Special Issue: “Advances in Homogeneous Catalysis”
Abstract
- (i)
- The selectivity of a selected process, which indirectly affects its efficiency, obtaining the desired product with minor amounts of by-products and waste, thus decreasing the energy consumption and maximizing the total atom economy [7] of the process.
- (ii)
- The tolerance to potentially degradable or sensitive functional groups, since catalytic reactions usually take place under milder reaction conditions than stoichiometric processes.
- (iii)
- The enantiomeric excess: the employment of chiral catalysts allows to transfer the chiral information from the catalyst to the desired enantiomerically pure compounds, which usually present high added value and are very interesting for the pharmaceutical industry.
Acknowledgments
Conflicts of Interest
References
- Van Leeuwen, P.W. Homogeneous Catalysis: Understanding the Art; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Hartwig, J.F. Organotransition Metal Chemistry: From Bonding to Catalysis; University Science Books: Sausalito, CA, USA, 2010. [Google Scholar]
- Dalko, P.I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Bommarius, A.S.; Riebel, B.R. Biocatalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Rothenberg, G. Catalysis: Concepts and Green Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Cole-Hamilton, D.J.; Tooze, R.P. Catalyst Separation, Recovery and Recycling. Chemistry and Process Design; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Trost, B.M. The atom economy-a search for synthetic efficiency. Science 1991, 254, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Song, X.R.; Yang, R.; Ding, H.; Bai, J.; Xiao, Q. An efficient approach to phosphorylated isoindoline fused with triazoles via Zn-Catalyzed cascade cyclization of 2–Propynol benzyl azides and diarylphosphine oxides. Molecules 2019, 24, 3526. [Google Scholar] [CrossRef] [PubMed]
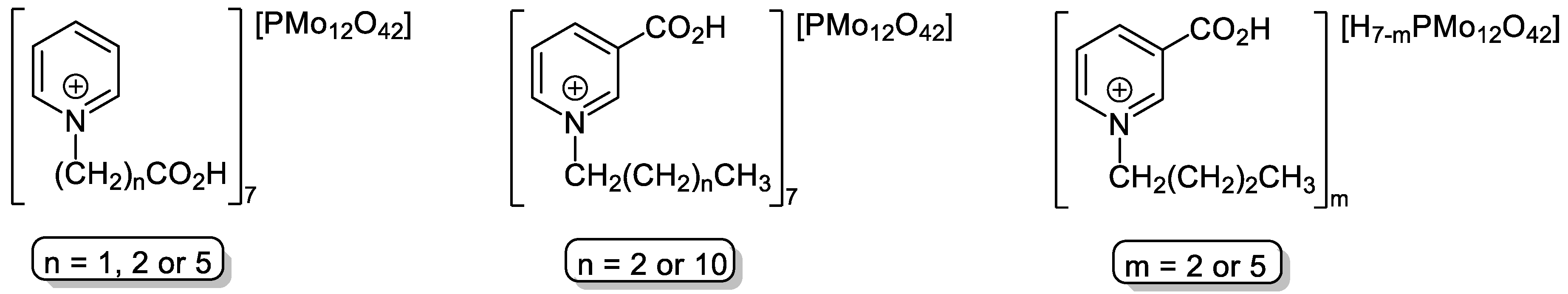
- Akopyan, A.; Eseva, E.; Polikarpova, P.; Kedalo, A.; Vutolkina, A.; Glotov, A. Deep oxidative desulfurization of fuels in the presence of brönsted acidic polyoxometalate-based ionic liquids. Molecules 2020, 25, 536. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Liu, T.; Chang, X.; Guo, W. An update of transition metal-catalyzed decarboxylative transformations of cyclic carbonates and carbamates. Molecules 2019, 24, 3930. [Google Scholar] [CrossRef] [PubMed]
- Nebra, N. High-Valent NiIII and NiIV species relevant to C–C and C–Heteroatom cross-coupling reactions: State of the art. Molecules 2020, 25, 1141. [Google Scholar] [CrossRef] [PubMed]
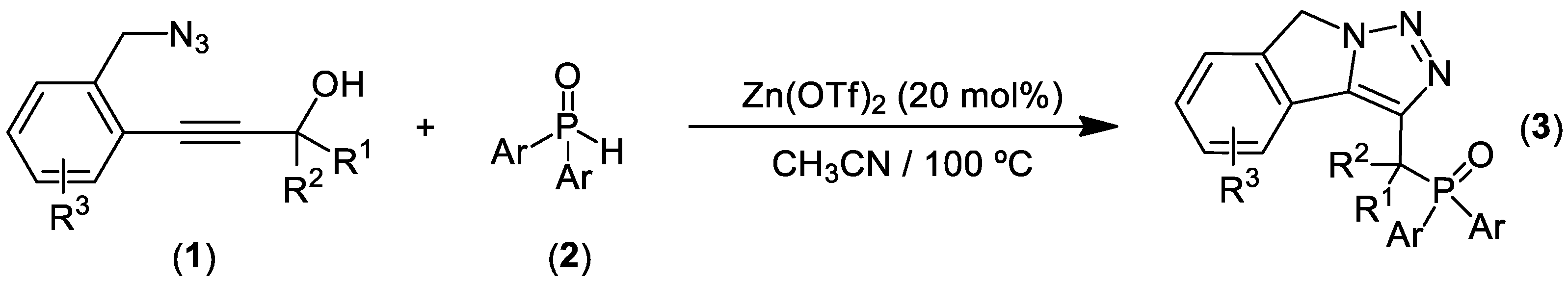
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Álvarez, J. Special Issue: “Advances in Homogeneous Catalysis”. Molecules 2020, 25, 1493. https://doi.org/10.3390/molecules25071493
García-Álvarez J. Special Issue: “Advances in Homogeneous Catalysis”. Molecules. 2020; 25(7):1493. https://doi.org/10.3390/molecules25071493
Chicago/Turabian StyleGarcía-Álvarez, Joaquín. 2020. "Special Issue: “Advances in Homogeneous Catalysis”" Molecules 25, no. 7: 1493. https://doi.org/10.3390/molecules25071493
APA StyleGarcía-Álvarez, J. (2020). Special Issue: “Advances in Homogeneous Catalysis”. Molecules, 25(7), 1493. https://doi.org/10.3390/molecules25071493




