; Nyrop, J.; Lin, F.; Rogers, H. Online analysis of flowing streams using microflow HPLC. Org. Proc. Res. Dev. 2009, 13, 1022–1025. [Google Scholar] [CrossRef]
- Werner, M.; Kuratli, C.; Martin, R.E.; Hochstrasser, R.; Wechsler, D.; Enderle, T.; Alanine, A.I.; Vogel, H. Seamless integration of dose-response screening and flow chemistry: Efficient generation of structure- activity relationship data of β-secretase (BACE1) inhibitors. Angew. Chem. Int. Ed. 2014, 53, 1704–1708. [Google Scholar] [CrossRef]
- Korn, M.; Gouveia, L.F.B.P.; de Oliveira, E.; Reis, B.F. Binary search in flow titration employing photometric end-point detection. Anal. Chim. Acta 1995, 313, 177–184. [Google Scholar] [CrossRef]
- Lima, M.J.A.; Reis, B.F.; Zagatto, E.A.G.; Kamogawa, M.Y. An automatic titration setup for the chemilumi- nometric determination of the copper complexation capacity in opaque solutions. Talanta 2020, 209, 120530. [Google Scholar] [CrossRef]
- Pons, C.; Forteza, R.; Cerda, V. Expert multi-syringe flow-injection system for the determination and speciation analysis of iron using chelating disks in water samples. Anal. Chim. Acta 2004, 524, 79–88. [Google Scholar] [CrossRef]
- Avivar, J.; Ferrer, L.; Casas, M.; Cerda, V. Smart thorium and uranium determination exploiting renewable solid-phase extraction applied to environmental samples in a wide concentration range. Anal. Bioanal. Chem. 2011, 400, 3585–3594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goodell, J.R.; McMullen, J.P.; Zaborenko, N.; Maloney, J.R.; Ho, C.-X.; Jensen, K.F.; Porco, J.A.; Beeler, A.B. Development of an automated microfluidic reaction platform for multidimensional screening: Reaction discovery employing bicycle [3.2.1]octanoid scaffolds. J. Org. Chem. 2009, 74, 6169–6180. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Carter, C.F.; Hopkin, M.D.; Burke, A.; Goode, J.G.; Baxendale, I.R.; Ley, S.V. A breakthrough method for the accurate addition of reagents in multi-step segmented flow processing. Chem. Sci. 2011, 2, 765–769. [Google Scholar] [CrossRef]
- Zhu, C.; Raghuvanshi, K.; Mason, D.; Rodgers, J.; Janka, M.E.; Abolhasani, M.; Coley, C.W. Flow chemistry-enabled studies of rhodium- catalyzed hydroformylation reactions. Chem. Commun. 2018, 54, 8567–8570. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.D.; Eckstein, R.J. Robotic sample preparation station. Anal. Chem. 1982, 54, 2347–2351. [Google Scholar] [CrossRef]
- Granchi, M.P.; Biggerstaff, J.A.; Hillard, L.H.; Grey, P. Use of a robot and flow injection for automated sample preparation and analysis of used oils by ICP emission spectrometry. Spectrochim. Acta 1987, 42B, 169–180. [Google Scholar] [CrossRef]
- Garcia-Mesa, J.A.; Luque de Castro, M.D.; Valcarcel, M. Coupled robot-flow injection analysis system for fully automated determination of total polyphenols in olive oil. Anal. Chem. 1993, 65, 3540–3542. [Google Scholar] [CrossRef]
- Velasco-Arjona, A.; Luque de Castro, M.D. A robotic-flow injection approach to the fully automated determination of starch in food. Anal. Chim. Acta 1996, 333, 205–213. [Google Scholar] [CrossRef]
- Prabhu, G.R.D.; Urban, P.L. The dawn of unmanned analytical laboratories. Trends Anal. Chem. 2017, 88, 41–52. [Google Scholar] [CrossRef]
- Kellici, S.; Gong, K.; Lin, T.; Brown, S.; Clark, R.J.H.; Vickers, M.; Cockcroft, J.K.; Middelkoop, V.; Barnes, P.; Perkins, J.M.; et al. High-throughput continuous hydrothermal flow synthesis of Zn-Ce oxides: Unprecedented solubility of Zn in the nanoparticle fluorite lattice. Phil Trans. R. Soc. A 2010, 368, 4331–4349. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.J.; Lin, T.; Brett, D.; Evans, J.R.; Cibin, G.; Dent, A.; Sankar, G.; Darr, J.A. A combinatorial nanoprecursor route for direct solid state chemistry: Discovery and electronic properties of new iron- doped lanthanum nickelates up to La4Ni2FeO10−δ. Solid State Ionics 2012, 225, 176–181. [Google Scholar] [CrossRef][Green Version]
- Urban, P.L. Universal electronics for miniature and automated chemical assays. Analyst 2015, 140, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M. Mobile-phone based chemical analysis – instrumental innovations and smartphone apps. Modern Chem. Appl. 2017, 5, 1000220. [Google Scholar] [CrossRef]
- Guo, T.; Patnaik, R.; Kuhlmann, K.; Rai, A.J.; Sia, S.K. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab. Chip 2015, 15, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; He, J.; Chen, X.; Qiao, Y.; Wang, F.; Xia, Q.; Yu, L.; Lu, Z.S. A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose 2019, 26, 4553–4562. [Google Scholar] [CrossRef]
- Alves, I.P.; Reis, N.M. Microfluidic smartphone quantitation of Escherichia coli in synthetic urine. Biosens. Bioelectron. 2019, 145, 111624. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Xing, D. Paper-based bipolar electrode-electrochemiluminescence (BPE-ECL) device with battery energy supply and smartphone read-out: A handheld ECL system for biochemical analysis at the point-of-care level. Sens. Actuat. B 2016, 237, 308–317. [Google Scholar] [CrossRef]
- Wongwilai, W.; Lapanantnoppakhun, S.; Grudpan, S.; Grudpan, K. Webcam camera as a detector for a simple lab-on-chip time based approach. Talanta 2010, 81, 1137–1141. [Google Scholar] [CrossRef]
- Lima, M.B.; Barreto, I.S.; Andrade, S.I.E.; Almeida, L.F.; Araujo, M.C.U. Using webcam CdTe quantum dots and flow-batch system for automatic spectrofluorimetric determination of N-acetyl-L-cysteine in pharma- ceutical formulations. J. Braz. Chem. Soc. 2014, 25, 1638–1646. [Google Scholar]
- Li, B.; Li, L.; Guan, A.; Dong, Q.; Ruan, K.; Hu, R.; Li, Z. A smartphone controlled handheld microfluidic liquid handling system. Lab. Chip 2014, 14, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, C.; Liu, F.; Zou, X.; Xu, Y.; Xu, X. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef] [PubMed]
- Ingham, R.J.; Battilocchio, C.; Fitzpatrick, D.E.; Hawkinsk, J.M.; Ley, S.V. A systems approach towards an intelligent and self-controlling platform for integrated continuous reaction sequences. Angew. Chem. Int. Ed. 2015, 54, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Ingham, R.J.; O’Brien, M.; Brownbe, D.L. Camera-enabled techniques for organic synthesis. Beilstein J. Org. Chem. 2013, 9, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Nacapricha, D.; Amornthammarong, N.; Sereenonchai, K.; Anujarawat, P.; Wilairat, P. Low cost telemetry with PC sound card for chemical analysis applications. Talanta 2007, 71, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas III, S.W.; Sindi, H.; Whitesides, G.M. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef]
- Lillehoj, P.B.; Huang, M.; Truong, N.; Ho, C. Rapid electrochemical detection on a mobile phone. Lab. Chip 2013, 13, 2950–2955. [Google Scholar] [CrossRef]
- Trojanowicz, M. Nanostructures in flow analysis. J. Flow-Inj. Anal. 2008, 25, 5–13. [Google Scholar]
- Frigerio, C.; Ribeiro, D.S.; Rodrigues, S.S.M.; Abreu, V.L.; Barbosa, J.; Prior, J.A.V.; Marques, K.; Santos, J.L. Application of quantum dots as analytical tools in automated chemical analysis: A review. Anal. Chim. Acta 2012, 735, 9–22. [Google Scholar] [CrossRef]
- Passos, M.L.C.; Pinto, P.C.A.G.; Santos, J.L.M.; Saraiva, M.L.M.F.S.; Araujo, A.R.T.S. Nanoparticle-based assays in automated flow systems: A review. Anal. Chim. Acta 2015, 889, 22–34. [Google Scholar] [CrossRef]
- Shahbazali, E.; Hessel, V.; Noël, Y.T.; Wang, Q. Metallic nanoparticles made in flow and their catalytic applications in micro-flow reactors for organic synthesis. Phys. Sci. Rev. 2016, 20150016. [Google Scholar] [CrossRef]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous hydrothermal synthesis of inorganic nanoparticles: Applications and future directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Yan, J.; Liu, D.; Wang, K.; Luo, G. Continuous synthesis of nanocrystals via flow chemistry technology. Small 2019, e1902828. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Oyarzan-Ampuero, F.; Larta, P.; Guerrero, S.; Cabuil, V. Flow chemistry to control the synthesis of nano and microparticles for biomedical applications. Curr. Topics Med. Chem. 2014, 14, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lee, S.M.; Yi, C.; Li, C. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications. Lab. Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Jensen, K.F. Continuous manufacturing – the Green Chemistry promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef]
- Rocha, F.R.P.; Zagatto, E.A.G. Flow analysis during the 60 years of Talanta. Talanta 2010, 206, 120186. [Google Scholar] [CrossRef]
- Cerda, V.; Ferrer, L.; Portugal, L.A.; de Souza, C.T.; Ferreira, S.L.C. Multisyringe flow injection analysis in spectro- analytical techniques – A review. Trends Anal. Chem. 2018, 98, 1–18. [Google Scholar] [CrossRef]
- Dias, T.R.; Melchert, W.R.; Kamogawa, M.Y.; Rocha, F.R.P.; Zagatto, E.A.G. Fluidized particles in flow analysis: Potentialities, limitations and applications. Talanta 2018, 184, 325–331. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Kołacińska, K.; Grate, J.W. A review of flow analysis methods for determination of radionuclides in nuclear wastes and nuclear reactor coolants. Talanta 2018, 183, 70–82. [Google Scholar] [CrossRef]
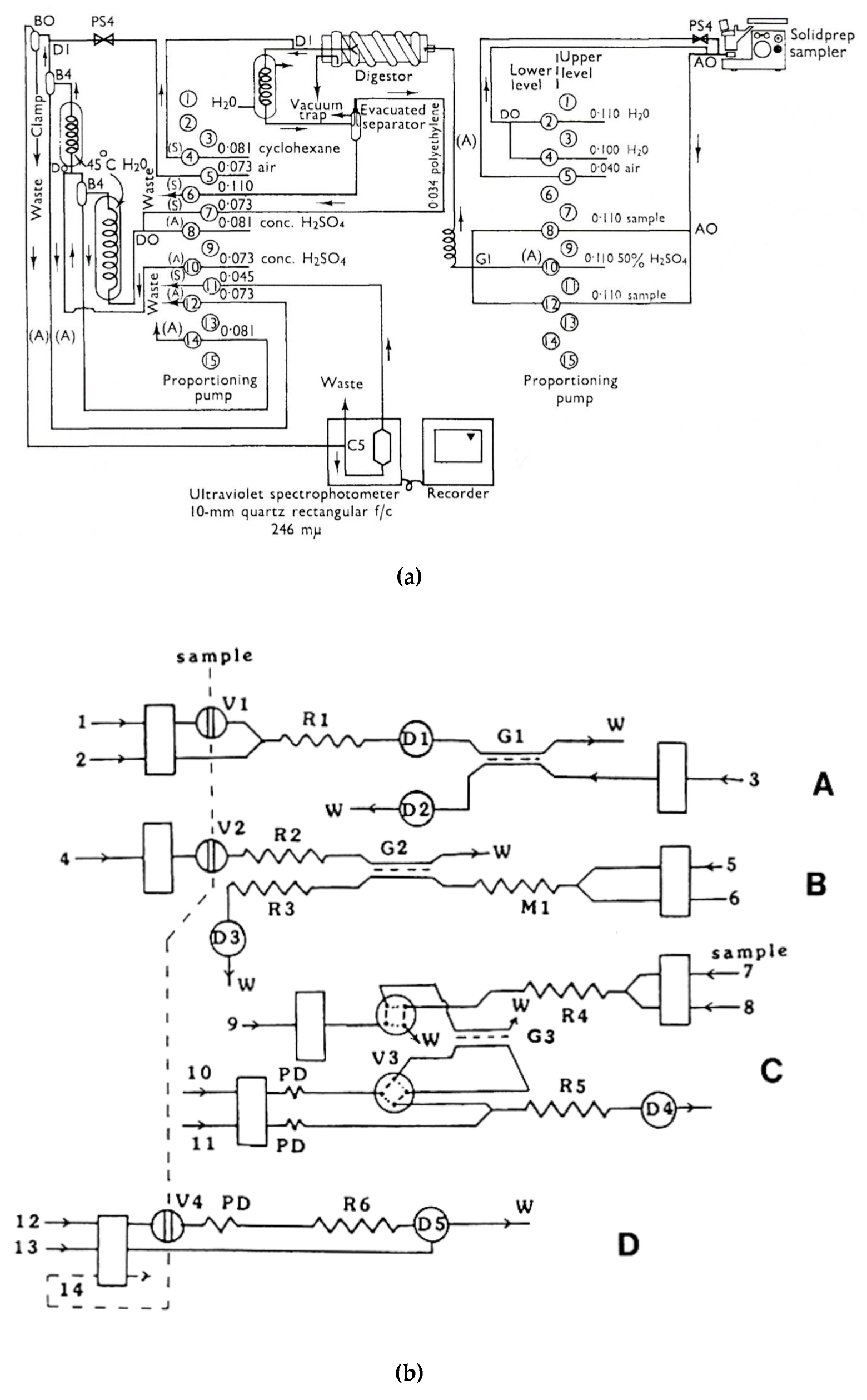
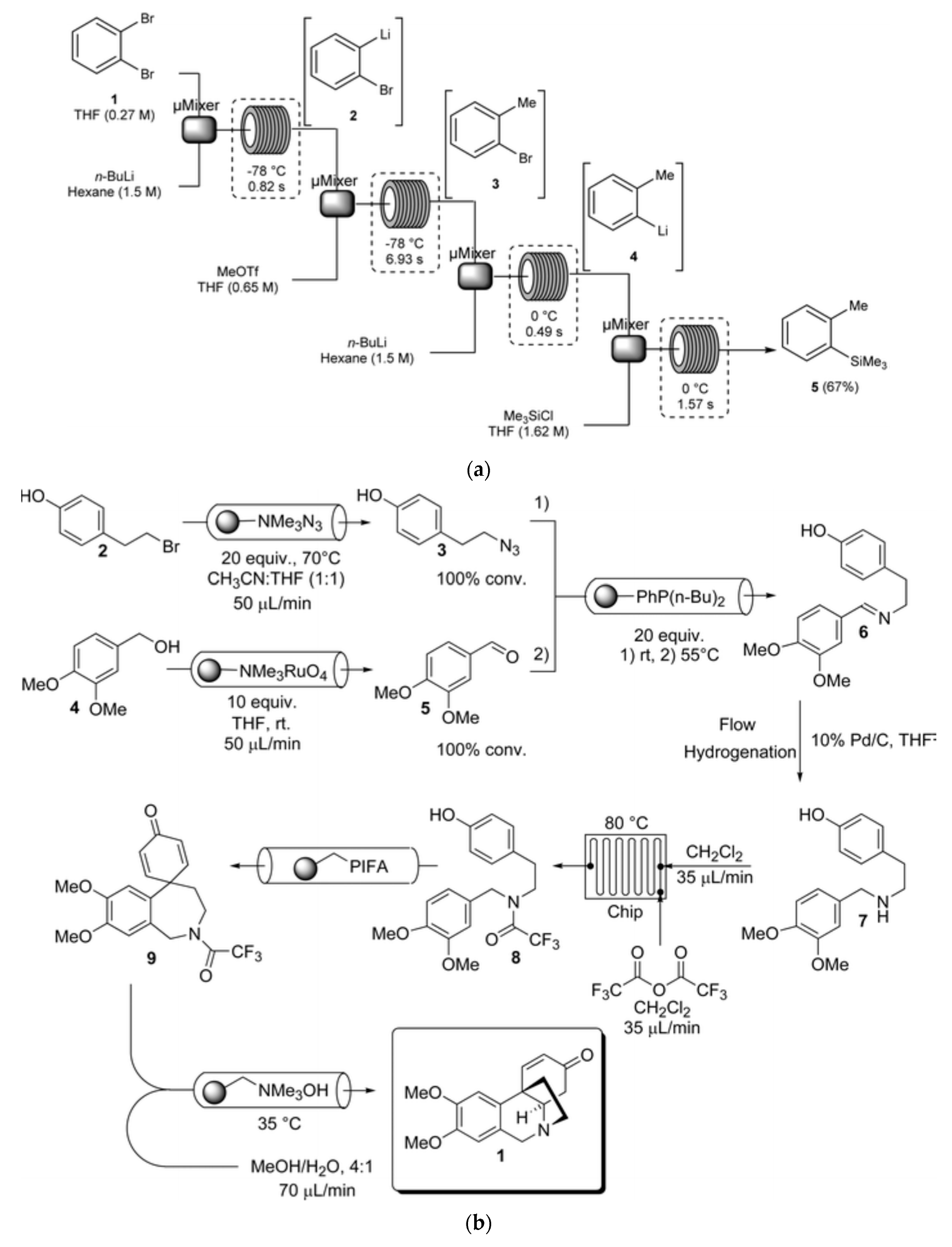
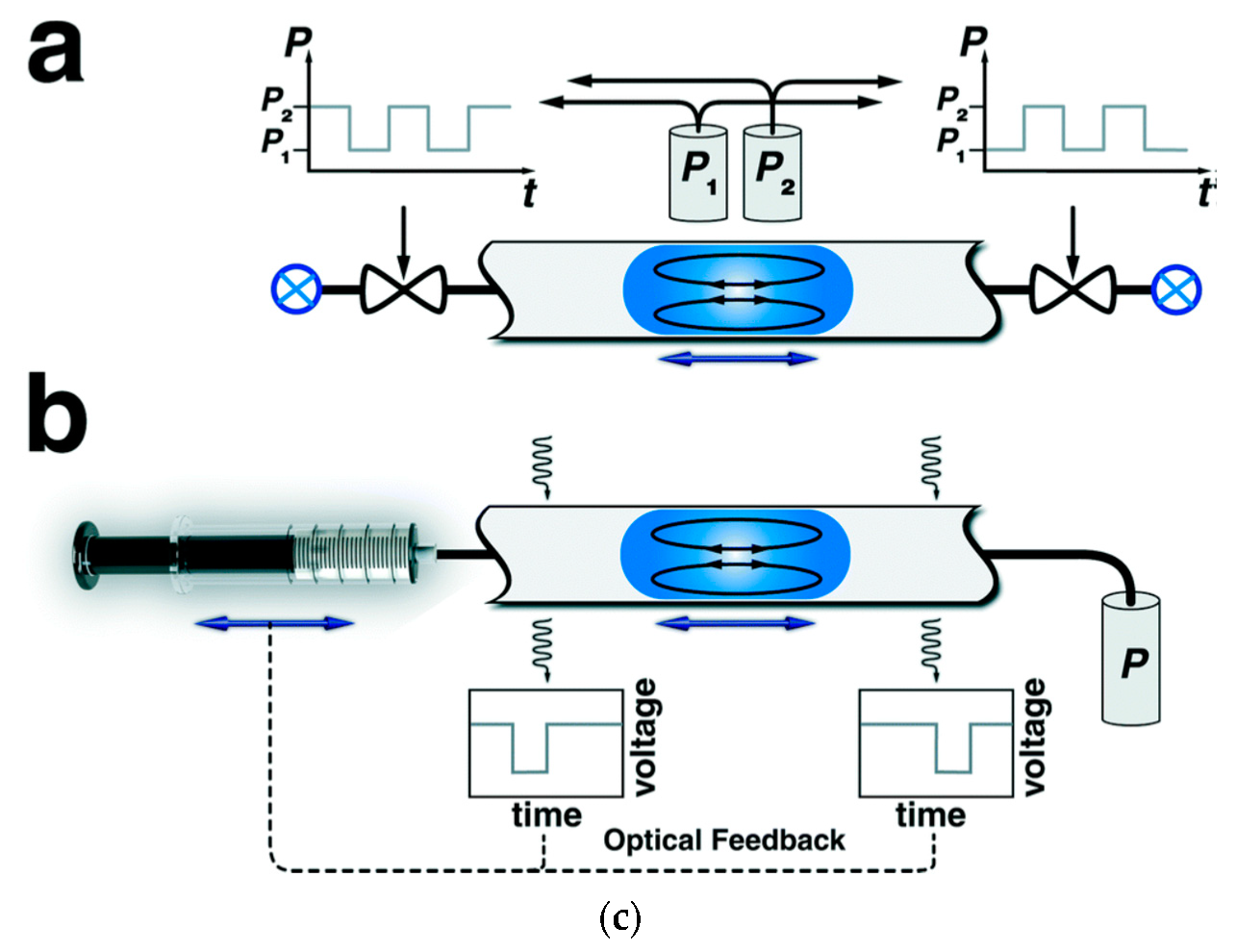

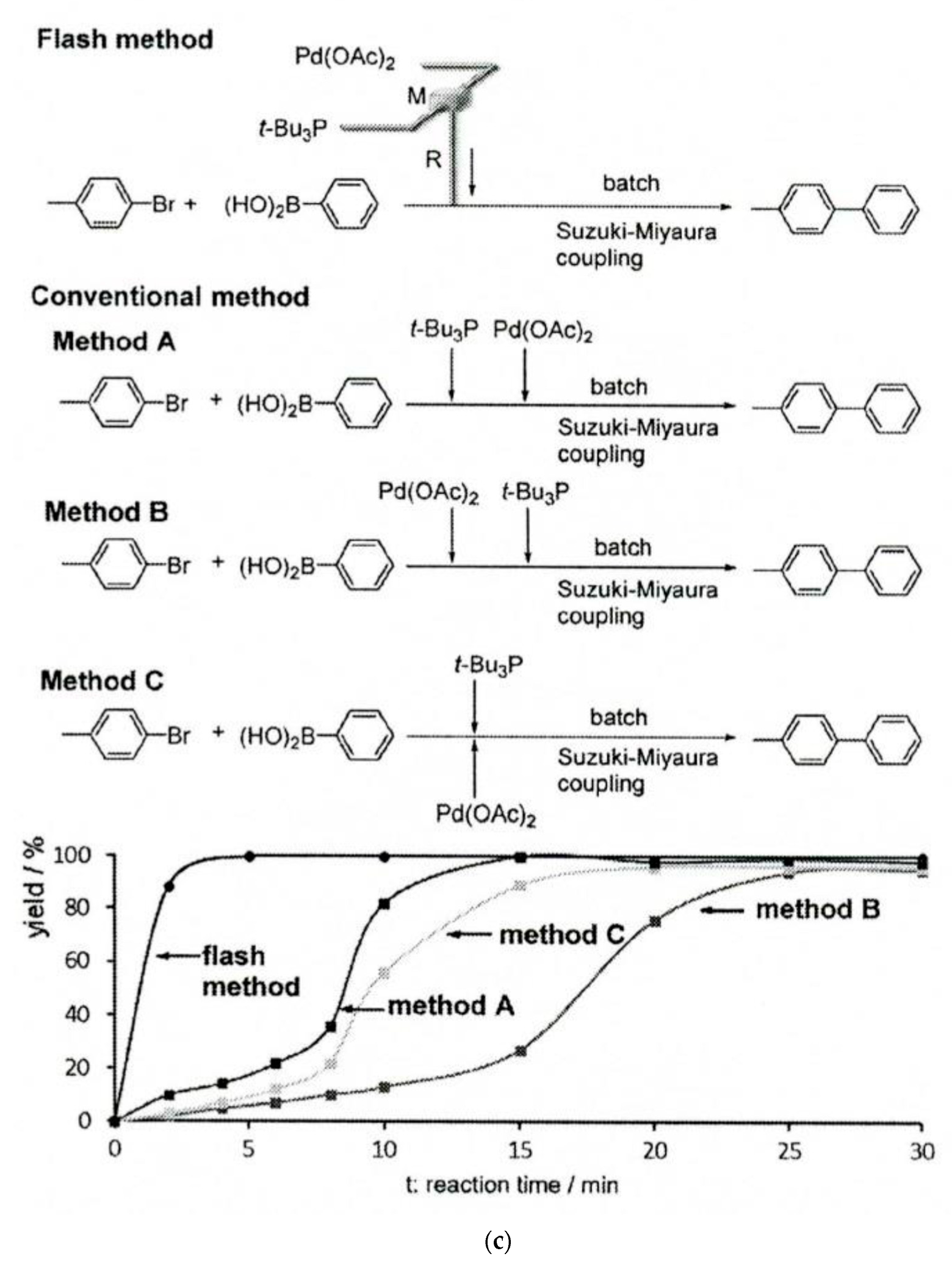


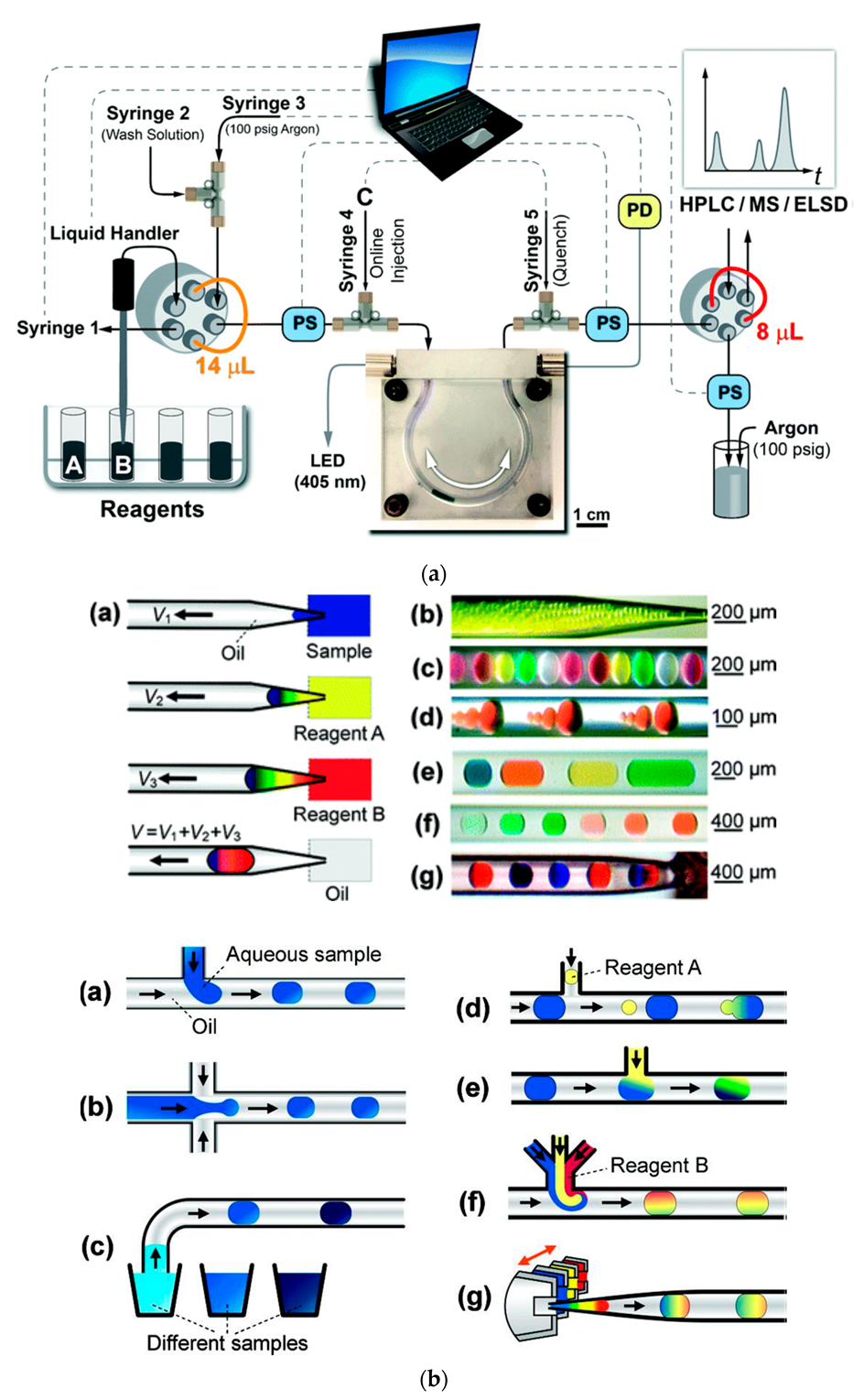


| Title | Author(s) | Publisher |
|---|---|---|
| Continuous Analysis of Chemical Process Systems | S. Siggia | Wiley, 1959 |
| Continuous-Flow Analysis. Theory and Practice | W.B. Furman | Marcel Dekker, 1976 |
| Automatic Chemical Analysis | J.K. Foreman, P.B. Stockwell | Ellis Horwood, 1975 |
| Automated Stream Analysis for Process Control | D.P. Manka | Academic Press, 1982 |
| Flow-Injection Analysis | J. Ruzicka, E.H. Hansen | Wiley, 1st Edition 1981, 2nd Edition, 1988 |
| Flow-Injection Analysis, Principles, and Applications | M. Valcarcel, M.D. Luque de Castro | Ellis Horwood, 1987 |
| Flow-Injection Atomic Spectroscopy | J.L. Burguera (Ed.) | Marcel Dekker, 1989 |
| Flow-Injection Analysis. A Practical Guide | B. Karlberg, G.E. Pacey | Elsevier, 1989 |
| Flow-Injection Separation and Preconcentration | Z.-L. Fang | VCH Verlag, 1993 |
| Flow-Injection Atomic Spectrometry | Z.-L. Fang | Wiley, 1994 |
| Flow-Injection Analysis of Pharmaceuticals: Automation in the Laboratory | J.M. Calatayud | Taylor and Francis, 1997 |
| Flow Analysis with Spectrometric Detectors | A. Sanz-Medel (Ed.), | Elsevier, 1999 |
| Flow-Injection Analysis. Instrumentation and Applications | M. Trojanowicz | World Scientific, 2000 |
| Advances in Flow Analysis | M. Trojanowicz (Ed.) | Wiley-VCH, 2008 |
| Advances in Flow-Injection Analysis and Related Techniques | S.D. Kolev, I.D. McKelvie (Eds.) | Elsevier, 2008 |
| Flow-Injection Analysis of Marine Samples | M.C. Yebra-Biurrun | Nova Science Publishers, 2009 |
| Flow Analysis with Spectrophotometric and Luminometric Detection | E.A.G. Zagatto, C.C. Oliveira, A. Townshend, P. Worsfold | Elsevier, 2011 |
| Flow Analysis: A Practical Guide | V. Cerda, L. Ferrer, J. Avivar, A. Cerda | Elsevier, 2014 |
| Flow-Injection Analysis of Food Additives | C. Ruiz-Capillas, L.M.L. Nollet (Eds.) | CRC Press, 2015. |
| Flow and Capillary Electrophoretic Analysis | P. Kościelniak, M. Trojanowicz (Eds.) | Nova Science Publishers, 2017 |
| Title | Author(s) | Publisher |
|---|---|---|
| Chemical reactions and processes under flow conditions | S.V.Luis, E. Garcia-Varga | RSC, 2010 |
| Micro reaction technology in organic synthesis | C. Viles, P. Watts | CRC Press, 2011 |
| Flow chemistry. Fundamentals | F. Darvas, V. Hessel, G. Dorman | De Gruyter, 2014 |
| Organometallic flow chemistry | T. Noël (Ed.) | Springer, 2016 |
| Continuous-flow chemistry in the research laboratory | T. Glasnov | Springer, 2016 |
| Sustainable flow chemistry: Methods and Applications | L. Vaccaro (Ed.) | Wiley, 2016 |
| Flow chemistry for the synthesis of heterocycles | U.K. Sharma, E.V. Van der Eycken (Eds.) | Springer, 2018 |
| Science of synthesis: Flow chemistry in organic synthesis | T.F. Jamison, G. Koch (Eds.) | Thieme,2018 |
| Flow chemistry: Integrated approaches for practical applications | S.V. Luis, E. Garcia-Verdugo | RSC, 2019 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trojanowicz, M. Flow Chemistry in Contemporary Chemical Sciences: A Real Variety of Its Applications. Molecules 2020, 25, 1434. https://doi.org/10.3390/molecules25061434
Trojanowicz M. Flow Chemistry in Contemporary Chemical Sciences: A Real Variety of Its Applications. Molecules. 2020; 25(6):1434. https://doi.org/10.3390/molecules25061434
Chicago/Turabian StyleTrojanowicz, Marek. 2020. "Flow Chemistry in Contemporary Chemical Sciences: A Real Variety of Its Applications" Molecules 25, no. 6: 1434. https://doi.org/10.3390/molecules25061434
APA StyleTrojanowicz, M. (2020). Flow Chemistry in Contemporary Chemical Sciences: A Real Variety of Its Applications. Molecules, 25(6), 1434. https://doi.org/10.3390/molecules25061434