Microwave Assisted Synthesis of 4-Phenylquinazolin-2(1H)-one Derivatives that Inhibit Vasopressor Tonus in Rat Thoracic Aorta
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
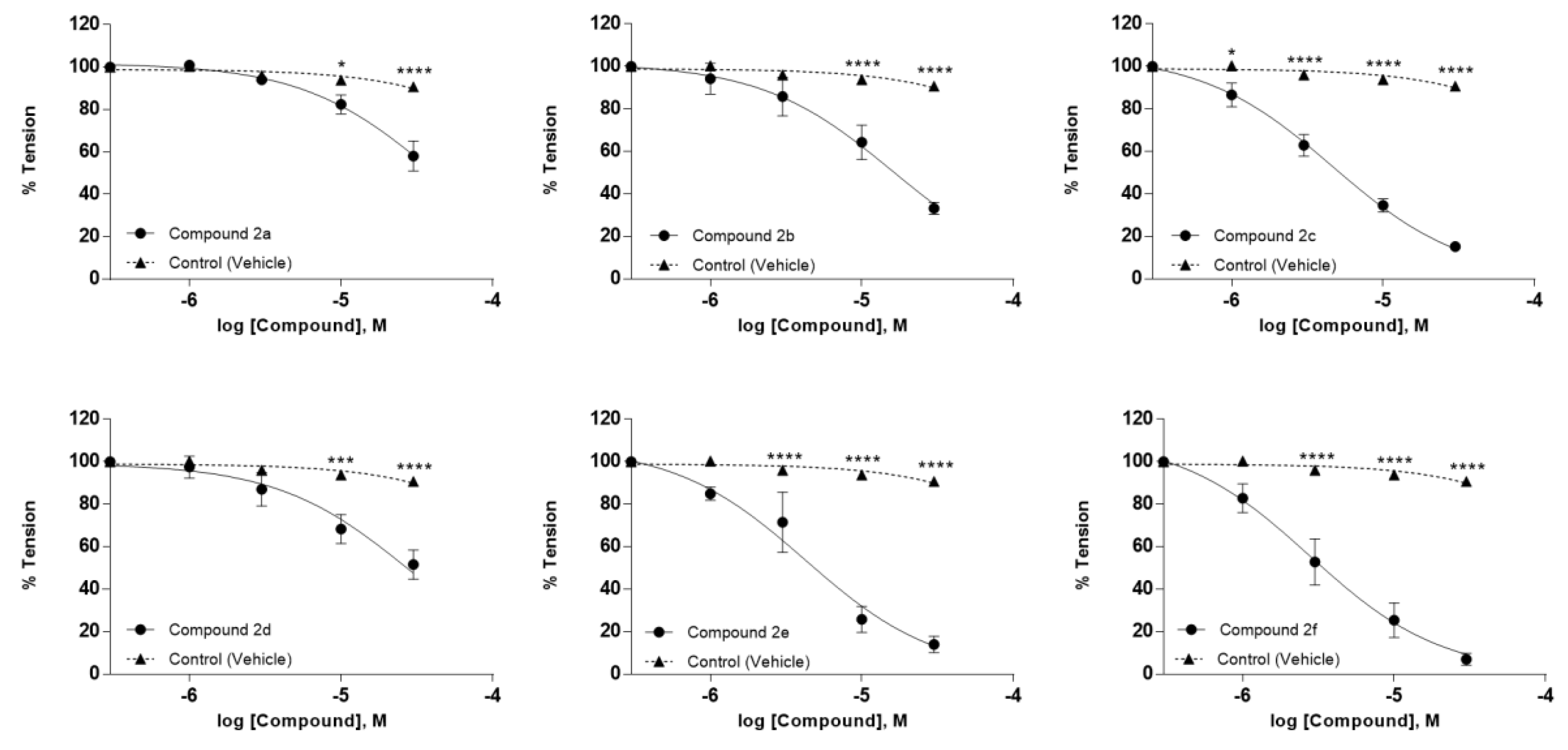
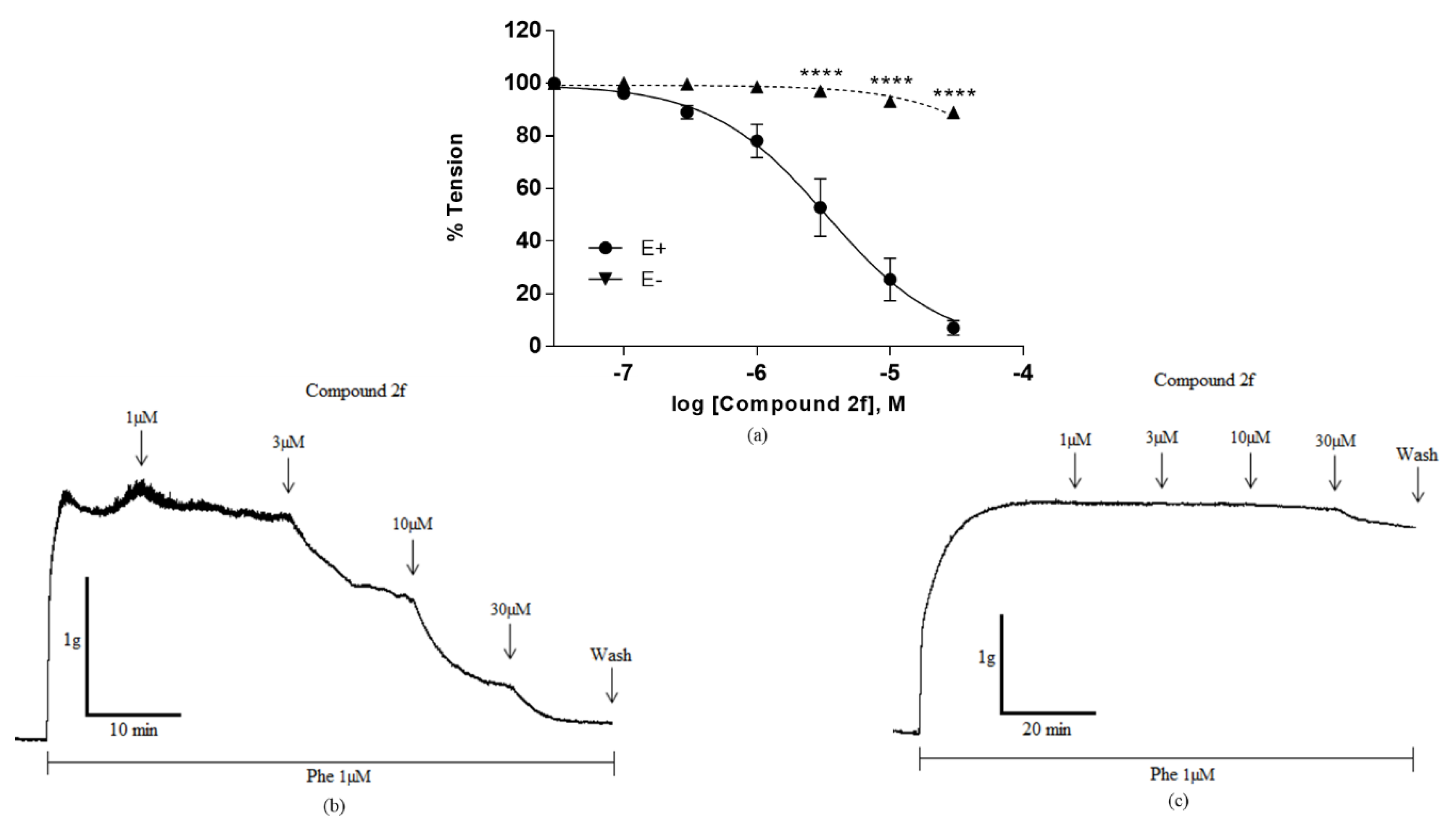
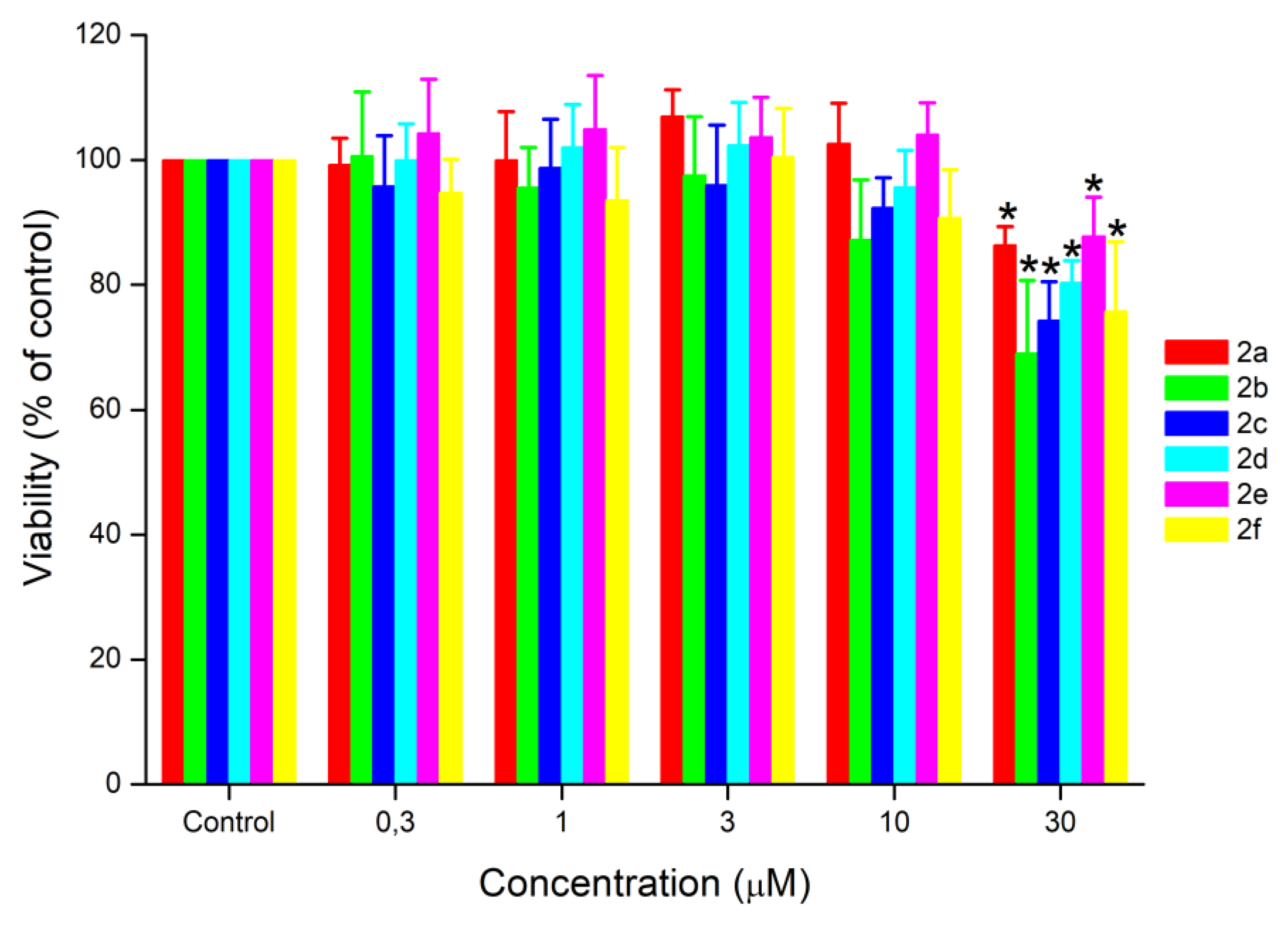
2.2. Biological Evaluation
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Preparation of 2a–f
Conventional
Microwave Irradiation
3.3. Sample Preparation
3.4. Animals
3.5. Compounds and Solution
3.6. Vascular Reactivity
3.7. Cytotoxicity
3.8. Expression of Data and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf, Z.; Igbal, J.; Saeed, A. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: An update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 2016, 24, 2361–2381. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Exp. Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, A.; Ashish, K.; Gill, N.S.; Rana, A.C. Quinazolinone: An Overview. Int. Res. J. Pharm. 2011, 2, 22–28. [Google Scholar]
- Pathak, S.; Malhotra, V.; Nath, R.; Shanker, K. Synthesis and Antihypertensive Activity of Novel Quinazolin-4(3H)-one Derivatives. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef]
- Jain, K.S.; Bariwal, J.B.; Kathiravan, M.K.; Phoujdar, M.S.; Sahne, R.S.; Chauhan, B.S.; Shah, A.K.; Yadav, M.R. Recent advances in selective α1-adrenoreceptor antagonists as antihypertensive agents. Bioorg. Med. Chem. 2008, 16, 4759–4800. [Google Scholar] [CrossRef]
- Seo, H.N.; Choi, J.Y.; Choe, Y.J.; Kim, Y.; Rhim, H.; Lee, S.H.; Kim, J.; Joo, D.J.; Lee, J.Y. Discovery of potent T-type calcium channel blocker. Bioorg. Med. Chem. Lett. 2007, 17, 5740–5743. [Google Scholar] [CrossRef]
- Venkatesh, R.; Kasaboina, S.; Gaikwad, H.; Janardhan, S.; Bantu, R.; Nagarapu, L.; Sastry, G.N.; Banerjee, S.K. Design and synthesis of 3- ( 3- (( 9 H -carbazol-4-yl) oxy ) -2- hydroxypropyl ) -2-phenylquinazolin-4 ( 3 H ) -one derivatives to induce ACE inhibitory activity. Eur. J. Med. Chem. 2015, 96, 22–29. [Google Scholar] [CrossRef]
- Paracha, T.U.; Pobsuk, N.; Salaloy, N.; Suphakun, P.; Pekthong, D.; Hannongbua, S.; Choowongkomon, K.; Khorana, N.; Temkitthawon, P.; Ingkaninan, K.; et al. Elucidation of vasodilation response and structure activity relationships of N2,N4 -disubstituted quinazoline 2,4-diamines in a rat pulmonary artery model. Molecules 2019, 24, 281. [Google Scholar] [CrossRef] [PubMed]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4 (3 H) -Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef] [PubMed]
- El-Messery, S.M.; Hassan, G.S.; Nagi, M.N.; Habib, E.S.E.; Al-Rashood, S.T.; El-Subbagh, H.I. Synthesis, biological evaluation and molecular modeling study of some new methoxylated 2-benzylthio-quinazoline-4(3H)-ones as nonclassical antifolates. Bioorg. Med. Chem. Lett. 2016, 26, 4815–4823. [Google Scholar] [CrossRef] [PubMed]
- Abuelizz, H.A.; Marzouk, M.; Ghabbour, H.; Al-Salahi, R. Synthesis and anticancer activity of new quinazoline derivatives. Saudi Pharm. J. 2017, 25, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Panneerselvam, T.; Alagarsamy, V.; Kunjiappan, S.; Parasuraman, P.; Murugan, I.; Kumar, D.P. Design, graph theoretical analysis, density functionality theories, Insilico modeling, synthesis, characterization and biological activities of novel thiazole fused quinazolinone derivatives. Drug Dev. Res. 2018, 79, 260–274. [Google Scholar] [CrossRef]
- Widler, L.; Altmann, E.; Beerli, R.; Breitenstein, W.; Bouhelal, R.; Buhl, T.; Gamse, R.; Gerspacher, M.; Halleux, C.; John, M.R.; et al. 1-Alkyl-4-phenyl-6-alkoxy-1H-quinazolin-2-ones: A novel series of potent calcium-sensing receptor antagonists. J. Med. Chem. 2010, 53, 2250–2263. [Google Scholar] [CrossRef]
- Sandoz, A.; Gamboni, G.; Schmid, M.W.; Sutter, B.A. Preparation of Quinazolin-2(1H)-Ones. U.S. Patent 4236006, 25 November 1980. [Google Scholar]
- Barthel, A.; Trieschmann, L.; Ströhl, D.; Kluge, R.; Böhm, G.; Csuk, R. Synthesis of dimeric quinazolin-2-one, 1,4-benzodiazepin-2-one, and isoalloxazine compounds as inhibitors of amyloid peptides association. Arch. Pharm. Chem. Life Sci. 2009, 342, 445–482. [Google Scholar] [CrossRef]
- Barrow, J.C.; Rittle, K.E.; Reger, T.S.; Yang, Z.Q.; Bondiskey, P.; McGaughey, G.B.; Bock, M.G.; Hartman, G.D.; Tang, C.; Ballard, J.; et al. Discovery of 4,4-disubstituted quinazolin-2-ones as T-type calcium channel antagonists. ACS Med. Chem. Lett. 2010, 1, 75–79. [Google Scholar] [CrossRef]
- Crespo, A.; Coelho, A.; Diz, P.M.; Fernández, F.; De Armas, H.N.; Sotelo, E. Convergent assembly of structurally diverse quinazolines. Org. Biomol. Chem. 2011, 9, 351–357. [Google Scholar] [CrossRef]
- Pham, T.T.; Walden, M.; Butler, C.; Diaz-Gonzalez, R.; Pérez-Moreno, G.; Ceballos-Pérez, G.; Gomez-Pérez, V.; García-Hernández, R.; Zecca, H.; Krakoff, E.; et al. Novel 1,2-dihydroquinazolin-2-ones: Design, synthesis, and biological evaluation against Trypanosoma brucei. Bioorg. Med. Chem. Lett. 2017, 27, 3629–3635. [Google Scholar] [CrossRef]
- Westman, J.; Lidstrom, P.; Tierney, J.; Whatey, B. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar]
- Kappe, C.O. My twenty years in microwave chemistry: From kitchen ovens to microwaves that aren´t microwaves. Chem. Rec. 2018, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Nava, E.; Llorens, S. The local regulation of vascular function: From an inside-outside to an outside-inside model. Front. Physiol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Busse, R. NO: The primary EDRF. J. Mol. Cell Cardiol. 1999, 31, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Quast, U.; Guillon, J.M.; Cavero, I. Cellular pharmacology of potassium channel openers in vascular smooth muscle. Cardiovasc. Res. 1994, 28, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.P.; Kargacin, G.J.; Kendrick-Jones, J.; Lincoln, T.M. Intracellular mechanisms involved in the regulation of vascular smooth muscle tone. Can. J. Physiol. Pharmacol. 1995, 73, 565–573. [Google Scholar] [CrossRef]
- Virdis, A.; Taddei, S. Endothelial Dysfunction in Resistance Arteries of Hypertensive Humans: Old and New Conspirators. J. Cardiovasc. Pharmacol. 2016, 67, 451–457. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Voronina, T.A.; Gordiichuk, G.N.; Andronati, S.A.; Garibova, T.L.; Zhilina, Z.I. Synthesis and pharmacological properties of some 4-phenyl-quinazoline-2-ones. Pharm. Chem. J. 1981, 15, 495–497. [Google Scholar] [CrossRef]
- Altmann, E.; Beerli, R.; Gerspacher, M.; Renaud, J.; Weiler, S.; Widler, L. Aryl-quinazoline/aryl-2amino-phenyl methanone derivatives. U.S. Patent WO 2004/056365A2, 08 July 2004. [Google Scholar]
Sample Availability: Samples of the compounds 4-phenylquinazolin-2(1H)-one derivates are available from Gabriel Resende. |



| Compound | Method | Yield (%) | Time |
|---|---|---|---|
| 2a | Conventional | 78 | 18 h |
| 2a | MW | 92 | 30 min |
| 2b | Conventional | 16 | 30 h |
| 2b | MW | 31 | 45 min |
| 2c | Conventional | 67 | 24 h |
| 2c | MW | 89 | 30 min |
| 2d | Conventional | 78 | 22 h |
| 2d | MW | 80 | 30 min |
| 2e | Conventional | 68 | 24 h |
| 2e | MW | 63 | 30 min |
| 2f | Conventional | 85 | 28 h |
| 2f | MW | 88 | 30 min |
| Compound ID | R1 | R2 | R3 | MW (g/mol) | IC50 (µM) |
|---|---|---|---|---|---|
| 2a | H | H | H | 222.24 | 39.72 ± 6.77 |
| 2b | NO2 | H | H | 267.24 | 16.39 ± 3.50 |
| 2c | Cl | Me | H | 270.71 | 4.31 ± 0.90 |
| 2d | Cl | F | H | 274.68 | 26.53 ± 8.98 |
| 2e | Br | H | H | 301.13 | 4.94 ± 1.21 |
| 2f | Cl | H | Me | 270.71 | 3.41 ± 0.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, R.; Menengat, T.; Andrade, G.; Cotrim, B.; Ponte, C.; Santos, W.C.; Resende, G. Microwave Assisted Synthesis of 4-Phenylquinazolin-2(1H)-one Derivatives that Inhibit Vasopressor Tonus in Rat Thoracic Aorta. Molecules 2020, 25, 1467. https://doi.org/10.3390/molecules25061467
Teixeira R, Menengat T, Andrade G, Cotrim B, Ponte C, Santos WC, Resende G. Microwave Assisted Synthesis of 4-Phenylquinazolin-2(1H)-one Derivatives that Inhibit Vasopressor Tonus in Rat Thoracic Aorta. Molecules. 2020; 25(6):1467. https://doi.org/10.3390/molecules25061467
Chicago/Turabian StyleTeixeira, Rafaela, Talita Menengat, Gabriel Andrade, Bruno Cotrim, Cristiano Ponte, Wilson C. Santos, and Gabriel Resende. 2020. "Microwave Assisted Synthesis of 4-Phenylquinazolin-2(1H)-one Derivatives that Inhibit Vasopressor Tonus in Rat Thoracic Aorta" Molecules 25, no. 6: 1467. https://doi.org/10.3390/molecules25061467
APA StyleTeixeira, R., Menengat, T., Andrade, G., Cotrim, B., Ponte, C., Santos, W. C., & Resende, G. (2020). Microwave Assisted Synthesis of 4-Phenylquinazolin-2(1H)-one Derivatives that Inhibit Vasopressor Tonus in Rat Thoracic Aorta. Molecules, 25(6), 1467. https://doi.org/10.3390/molecules25061467






