Fatty Acid Content and Composition of the Morphological Fractions of Cistus Ladanifer L. and Its Seasonal Variation
Abstract
1. Introduction
2. Results
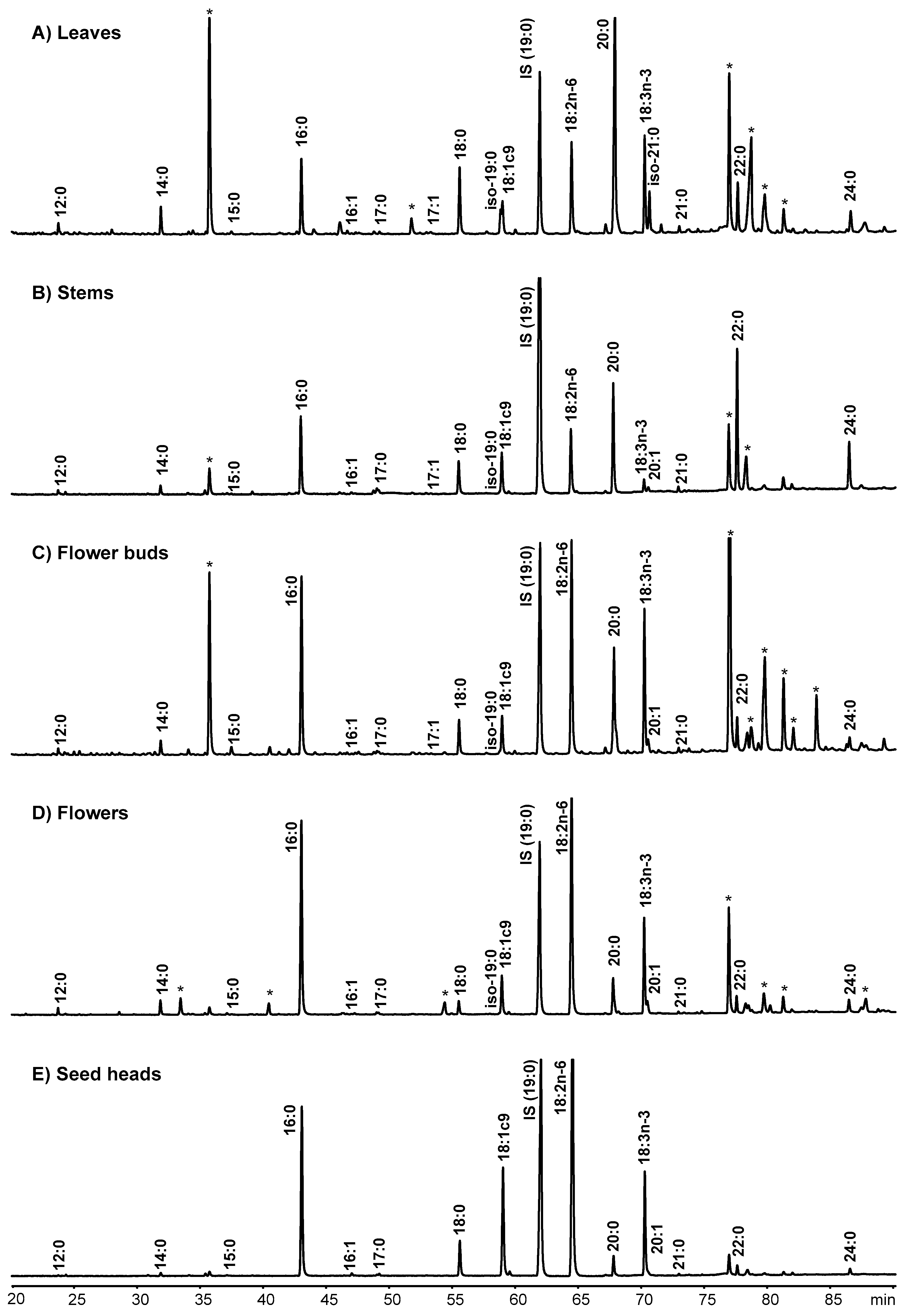
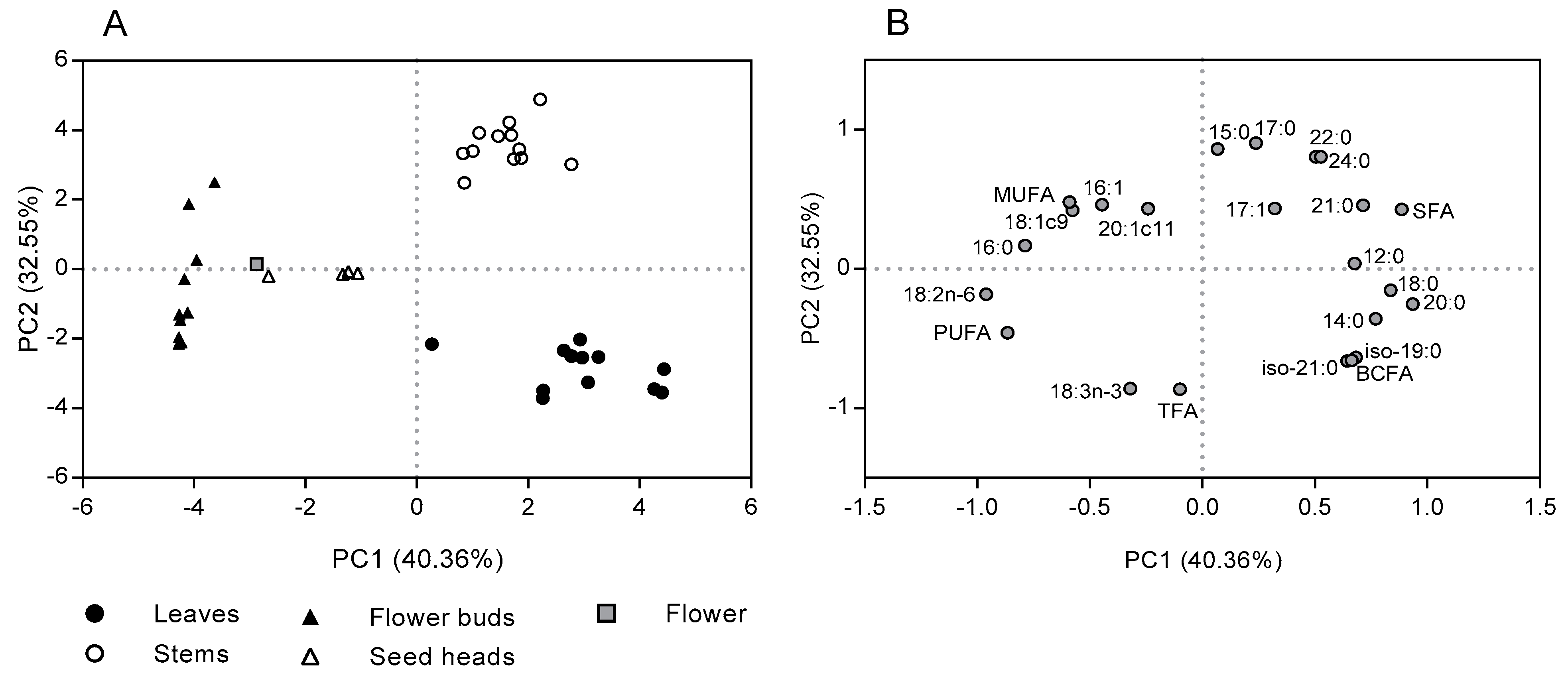
2.1. Total Fatty Acid Content and Composition of the Morphological Fractions of Cistus Ladanifer
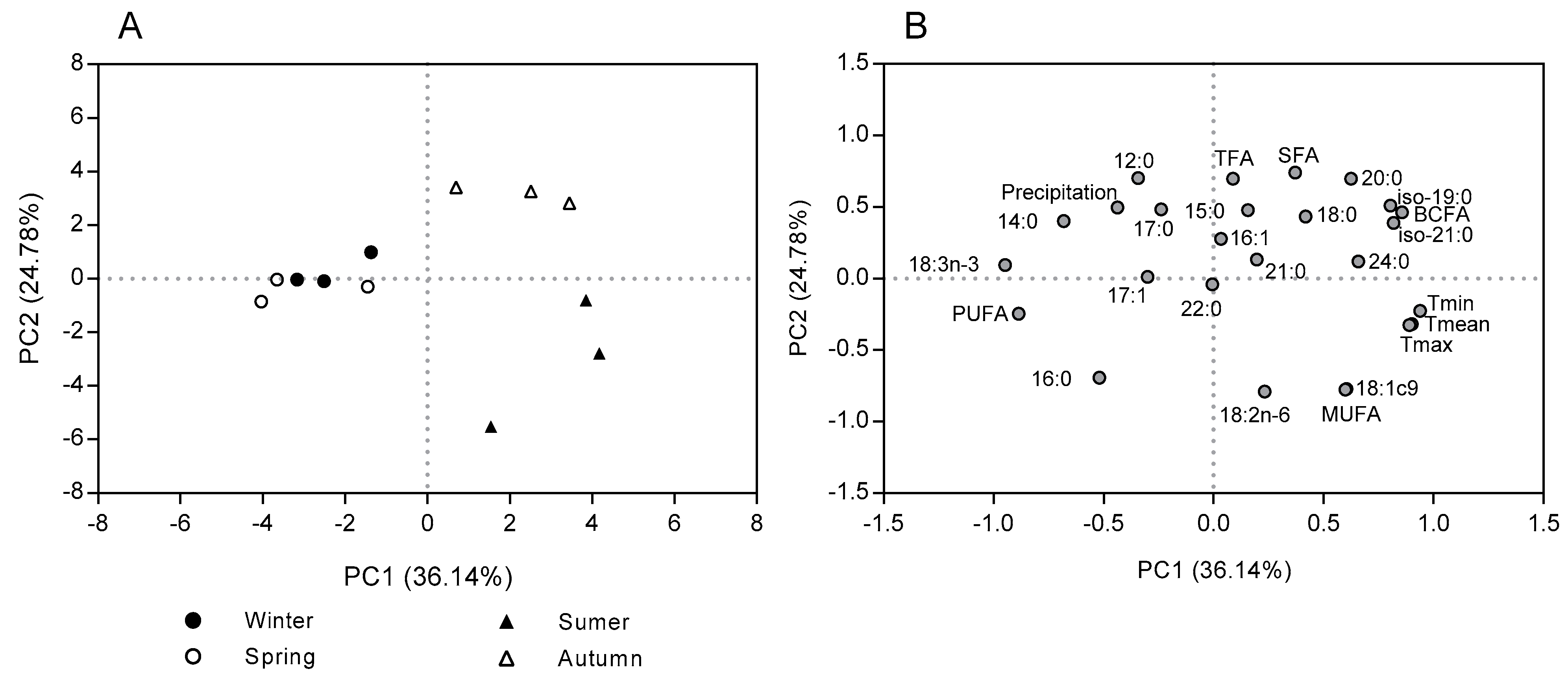
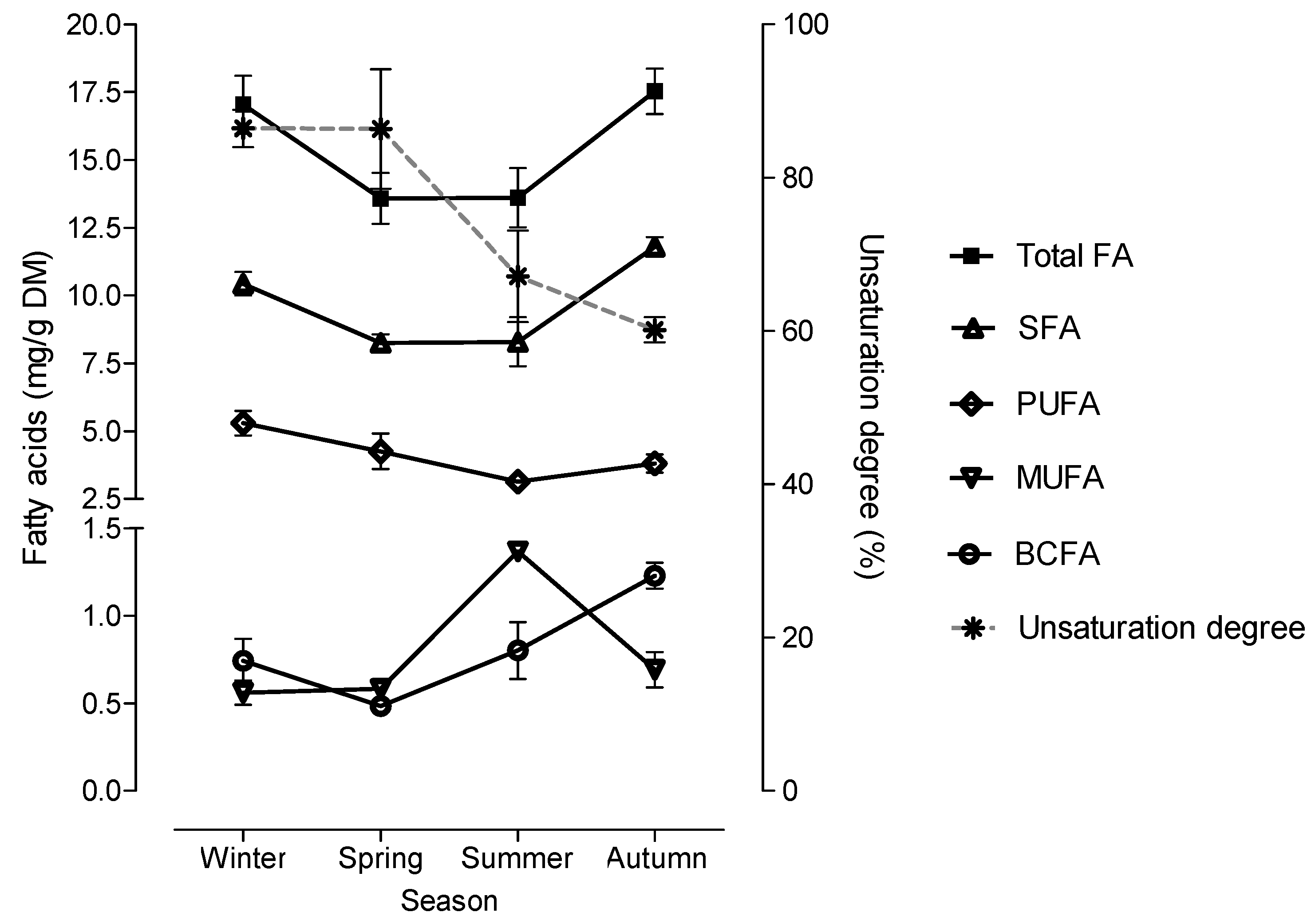
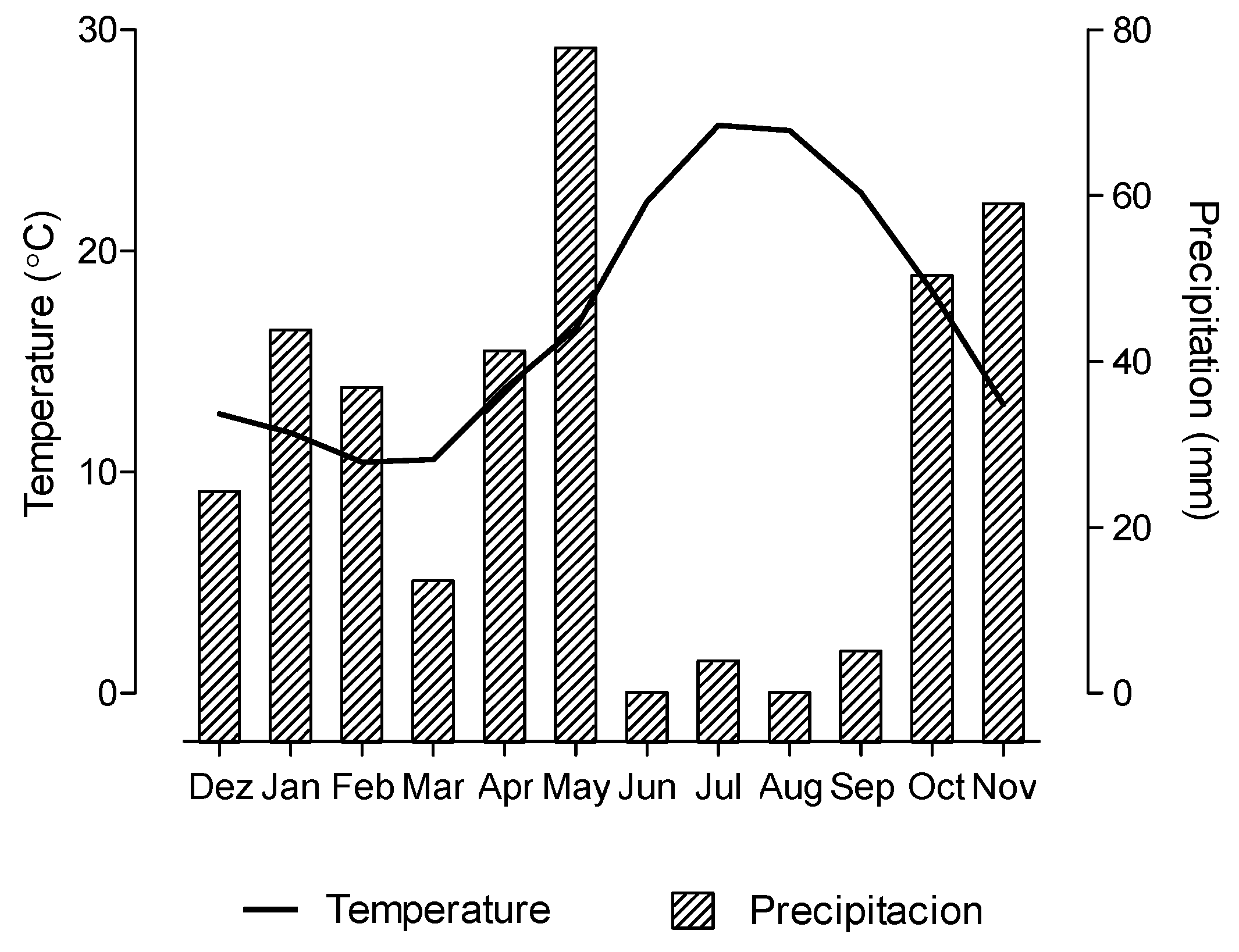
2.2. Seasonal Variation in the Fatty Acid Content and Composition in the Morphological Fractions of Cistus Ladanifer
3. Discussion
4. Materials and Methods
4.1. Plant Material Sampling
4.2. Fatty Acid Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frazão, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabarís, C.; Gonçalves, J.C.; Delgado, F. Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri, A. Chemical composition and antioxidant activity of essential oil, various organic extracts of Cistus ladanifer and Cistus libanotis growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12. [Google Scholar] [CrossRef]
- Amensour, M.; Sendra, E.; Perez-Alvarez, J.A.; Skali-Senhaji, N.; Abrini, J.; Fernandez-Lopez, J. Antioxidant activity and chemical content of methanol and ethanol extracts from leaves of rockrose (Cistus ladaniferus). Plant Foods Hum. Nutr. 2010, 65, 170–178. [Google Scholar] [CrossRef]
- Guerreiro, O.; Dentinho, M.T.P.; Moreira, O.C.; Guerra, A.R.; Ramos, P.A.B.; Bessa, R.J.B.; Duarte, M.F.; Jerónimo, E. Potential of Cistus ladanifer L. (rockrose) in small ruminant diets—Effect of season and plant age on chemical composition, in vitro digestibility and antioxidant activity. Grass Forage Sci. 2016, 71, 437–447. [Google Scholar] [CrossRef]
- Ferreira, S.; Santos, J.; Duarte, A.; Duarte, A.P.; Queiroz, J.A.; Domingues, F.C. Screening of antimicrobial activity of Cistus ladanifer and Arbutus unedo extracts. Nat. Prod. Res. 2011, 26, 1558–1560. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Morales-Soto, A.; Barrajon-Catalan, E.; Roldan-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Saura, D.; Guillen, E.; Fernandez-Gutierrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef]
- Belmokhtar, M.; Bouanani, N.E.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Mateo, P.; Fischmeister, R.; Legssyer, A. Antihypertensive and endothelium-dependent vasodilator effects of aqueous extract of Cistus ladaniferus. Biochem. Biophys. Res. Commun. 2009, 389, 145–149. [Google Scholar] [CrossRef] [PubMed]
- El Youbi, A.E.; El Mansouri, L.; Boukhira, S.; Daoudi, A.; Bousta, D. In Vivo Anti-Inflammatory and Analgesic Effects of Aqueous Extract of Cistus ladanifer L. From Morocco. Am. J. Ther. 2016, 23, E1554–E1559. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, E.; Alves, S.P.; Dentinho, M.T.P.; Martins, S.V.; Prates, J.A.M.; Santos-Silva, J.; Bessa, R.J.B. The effect of grape seed extract and Cistus ladanifer L. and vegetable oil supplementation on fatty acid composition of abomasal digesta and intramuscular fat of lambs. J. Agric. Food Chem. 2010, 58, 10710–10721. [Google Scholar] [CrossRef] [PubMed]
- Dentinho, M.T.P.; Belo, A.T.; Bessa, R.J.B. Digestion, ruminal fermentation and microbial nitrogen supply in sheep fed soybean meal treated with Cistus ladanifer L. tannins. Small Rumin. Res. 2014, 119, 57–64. [Google Scholar] [CrossRef]
- Francisco, A.; Alves, S.P.; Portugal, P.V.; Dentinho, M.T.; Jerónimo, E.; Sengo, S.; Almeida, J.; Bressan, M.C.; Pires, V.M.R.; Alfaia, C.M.; et al. Effects of dietary inclusion of citrus pulp and rockrose soft stems and leaves on lamb meat quality and fatty acid composition. Animal 2018, 12, 872–881. [Google Scholar] [CrossRef]
- Francisco, A.; Dentinho, M.T.; Alves, S.P.; Portugal, P.V.; Fernandes, F.; Sengo, S.; Jerónimo, E.; Oliveira, M.A.; Costa, P.; Sequeira, A.; et al. Growth performance, carcass and meat quality of lambs supplemented with increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Meat Sci. 2015, 100, 275–282. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Ferro, M.D.; Paulino, A.F.C.; Chaves, H.T.; Evtuguin, D.V.; Xavier, A.M.R.B. Comparative study on hydrolysis and bioethanol production from cardoon and rockrose pretreated by dilute acid hydrolysis. Ind. Crops Prod. 2018, 111, 633–641. [Google Scholar] [CrossRef]
- Mariotti, J.P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Composition of the essential oil of Cistus ladaniferus L. cultivated in Corsica (France). Flavour Fragr. J. 1997, 12, 147–151. [Google Scholar] [CrossRef]
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-grown Cistus ladanifer essential oil. J. Essent. Oil Res. 2005, 17, 160–165. [Google Scholar] [CrossRef]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, A.; da Costa, P.M.; Belo, A.D.F. Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from Eucalyptus globulus LABILL. and seven Mediterranean aromatic plants. Chem. Biodivers. 2017, 14, 12. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C.; Gutiérrez-merino, C. Seasonal-Variation of Exudate of Cistus ladanifer. J. Chem. Ecol. 1993, 19, 2577–2591. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Escudero, J.C.; Gutiérrez-Merino, C. Role of ecological variables in the seasonal variation of flavonoid content of Cistus ladanifer exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Sosa, T.; Alias, J.C.; Escudero, J.C.; Chaves, N. Interpopulational variation in the flavonoid composition of Cistus ladanifer L. exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal variation of Cistus ladanifer L. Diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dentinho, M.T.P.; Navas, D.; Potes, J. Chemical and nutritional evaluation of food complements for large cattle breeding, in Montado de azinho area. Pastagens Forrag. 2005, 26–27, 41–46. [Google Scholar]
- Guerreiro, O.; Alves, S.P.; Duarte, M.F.; Bessa, R.J.B.; Jerónimo, E. Cistus ladanifer L. shrub is rich in saturated and branched chain fatty acids and their concentration increases in the Mediterranean dry season. Lipids 2015, 50, 493–501. [Google Scholar] [CrossRef]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004, 50, 137–143. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Chen, X.; Chen, H.; Huang, M.; Zheng, J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res. 2000, 60, 505–509. [Google Scholar]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef]
- Cabezudo, B.; Navarro, T.; Pérez Latorre, A.; Nieto Caldera, J.M.; Orshan, G. Estudios fenomorfológicos en la vegetación del sur de España. I. Cistus L. (Phenomorphologic studies in vegetation of South of Spain. I. Cistus L.). Acta Bot. Malacit. 1992, 17, 229–237. [Google Scholar]
- Talavera, S.; Gibbs, P.E.; Herrera, J. Reproductive biology of Cistus Ladanifer (Cistaceae). Plant Syst. Evol. 1993, 186, 123–134. [Google Scholar] [CrossRef]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and txonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.H.; Brenna, J.T. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Eibler, D.; Seyfried, C.; Kaffarnik, S.; Vetter, W. anteiso-Fatty acids in Brussels Sprouts (Brassica oleracea var. gemmifera L.): Quantities, enantioselectivities, and stable carbon isotope ratios. J. Agric. Food Chem. 2015, 63, 8921–8929. [Google Scholar] [PubMed]
- Bonvehí, J.S.; Jordà, R.E. Nutrient composition and microbiological quality of honeybee-collected pollen in Spain. J. Agric. Food Chem. 1997, 45, 725–732. [Google Scholar] [CrossRef]
- Barbosa, S.T.R.; Silvestre, A.J.D.; Simões, M.M.Q.; Estevinho, M.L.M.F. Composition and antibacterial activity ofthe lipophilic fraction of honeybee pollen from native species of Montesinho natural park. Int. J. Agric. Res. 2006, 1, 471–479. [Google Scholar]
- Demoly, J.P.; Montserrat, P. Cistus. Flora Iber. 1993, 3, 319–337. [Google Scholar]
- Guzman, B.; Vargas, P. Long-distance colonization of the Western Mediterranean by Cistus ladanifer (Cistaceae) despite the absence of special dispersal mechanisms. J. Biogeogr. 2009, 36, 954–968. [Google Scholar] [CrossRef]
- Bastida, F.; Talavera, S. Temporal and spatial patterns of seed dispersal in two Cistus species (Cistaceae). Ann. Bot. 2002, 89, 427–434. [Google Scholar] [CrossRef]
- Krollmann, P.; Gulz, P.G. Composition of seed lipids from species of the Genus Cistus L. (Cistaceae). Z. Pflanzenphysiol. 1983, 110, 469–474. [Google Scholar] [CrossRef]
- Sauvant, D.; Perez, J.-M.; Tran, G. Tables of Composition and Nutritional Value of Feed Materials: Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses and Fish; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004. [Google Scholar]
- Carvalho, C.C.C.R.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chapman, K. Lipid signaling in plants. Front. Plant Sci. 2013, 4, 216. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S. Temperature perception and signal transduction in plants. New Phytol. 2008, 179, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Falcone, D.; Ogas, J.; Somerville, C. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, R.W. Effect of growth temperature on fatty acid composition of leaf lipids in Atriplex Lentiformis (Torr.) Wats. Plant Physiol. 1978, 61, 484–486. [Google Scholar] [CrossRef]
- Dakhma, W.S.; Zarrouk, M.; Cherif, A. Effects of drought-Stress on lipids in rape leaves. Phytochemistry 1995, 40, 1383–1386. [Google Scholar] [CrossRef]
- Radunz, A. On the function of methyl-branched chain fatty acids in phospholipids of cell membranes of higher plants. In The Metabolism, Structure, and Function of Plant Lipids; Stumpf, P., Mudd, J.B., Nes, W.D., Eds.; Springer: New York, NY, USA, 1987; pp. 197–200. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Winter | Spring | Summer | Autumn | SEM | p Values | |
|---|---|---|---|---|---|---|
| Total FA | 17.0b | 13.6a | 13.6a | 17.5b | 0.99 | 0.037 |
| 12:0 | 0.12 | 0.10 | 0.03 | 0.14 | 0.026 | 0.091 |
| 14:0 | 0.64b | 0.64b | 0.29a | 0.55b | 0.064 | 0.015 |
| 15:0 | - | 0.003 | - | 0.007 | 0.0024 | 0.219 |
| 16:0 | 2.22 | 2.01 | 1.82 | 1.89 | 0.102 | 0.103 |
| 16:1 | - | 0.003 | - | 0.007 | 0.0037 | 0.561 |
| 17:0 | 0.013 | 0.033 | 0.007 | 0.033 | 0.0089 | 0.140 |
| 17:1 | 0.007 | 0.027 | 0.007 | 0.010 | 0.0112 | 0.561 |
| 18:0 | 1.18a | 1.08a | 1.07a | 1.73b | 0.088 | 0.002 |
| iso-19:0 | 0.28a | 0.20a | 0.31a | 0.50b | 0.040 | 0.004 |
| 18:1 cis-9 | 0.55a | 0.55a | 1.36b | 0.68a | 0.064 | <0.001 |
| 18:2n-6 | 1.67 | 1.50 | 1.80 | 1.57 | 0.231 | 0.805 |
| 20:0 | 4.89bc | 3.31a | 3.89ab | 5.97c | 0.346 | 0.003 |
| 18:3n-3 | 3.62c | 2.76bc | 1.34a | 2.24ab | 0.310 | 0.005 |
| iso-21:0 | 0.46a | 0.29a | 0.49ab | 0.73b | 0.077 | 0.024 |
| 21:0 | 0.06 | 0.10 | 0.08 | 0.11 | 0.034 | 0.794 |
| 22:0 | 0.95 | 0.70 | 0.74 | 0.92 | 0.070 | 0.086 |
| 24:0 | 0.38 | 0.27 | 0.35 | 0.46 | 0.045 | 0.124 |
| Partial sums | ||||||
| n-SFA | 10.4b | 8.26a | 8.29a | 12.0b | 0.558 | 0.005 |
| BCFA | 0.74a | 0.49a | 0.80a | 1.23b | 0.110 | 0.009 |
| MUFA | 0.56a | 0.58a | 1.37b | 0.69a | 0.067 | <0.001 |
| PUFA | 5.29b | 4.26ab | 3.15a | 3.81a | 0.434 | 0.044 |
| Winter | Spring | Summer | Autumn | SEM | p Values | |
|---|---|---|---|---|---|---|
| Total FA | 3.60 | 3.18 | 3.71 | 3.35 | 0.433 | 0.818 |
| 12:0 | 0.013a | 0.010a | 0.022b | 0.020b | 0.0019 | 0.008 |
| 14:0 | 0.057 | 0.051 | 0.058 | 0.056 | 0.0054 | 0.771 |
| 15:0 | 0.007 | 0.008 | 0.007 | 0.006 | 0.0009 | 0.510 |
| 16:0 | 0.64 | 0.58 | 0.53 | 0.49 | 0.052 | 0.282 |
| 16:1 | 0.006 | 0.006 | 0.006 | 0.007 | 0.0011 | 0.952 |
| 17:0 | 0.018 | 0.019 | 0.021 | 0.019 | 0.001 | 0.234 |
| 18:0 | 0.23 | 0.20 | 0.20 | 0.25 | 0.029 | 0.495 |
| iso-19:0 | 0.002 | 0.005 | 0.004 | 0.009 | 0.0026 | 0.284 |
| 18:1 cis-9 | 0.25a | 0.30ab | 0.42b | 0.25a | 0.036 | 0.038 |
| 18:2n-6 | 0.49 | 0.43 | 0.39 | 0.35 | 0.066 | 0.514 |
| 20:0 | 0.69 | 0.52 | 0.62 | 0.64 | 0.133 | 0.828 |
| 20:1 | 0.015 | 0.021 | 0.018 | 0.024 | 0.0061 | 0.760 |
| 18:3n-3 | 0.11b | 0.12b | 0.07a | 0.06a | 0.012 | 0.011 |
| 21:0 | 0.030 | 0.025 | 0.029 | 0.027 | 0.0042 | 0.857 |
| 22:0 | 0.77 | 0.66 | 1.01 | 0.84 | 0.104 | 0.206 |
| 24:0 | 0.27 | 0.22 | 0.33 | 0.30 | 0.040 | 0.301 |
| Partial sums | ||||||
| n-SFA | 2.73 | 2.29 | 2.82 | 2.65 | 0.335 | 0.702 |
| MUFA | 0.27 | 0.33 | 0.45 | 0.29 | 0.044 | 0.079 |
| PUFA | 0.60 | 0.56 | 0.45 | 0.41 | 0.077 | 0.322 |
| Flower Buds | Flowers | ||||
|---|---|---|---|---|---|
| Winter | Spring | SEM | p Values | Spring | |
| Total FA | 12.0 | 11.8 | 1.07 | 0.900 | 14.6 |
| 12:0 | 0.08 | 0.07 | 0.028 | 0.732 | 0.10 |
| 14:0 | 0.21 | 0.24 | 0.010 | 0.150 | 0.25 |
| 15:0 | 0.016 | 0.021 | 0.0025 | 0.293 | 0.033 |
| 16:0 | 2.71 | 2.92 | 0.204 | 0.551 | 4.07 |
| 16:1 | 0.011 | 0.008 | 0.0011 | 0.198 | 0.012 |
| 17:0 | 0.028 | 0.027 | 0.0072 | 0.931 | 0.025 |
| 18:0 | 0.51 | 0.57 | 0.107 | 0.704 | 0.26 |
| iso-19:0 | 0.030 | 0.037 | 0.0111 | 0.679 | 0.021 |
| 18:1 cis-9 | 0.55 | 1.04 | 0.096 | 0.069 | 0.84 |
| 18:2n-6 | 3.47 | 3.90 | 0.147 | 0.174 | 5.41 |
| 20:0 | 1.69 | 1.13 | 0.271 | 0.280 | 0.87 |
| 20:1 | 0.19 | 0.076 | 0.0439 | 0.213 | 0.27 |
| 18:3n-3 | 1.82 | 1.32 | 0.229 | 0.265 | 1.83 |
| 21:0 | 0.056 | 0.040 | 0.0115 | 0.429 | 0.030 |
| 22:0 | 0.44 | 0.27 | 0.055 | 0.149 | 0.31 |
| 24:0 | 0.21 | 0.15 | 0.025 | 0.230 | 0.25 |
| Partial sums | |||||
| n-SFA | 5.95 | 5.42 | 0.710 | 0.653 | 6.20 |
| MUFA | 0.76 | 1.13 | 0.117 | 0.155 | 1.14 |
| PUFA | 5.28 | 5.22 | 0.376 | 0.912 | 7.24 |
| Winter | Spring | Summer | Autumn | SEM | p Values | |
|---|---|---|---|---|---|---|
| Total FA | 9.70 | 12.6 | 22.7 | 22.3 | 4.122 | 0.069 |
| 12:0 | 0.007 | 0.007 | 0.011 | 0.003 | 0.0022 | 0.089 |
| 14:0 | 0.04 | 0.07 | 0.10 | 0.06 | 0.019 | 0.098 |
| 15:0 | 0.008 | 0.009 | 0.004 | 0.006 | 0.0035 | 0.702 |
| 16:0 | 1.92 | 2.70 | 4.93 | 4.59 | 0.883 | 0.062 |
| 16:1 | 0.04 | 0.04 | 0.03 | 0.03 | 0.008 | 0.683 |
| 17:0 | 0.02 | 0.02 | 0.03 | 0.03 | 0.003 | 0.078 |
| 18:0 | 0.45 | 0.49 | 0.80 | 0.85 | 0.134 | 0.088 |
| 18:1 cis-9 | 1.55 | 1.38 | 1.99 | 2.06 | 0.297 | 0.272 |
| 18:2n-6 | 4.05 | 5.82 | 11.2 | 11.1 | 2.183 | 0.061 |
| 20:0 | 0.29 | 0.23 | 0.29 | 0.26 | 0.043 | 0.621 |
| 20:1 | 0.04 | 0.05 | 0.08 | - | 0.022 | 0.431 |
| 18:3n-3 | 1.02 | 1.60 | 3.08 | 3.13 | 0.623 | 0.054 |
| 21:0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.003 | 0.911 |
| 22:0 | 0.14 | 0.12 | 0.13 | 0.12 | 0.015 | 0.622 |
| 24:0 | 0.10 | 0.08 | 0.08 | 0.08 | 0.010 | 0.362 |
| Partial sums | ||||||
| n-SFA | 2.99 | 3.73 | 6.40 | 6.01 | 1.057 | 0.074 |
| MUFA | 1.64 | 1.48 | 2.10 | 2.10 | 0.310 | 0.330 |
| PUFA | 5.08 | 7.42 | 14.2 | 14.2 | 2.805 | 0.059 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerónimo, E.; Cachucho, L.; Soldado, D.; Guerreiro, O.; Bessa, R.J.B.; Alves, S.P. Fatty Acid Content and Composition of the Morphological Fractions of Cistus Ladanifer L. and Its Seasonal Variation. Molecules 2020, 25, 1550. https://doi.org/10.3390/molecules25071550
Jerónimo E, Cachucho L, Soldado D, Guerreiro O, Bessa RJB, Alves SP. Fatty Acid Content and Composition of the Morphological Fractions of Cistus Ladanifer L. and Its Seasonal Variation. Molecules. 2020; 25(7):1550. https://doi.org/10.3390/molecules25071550
Chicago/Turabian StyleJerónimo, Eliana, Liliana Cachucho, David Soldado, Olinda Guerreiro, Rui J. B. Bessa, and Susana P. Alves. 2020. "Fatty Acid Content and Composition of the Morphological Fractions of Cistus Ladanifer L. and Its Seasonal Variation" Molecules 25, no. 7: 1550. https://doi.org/10.3390/molecules25071550
APA StyleJerónimo, E., Cachucho, L., Soldado, D., Guerreiro, O., Bessa, R. J. B., & Alves, S. P. (2020). Fatty Acid Content and Composition of the Morphological Fractions of Cistus Ladanifer L. and Its Seasonal Variation. Molecules, 25(7), 1550. https://doi.org/10.3390/molecules25071550







