Antimalarial Agents as Therapeutic Tools Against Toxoplasmosis—A Short Bridge between Two Distant Illnesses
Abstract
1. Introduction
2. Treatment
2.1. Currently Available Drugs
2.2. Drug Repurposing in Toxoplasmosis Therapy
2.2.1. Can Antimalarials Treat Toxoplasmosis?
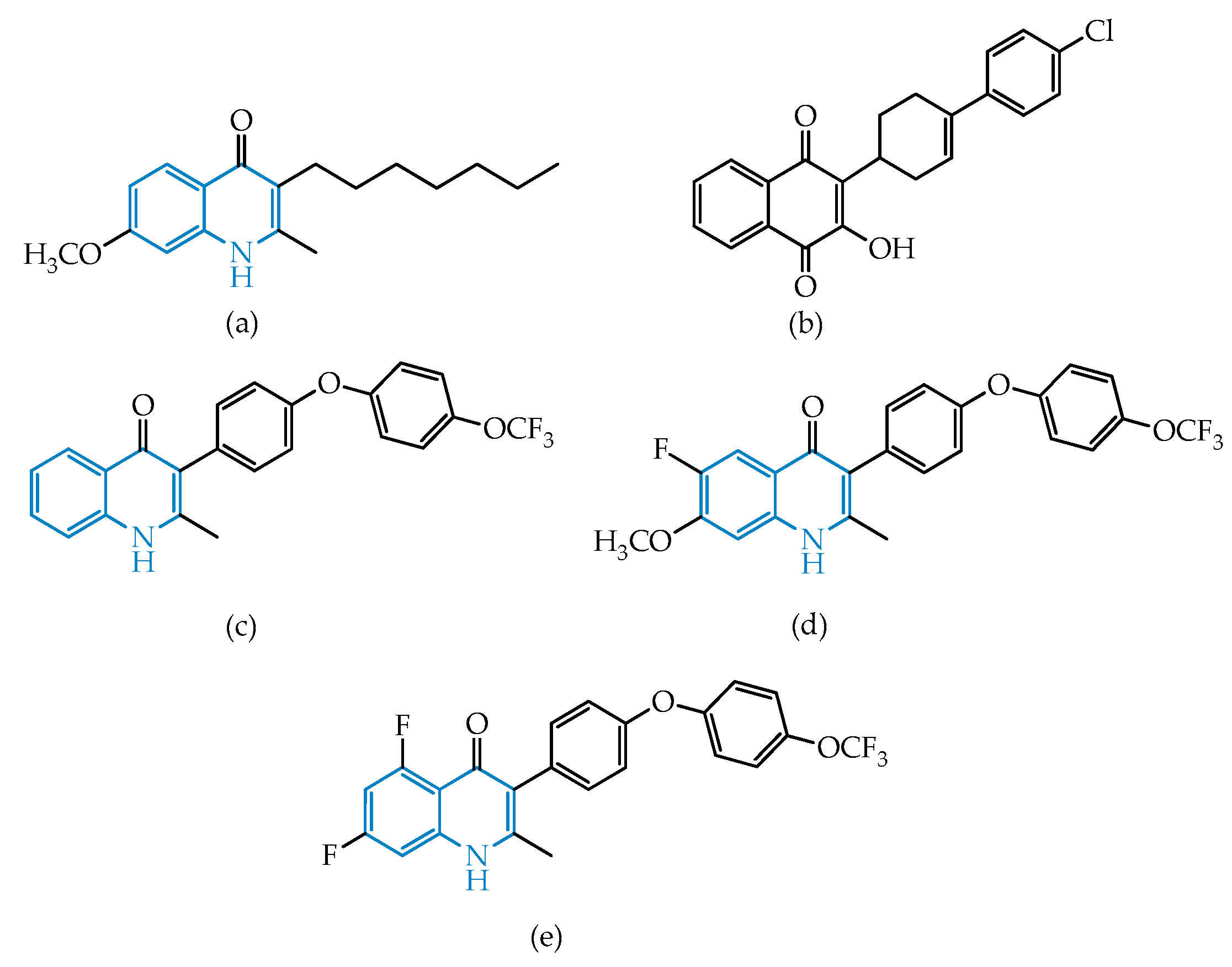
2.2.2. Quinolines
Endochin-Like Oxoquinolines (ELQs) and Quinoline-Based PPQ-8
2.2.3. Artemisinin and Related Endoperoxides
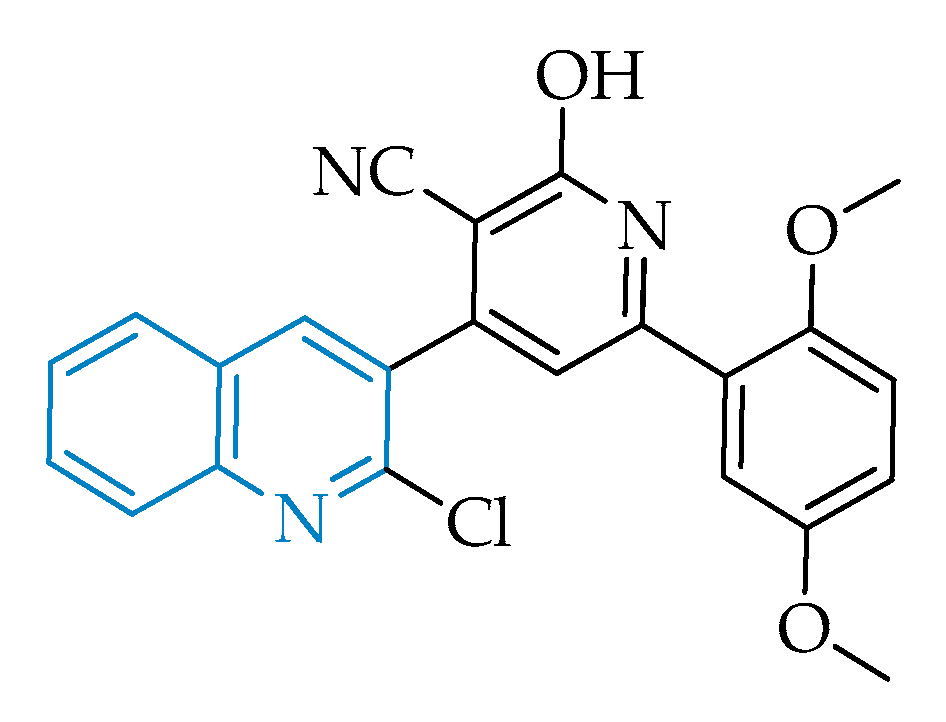
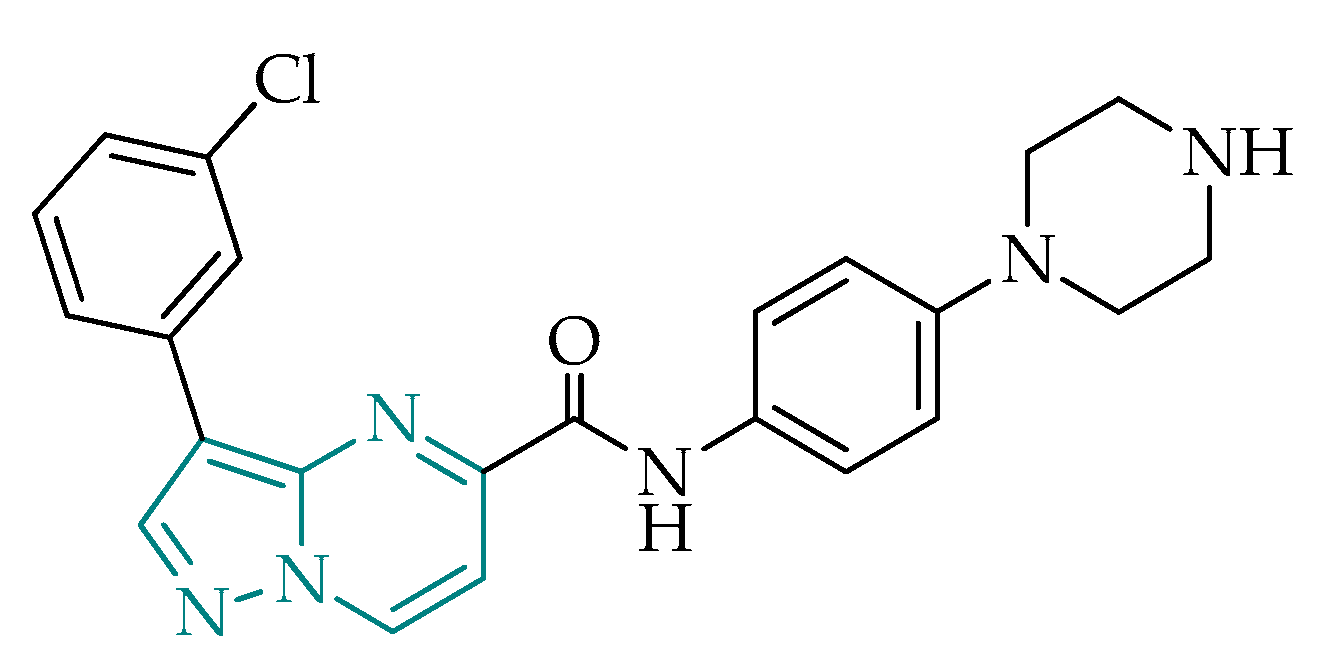
2.2.4. Pyrazolo[1,5-a]pyrimidines
2.2.5. Immunomodulatory Agents
Antimicrobial Peptides
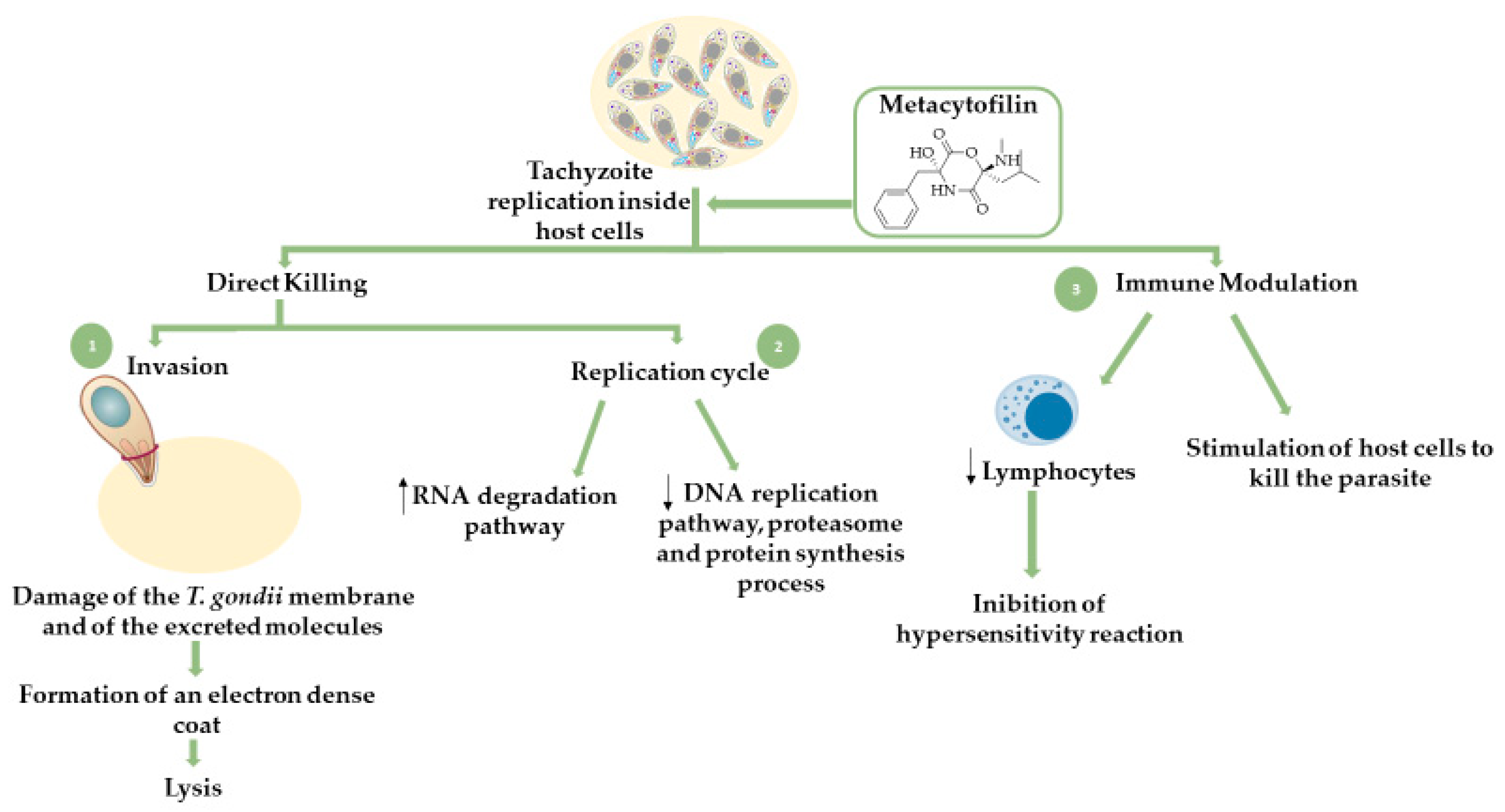
Fungal Secondary Metabolites
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Rajapakse, S.; Chrishan Shivanthan, M.; Samaranayake, N.; Rodrigo, C.; Deepika Fernando, S. Antibiotics for human toxoplasmosis: A systematic review of randomized trials. Pathog. Glob. Health 2013, 107, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Chimelli, L. A Morphological Approach to the Diagnosis of Protozoal Infections of the Central Nervous System. Patholog. Res. Int. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Seeber, F.; Steinfelder, S. Recent advances in understanding apicomplexan parasites. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef]
- Loo, C.S.N.; Lam, N.S.K.; Yu, D.; Su, X.; Lu, F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharm. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R D 2017, 17, 523–544. [Google Scholar] [CrossRef]
- Castro, P.D.J.; Dubey, J.P. Toxoplasma gondii – the facts. Companion Anim. 2019, 24, 300–305. [Google Scholar] [CrossRef]
- Pleyer, U.; Groß, U.; Schlüter, D.; Wilking, H.; Seeber, F. Toxoplasmosis in Germany—epidemiology, diagnosis, risk factors, and treatment. Dtsch. Arztebl. Int. 2019, 116, 435–444. [Google Scholar]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet. 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis, and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.M. Congenital toxoplasmosis: A review. Neonatal Netw. 2015, 34, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Saso, A.; Bamford, A.; Grewal, K.; Noori, M.; Hatcher, J.; D’arco, F.; Guy, E.; Lyall, H. Fifteen minute consultation: Management of the infant born to a mother with toxoplasmosis in pregnancy. Arch. Dis Child. Educ Pract Ed. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bay-Richter, C.; Buttenschøn, H.N.; Mors, O.; Eskelund, A.; Budac, D.; Kærlev, L.; Wegener, G. Latent toxoplasmosis and psychiatric symptoms – A role of tryptophan metabolism? J. Psychiatr. Res. 2019, 110, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chorlton, S.D. Toxoplasma gondii and schizophrenia: A review of published RCTs. Parasitol. Res. 2017, 116, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Fuglewicz, A.J.; Piotrowski, P.; Stodolak, A. Relationship between toxoplasmosis and schizophrenia: A review. Adv. Clin. Exp. Med. 2017, 26, 1033–1038. [Google Scholar] [CrossRef]
- Neville, A.J.; Zach, S.J.; Wang, X.; Larson, J.J.; Judge, A.K.; Davis, L.A.; Vennerstrom, J.L.; Davis, P.H. Clinically Available Medicines Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2015, 59, 7161–7169. [Google Scholar] [CrossRef]
- Brüne, M. Latent Toxoplasmosis: Host-parasite interaction and psychopathology. Evol. Med. Public Heal. 2019, 2019, 212–213. [Google Scholar] [CrossRef]
- Montazeri, M.; Sharif, M.; Sarvi, S.; Mehrzadi, S.; Ahmadpour, E.; Daryani, A. A systematic review of in vitro and in vivo activities of anti-Toxoplasma drugs and compounds (2006–2016). Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Wei, H.X.; Wei, S.S.; Lindsay, D.S.; Peng, H.J. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Shiojiri, D.; Kinai, E.; Teruya, K.; Kikuchi, Y.; Oka, S. Combination of clindamycin and azithromycin as alternative treatment for Toxoplasma gondii encephalitis. Emerg. Infect. Dis. 2019, 25, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, Y.A.; Read, J.S.; Byington, C.L.; Barnett, E.D.; Davies, H.D.; Edwards, K.M.; Lynfield, R.; Munoz, F.M.; Nolt, D.; Nyquist, A.C.; et al. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Kolísko, M.; Hehenberger, E.; Irwin, N.A.T.; Leander, B.S.; Kristmundsson, A.; Freeman, M.A.; Keeling, P.J. Multiple Independent Origins of Apicomplexan-Like Parasites. Curr. Biol. 2019, 29, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Amberg-Johnson, K.; Yeh, E. Host Cell Metabolism Contributes to Delayed-Death Kinetics of Apicoplast Inhibitors in Toxoplasma gondii. Antimicrob Agents Chemother. 2019, 63. [Google Scholar]
- Opsenica, D.; Radivojevic, J.; Matic, I.; Stajner, T.; Knezevic-Usaj, S.; Djurkovic-Djakovic, O.; Solaja, B. Tetraoxanes as inhibitors of apicomplexan parasites Plasmodium falciparum and Toxoplasma gondii and anti-cancer molecules. J. Serbian Chem. Soc. 2015, 80, 1339–1359. [Google Scholar] [CrossRef]
- Alday, H.; Doggett, J. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Devel. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef]
- Jain, V.; Yogavel, M.; Oshima, Y.; Kikuchi, H.; Touquet, B.; Hakimi, M.A.; Sharma, A. Structure of prolyl-tRNA synthetase-halofuginone complex provides basis for development of drugs against malaria and toxoplasmosis. Structure 2015, 23, 819–829. [Google Scholar] [CrossRef]
- Brown, C.E.; McNulty, J.; Bordón, C.; Yolken, R.; Jones-Brando, L. Enol ethers as carbonyl surrogates in a modification of the Povarov synthesis of 3-aryl quinolines and their anti-Toxoplasma activity. Org. Biomol. Chem. 2016, 14, 5951–5955. [Google Scholar] [CrossRef]
- Spalenka, J.; Escotte-Binet, S.; Bakiri, A.; Hubert, J.; Renault, J.-H.; Velard, F.; Duchateau, S.; Aubert, D.; Huguenin, A.; Villenaa, I. Discovery of New Inhibitors of Toxoplasma Gondii via the Pathogen Box. Antimicrob Agents Chemother. 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Lapinskas, P.J.; Ben-Harari, R.R. Perspective on Current and Emerging Drugs in the Treatment of Acute and Chronic Toxoplasmosis. Postgrad. Med. 2019, 131, 589–596. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of Toxoplasmosis: Current Options and Future Perspectives. Food Waterborne Parasitol. 2019, 15. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, T.; Zhai, S.Q.; Li, C.H. Recent Progress on Anti-Toxoplasma Drugs Discovery: Design, Synthesis and Screening. Eur. J. Med. Chem. 2019, 183, 111711. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R.; Dobson, M.J. The History of Antimalarial Drugs. In Antimalarial Chemotherapy; Philip, J., Rosenthal, M.D., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 15–25. [Google Scholar]
- Hofheinz, W.; Merkli, B. Quinine and Quinine Analogues. In Antimalarial Drugs II: Current Antimalarials and New Drug Developments; Peters, W., Rogers, W.H.G., Eds.; Springer: Berlin/Heidelberg, Germany; London, UK, 1984; pp. 61–81. [Google Scholar]
- O’Neill, P.M.; Bray, P.G.; Hawley, S.R.; Ward, S.A.; Park, B.K. 4-Aminoquinolines-Past, Present, and Future: A Chemical Perspective. Pharmacol. Ther. 1998, 77, 29–58. [Google Scholar] [CrossRef]
- Horta, P.; Secrieru, A.; Coninckx, A.; Cristiano, M.L.S. Quinolones for Applications in Medicinal Chemistry: Synthesis and Structure. In Targets in Heterocyclic Systems; Attanasi, O., Merino, P., Spinelli, D., Eds.; Società Chimica Italiana: Rome, Italy, 2018; pp. 260–297. [Google Scholar]
- Elgawad, H.A.; Alhusseiny, S.M.; Taman, A.; Youssef, M.Y.; Mansour, B.; Massoud, M.; Handousa, A. Biological Evaluation of Newly Synthesized Quinoline–Based Compound PPQ-8 in Acute and Chronic Toxoplasmosis: An Experimental Study. Exp. Parasitol. 2019, 206, 107756. [Google Scholar] [CrossRef]
- McConnell, E.V.; Bruzual, I.; Pou, S.; Winter, R.; Dodean, R.A.; Smilkstein, M.J.; Krollenbrock, A.; Nilsen, A.; Zakharov, L.N.; Riscoe, M.K.; et al. Targeted Structure-Activity Analysis of Endochin-like Quinolones Reveals Potent Qi and Qo Site Inhibitors of Toxoplasma gondii and Plasmodium falciparum Cytochrome bc1 and Identifies ELQ-400 as a Remarkably Effective Compound against Acute Experimental Toxoplasmosis”. ACS Infect. Dis. 2018, 4, 1574–1584. [Google Scholar] [PubMed]
- Alday, P.H.; Bruzual, I.; Nilsen, A.; Pou, S.; Winter, R.; Mamoun, C.B.; Riscoe, M.K.; Doggett, J.S. Genetic Evidence for Cytochrome b Qi Site Inhibition by 4(1H)-Quinolone-3- Diarylethers and Antimycin in Toxoplasma gondii. Antimicrob. Agents Chemother. 2017, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Doggett, J.S.; Nilsen, A.; Forquer, I.; Wegmann, K.W.; Jones-Brando, L.; Yolken, R.H.; Bordón, C.; Charman, S.A.; Katneni, K.; Schultz, T.; et al. Endochin-like Quinolones Are Highly Efficacious against Acute and Latent Experimental Toxoplasmosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15936–15941. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-L.; Cheng, X.-W.; Wu, J.-B.; Liu, M.; Zhang, F.-Z.; Xu, Z.; Feng, L.-S. Antiplasmodial and Antimalarial Activities of Quinolone Derivatives: An Overview. Eur. J. Med. Chem. 2018, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salzer, W.; Timmler, H.; Andersag, H. Über Einen Neuen, Gegen Vogelmalaria Wirksamen Verbindungstypus. Chem. Ber. 1948, 81, 12–19. [Google Scholar] [CrossRef]
- Casey, A.C. Synthesis of Some 4-Quinolones and Related Structures for Evaluation as Potential Antimalarial Agents; University of Bridgeport: Bridgeport, CT, USA, 1974. [Google Scholar]
- Winter, R.W.; Kelly, J.X.; Smilkstein, M.J.; Dodean, R.; Hinrichs, D.; Riscoe, M.K. Antimalarial Quinolones: Synthesis, Potency, and Mechanistic Studies. Exp. Parasitol. 2008, 118, 487–497. [Google Scholar] [CrossRef]
- Beteck, R.M.; Smit, F.J.; Haynes, R.K.; N’Da, D.D. Recent Progress in the Development of Anti-Malarial Quinolones. Malar. J. 2014, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Cooley, J.W.; Lee, D.-W.; Daldal, F. Across Membrane Communication between the Qo and Qi Active Sites of Cytochrome Bc1. Biochemistry 2009, 48, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Trumpower, B.L. Cytochrome Bc1 Complexes of Microorganisms. Microbiol. Rev. 1990, 54, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Sodero, A.C.R.; Abrahim-Vieira, B.; Torres, P.H.M.; Pascutti, P.G.; Garcia, C.R.S.; Ferreira, V.F.; da Rocha, D.R.; Ferreira, S.B.; Silva, F.P. Insights into Cytochrome Bc1 Complex Binding Mode of Antimalarial 2-Hydroxy-1,4-Naphthoquinones through Molecular Modelling. Mem. Inst. Oswaldo Cruz 2017, 112, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Frueh, L.; Li, Y.; Mather, M.W.; Li, Q.; Pou, S.; Nilsen, A.; Rolf, W.; Forquer, I.P.; Pershing, A.M.; Xie, L.H.; et al. Alkoxycarbonate Ester Prodrugs of Preclinical Drug Candidate ELQ-300 for Prophylaxis and Treatment of Malaria. ACS Infect. Dis. 2017, 3, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Stickles, A.M.; Ting, L.M.; Morrisey, J.M.; Li, Y.; Mather, M.W.; Meermeier, E.; Pershing, A.M.; Forquer, I.P.; Miley, G.P.; Pou, S.; et al. Inhibition of Cytochrome Bc1 as a Strategy for Single-Dose, Multi-Stage Antimalarial Therapy. Am. J. Trop. Med. Hyg. 2015, 92, 1195–1201. [Google Scholar] [CrossRef]
- Stickles, A.M.; De Almeida, M.J.; Morrisey, J.M.; Sheridan, K.A.; Forquer, I.P.; Nilsen, A.; Winter, R.W.; Burrows, J.N.; Fidock, D.A.; Vaidya, A.B.; et al. Subtle Changes in Endochin-like Quinolone Structure Alter the Site of Inhibition within the Cytochrome Bc1 Complex of Plasmodium Falciparum. Antimicrob. Agents Chemother. 2015, 59, 1977–1982. [Google Scholar] [CrossRef]
- Cowley, R.; Leung, S.; Fisher, N.; Al-Helal, M.; Berry, N.G.; Lawrenson, A.S.; Sharma, R.; Shone, A.E.; Ward, S.A.; Biagini, G.A.; et al. The Development of Quinolone Esters as Novel Antimalarial Agents Targeting the Plasmodium Falciparum Bc1 Protein Complex. Medchemcomm 2012, 3, 39–44. [Google Scholar] [CrossRef]
- Horta, P.; Kuş, N.; Henriques, M.S.C.; Paixão, J.A.; Coelho, L.; Nogueira, F.; O’Neill, P.M.; Fausto, R.; Cristiano, M.L.S. Quinolone-Hydroxyquinoline Tautomerism in Quinolone 3-Esters. Preserving the 4-Oxoquinoline Structure To Retain Antimalarial Activity. J. Org. Chem. 2015, 80, 12244–12257. [Google Scholar] [CrossRef]
- Song, Z.; Iorga, B.I.; Mounkoro, P.; Fisher, N.; Meunier, B. The Antimalarial Compound ELQ-400 Is an Unusual Inhibitor of the Bc 1 Complex, Targeting Both Q o and Q i Sites. FEBS Lett. 2018, 592, 1346–1356. [Google Scholar] [CrossRef]
- Gozalbes, R.; Brun-Pascaud, M.; Garcia-Domenech, R.; Galvez, J.; Girard, P.M.; Doucet, J.P.; Derouin, F. Anti-Toxoplasma Activities of 24 Quinolones and Fluoroquinolones in Vitro: Prediction of Activity by Molecular Topology and Virtual Computational Techniques. Antimicrob. Agents Chemother. 2000, 44, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L. Qinghaosu (Artemisinin): An Antimalarial Drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R. Artemisinin: Mechanisms of Action, Resistance and Toxicity. Int. J. Parasitol. 2002, 32, 1655–1660. [Google Scholar] [CrossRef]
- Meshnick, S.R.; Taylor, T.E.; Kamchonwongpaisan, S. Artemisinin and the Antimalarial Endoperoxides: From Herbal Remedy to Targeted Chemotherapy. Microbiol. Rev. 1996, 60, 301–315. [Google Scholar] [CrossRef]
- Cortes, S.; Albuquerque, A.; Cabral, L.I.L.; Lopes, L.; Campino, L.; Cristiano, M.L.S. In Vitro Susceptibility of Leishmania Infantum to Artemisinin Derivatives and Selected Trioxolanes. Antimicrob. Agents Chemother. 2015, 59, 5032–5035. [Google Scholar] [CrossRef]
- Lobo, L.; Cabral, L.I.L.; Sena, M.I.; Guerreiro, B.; Rodrigues, A.S.; De Andrade-Neto, V.F.; Cristiano, M.L.S.; Nogueira, F. New Endoperoxides Highly Active in Vivo and in Vitro against Artemisinin-Resistant Plasmodium Falciparum. Malar. J. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- Giannangelo, C.; Anderson, D.; Wang, X.; Vennerstrom, J.L.; Charman, S.A.; Creek, D.J. Ozonide Antimalarials Alkylate Heme in the Malaria Parasite Plasmodium Falciparum. ACS Infect. Dis. 2019, 5, 2076–2086. [Google Scholar] [CrossRef]
- Ashley, E.A.; Phyo, A.P. Drugs in Development for Malaria. Drugs 2018, 78, 861–879. [Google Scholar] [CrossRef]
- Araújo, N.C.P.; Barton, V.; Jones, M.; Stocks, P.A.; Ward, S.A.; Davies, J.; Bray, P.G.; Shone, A.E.; Cristiano, M.L.S.; O’Neill, P.M. Semi-Synthetic and Synthetic 1,2,4-Trioxaquines and 1,2,4-Trioxolaquines: Synthesis, Preliminary SAR and Comparison with Acridine Endoperoxide Conjugates. Bioorganic Med. Chem. Lett. 2009, 19, 2038–2043. [Google Scholar] [CrossRef]
- Schultz, T.L.; Hencken, C.P.; Woodard, L.E.; Posner, G.H.; Yolken, R.H.; Jones-Brando, L.; Carruthers, V.B. A Thiazole Derivative of Artemisinin Moderately Reduces Toxoplasma Gondii Cyst Burden in Infected Mice. J. Parasitol. 2014, 100, 516–521. [Google Scholar] [CrossRef]
- Deng, H.; Huang, X.; Jin, C.; Jin, C.M.; Quan, Z.S. Synthesis, in Vitro and in Vivo Biological Evaluation of Dihydroartemisinin Derivatives with Potential Anti-Toxoplasma Gondii Agents. Bioorg. Chem. 2019, 94, 103467. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Wing, C.C.; Haynes, R.K.; Sibley, L.D. Artemisone and Artemiside Control Acute and Reactivated Toxoplasmosis in a Murine Model. Antimicrob. Agents Chemother. 2009, 53, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, J.G.; Bordón, C.; Posner, G.H.; Yolken, R.; Jones-Brando, L. Artemisinin Derivatives Inhibit Toxoplasma Gondii in Vitro at Multiple Steps in the Lytic Cycle. J. Antimicrob. Chemother. 2009, 63, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, K.; Krug, E.C.; Marr, J.J.; Berens, R.L. Inhibition of Growth of Toxoplasma Gondii by Qinghaosu and Derivatives. Antimicrob. Agents Chemother. 1990, 34, 1961–1965. [Google Scholar]
- Mahmoud, D.M.; Mahmoud, M.S.; Ezz-el-din, H.M.; Abo-zahra, F.A. Artesunate Effect on RH Virulent and ME49 Non-Virulent Strains of Toxoplasma Gondii: In Vitro and in Vivo Experimental Studies. Sci. Parasitol. 2016, 17, 83–92. [Google Scholar]
- Haynes, R.K.; Krishna, S. Artemisinins: Activities and Actions. Microbes Infect. 2004, 6, 1339–1346. [Google Scholar] [CrossRef]
- Nagamune, K.; Moreno, S.N.J.; Sibley, L.D. Artemisinin-Resistant Mutants of Toxoplasma Gondii Have Altered Calcium Homeostasis. Antimicrob. Agents Chemother. 2007, 51, 3816–3823. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Barton, V.E.; Ward, S.A. The Molecular Mechanism of Action of Artemisinin - The Debate Continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef]
- Secrieru, A.; O’Neill, P.M.; Cristiano, M.L.S. Revisiting the Structure and Chemistry of 3(5)-Substituted Pyrazoles. Molecules 2020, 25, 42. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Smith, P.W. Perspective: Challenges and Opportunities in TB Drug Discovery from Phenotypic Screening. Bioorganic Med. Chem. 2015, 23, 5087–5097. [Google Scholar] [CrossRef]
- Candice, S.D.M.; Feng, T.S.; Van Der Westhuyzen, R.; Gessner, R.K.; Street, L.J.; Morgans, G.L.; Warner, D.F.; Moosa, A.; Naran, K.; Lawrence, N.; et al. Aminopyrazolo[1,5-a]Pyrimidines as Potential Inhibitors of Mycobacterium Tuberculosis: Structure Activity Relationships and ADME Characterization. Bioorganic Med. Chem. 2015, 23, 7240–7250. [Google Scholar]
- Pinheiro, L.C.S.; Feitosa, L.M.; Gandi, M.O.; Silveira, F.F.; Boechat, N. The Development of Novel Compounds against Malaria: Quinolines, Triazolpyridines, Pyrazolopyridines and Pyrazolopyrimidines. Molecules 2019, 24, 4095. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, L.F.S.P.; Coutinho, J.P.; Jabor, V.A.P.; Feliciano, P.R.; Nonato, M.C.; Kaiser, C.R.; Menezes, C.M.S.; Hammes, A.S.O.; Caffarena, E.R.; Hoelz, L.V.B.; et al. Evaluation of 7-Arylaminopyrazolo[1,5-a]Pyrimidines as Anti-Plasmodium Falciparum, Antimalarial, and Pf-Dihydroorotate Dehydrogenase Inhibitors. Eur. J. Med. Chem. 2017, 126, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, E.; Urdaneta, N.; Herrera, J.C. In Vitro Anti-Leishmanial and Anti-Trypanosomal Activity of Hydrazones, Pyrazoles, Pyrazolo[1,5-a]Pyrimidines and Pyrazolo[3,4-b]Pyridine, Synthesized from 6-Substituted-3-Formylchromones. In Proceedings of the the 2nd International Electronic Conference on Medical Chemistry, Chemistry Department, Simón Bolívar University, Caracas, Venezuela, 1–30 November 2016; Available online: https://pdfs.semanticscholar.org/d983/af79e74b52e542975cbdc37f43949bb04814.pdf (accessed on 15 March 2020).
- Duffy, S.; Sykes, M.L.; Jones, A.J.; Shelper, T.B.; Simpson, M.; Lang, R.; Poulsen, S.A.; Sleebs, B.E.; Avery, V.M. Screening the Medicines for Malaria Venture Pathogen Box across Multiple Pathogens Reclassifies Starting Points for Open-Source Drug Discovery. Antimicrob. Agents Chemother. 2017, 61, 1–22. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Molecular Biology. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where Do We Stand Now? Protein Pept. Lett. 2019, 27, 120–134. [Google Scholar] [CrossRef]
- Sabiá Júnior, E.F.; Menezes, L.F.S.; de Araújo, I.F.S.; Schwartz, E.F. Natural Occurrence in Venomous Arthropods of Antimicrobial Peptides Active against Protozoan Parasites. Toxins 2019, 11, 563. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune Modulation by Multifaceted Cationic Host Defense (Antimicrobial) Peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Fang, Y.; He, X.; Zhang, P.; Shen, C.; Mwangi, J.; Xu, C.; Mo, G.; Lai, R.; Zhang, Z. In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin. Toxins 2019, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Aguiar, L.; Gomes, P. Antimicrobial Peptides: A New Class of Antimalarial Drugs? Front. Pharmacol. 2014, 5, 275. [Google Scholar] [CrossRef] [PubMed]
- Mirski, T.; Niemcewicz, M.; Bartoszcze, M.; Gryko, R.; Michalski, A. Utilisation of Peptides against Microbial Infections – a Review. Ann. Agric. Environ. Med. 2018, 25, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.W.; Ke, H. Novel Defense Peptides from Platelets Kill Malaria Parasites. Trends Parasitol. 2018, 34, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Pulido, D.; Rivas, L.; Andreu, D. Antimicrobial Peptide Action on Parasites. Curr. Drug Targets 2012, 13, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, J.; Salzet, M. Antimicrobial Peptides versus Parasitic Infections? Trends Parasitol. 2002, 18, 475–476. [Google Scholar] [CrossRef]
- Rautenbach, M.; Vlok, N.M.; Stander, M.; Hoppe, H.C. Inhibition of Malaria Parasite Blood Stages by Tyrocidines, Membrane-Active Cyclic Peptide Antibiotics from Bacillus Brevis. Biochim. Biophys. Acta - Biomembr. 2007, 1768, 1488–1497. [Google Scholar] [CrossRef]
- Conde, R.; Zamudio, F.Z.; Rodr, M.H. Scorpine, an Anti-Malaria and Anti-Bacterial Agent Purified from Scorpion Venom. FEBS Lett. 2001, 471, 165–168. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Tullio, V.; Giribaldi, G. Human and Mosquito Lysozymes: Old Molecules for New Approaches against Malaria. In Human and Mosquito Lysozymes; Prato, M., Ed.; Springer: Cham, Switzerland; Heidelber, Germany, 2015; pp. 91–101. [Google Scholar]
- Giovati, L.; Ciociola, T.; Magliani, W.; Conti, S. Antimicrobial Peptides with Antiprotozoal Activity: Current State and Future Perspectives. Future Med. Chem. 2018, 10, 2569–2572. [Google Scholar] [CrossRef]
- Tonk, M.; Pierrot, C.; Cabezas-Cruz, A.; Rahnamaeian, M.; Khalife, J.; Vilcinskas, A. The Drosophila Melanogaster Antimicrobial Peptides Mtk-1 and Mtk-2 Are Active against the Malarial Parasite Plasmodium Falciparum. Parasitol. Res. 2019, 118, 1993–1998. [Google Scholar] [CrossRef]
- Couto, J.; Tonk, M.; Ferrolho, J.; Antunes, S.; Vilcinskas, A.; de la Fuente, J.; Domingos, A.; Cabezas-Cruz, A. Antiplasmodial Activity of Tick Defensins in a Mouse Model of Malaria. Ticks Tick. Borne. Dis. 2018, 9, 844–849. [Google Scholar] [CrossRef] [PubMed]
- De León-Nava, M.A.; Romero-Núñez, E.; Luna-Nophal, A.; Bernáldez-Sarabia, J.; Sánchez-Campos, L.N.; Licea-Navarro, A.F.; Morales-Montor, J.; Muñiz-Hernández, S. In Vitro Effect of the Synthetic Cal14.1a Conotoxin, Derived from Conus Californicus, on the Human Parasite Toxoplasma Gondii. Mar. Drugs 2016, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Rahman, M.M.; Battur, B.; Boldbaatar, D.; Liao, M.; Umemiya-Shirafuji, R.; Xuan, X.; Fujisaki, K. Parasiticidal Activity of Human α-Defensin-5 against Toxoplasma Gondii. Vitr. Cell. Dev. Biol. Anim. 2010, 46, 560–565. [Google Scholar] [CrossRef]
- Tanaka, T.; Maeda, H.; Matsuo, T.; Boldbattar, D.; Umemiya-Shirafuji, R.; Kume, A.; Suzuki, H.; Xuan, X.; Tsuji, N.; Fujisaki, K. Parasiticidal Activity of Haemaphysalis Longicornis Longicin P4 Peptide against Toxoplasma Gondii. Peptides 2012, 34, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Seeber, F. An Enzyme-Release Assay for the Assessment of the Lytic Activities of Complement or Antimicrobial Peptides on Extracellular Toxoplasma Gondii. J. Microbiol. Methods 2000, 39, 189–196. [Google Scholar] [CrossRef]
- Giovati, L.; Santinoli, C.; Mangia, C.; Vismarra, A.; Belletti, S.; D’Adda, T.; Fumarola, C.; Ciociola, T.; Bacci, C.; Magliani, W.; et al. Novel Activity of a Synthetic Decapeptide against Toxoplasma Gondii Tachyzoites. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Khaleghi Rostamkolaie, L.; Hamidinejat, H.; Razi Jalali, M.H.; Jafari, H.; Najafzadeh Varzi, H.; Seifi Abadshapouri, M.R. In Vitro Therapeutic Effect of Hemiscorpius Lepturus Venom on Tachyzoites of Toxoplasma Gondii. J. Parasit. Dis. 2019, 43, 472–478. [Google Scholar] [CrossRef]
- Liu, R.; Ni, Y.; Song, J.; Xu, Z.; Qiu, J.; Wang, L.; Zhu, Y.; Huang, Y.; Ji, M.; Chen, Y. Research on the Effect and Mechanism of Antimicrobial Peptides HPRP-A1/A2 Work against Toxoplasma Gondii Infection. Parasite Immunol. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Y.; Tang, Y.; Wu, M.; Guan, J.; Li, X.; Wang, Z.; Jiang, J.; Deng, M.; Duan, Z.; et al. Anti-Toxoplasma Gondii Effect of Two Spider Venoms in Vitro and in Vivo. Toxicon 2019, 166, 9–14. [Google Scholar] [CrossRef]
- Lee, Y.; Yamada, H.; Pradipta, A.; Ma, J.S.; Okamoto, M.; Nagaoka, H.; Takashima, E.; Standley, D.M.; Sasai, M.; Takei, K.; et al. Initial Phospholipid-Dependent Irgb6 Targeting to Toxoplasma Gondii Vacuoles Mediates Host Defense. Life Sci. Alliance 2020, 3, 1–16. [Google Scholar] [CrossRef]
- Tang, Y.; Hou, S.; Li, X.; Wu, M.; Ma, B.; Wang, Z.; Jiang, J.; Deng, M.; Duan, Z.; Tang, X.; et al. Anti-Parasitic Effect on Toxoplasma Gondii Induced by a Spider Peptide Lycosin-I. Exp. Parasitol. 2019, 198, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xue, Y.; Li, X.; Li, J.; Zhao, Z.; Quan, C.; Gao, W.; Zu, X.; Bai, X.; Feng, S. Fungi-Derived Lipopeptide Antibiotics Developed since 2000. Peptides 2019, 113, 52–65. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Dzodic, P.; Pavlovic, V.; Cherneva, E.; Yancheva, D. Cyclodidepsipeptides with a Promising Scaffold in Medicinal Chemistry. Amino Acids. 2014, 46, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Leesombun, A.; Iijima, M.; Umeda, K.; Kondoh, D.; Pagmadulam, B.; Abdou, A.M.; Suzuki, Y.; Ohba, S.; Isshiki, K.; Kimura, T.; et al. Metacytofilin Is a Potent Therapeutic Drug Candidate for Toxoplasmosis. J. Infect. Dis. 2019, 221, 766–774. [Google Scholar] [CrossRef]
- Donzelli, B.G.G.; Krasnoff, S.B. Molecular Genetics of Secondary Chemistry in Metarhizium Fungi. In Advances in Genetics; Brian., L., Raymond, S.L., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 94, pp. 365–436. [Google Scholar]
- Iijima, M.; Masuda, T.; Ishizuka, M.; Takeuchi, T.; Nakamura, H.; Naganawa, H.; Kurasawa, S.; Okami, Y.; Iitaka, Y. Metacytofilin, a Novel Immunomodulator Produced by Metarhizium Sp. TA2759. J. Antibiot. 1992, 45, 1553–1556. [Google Scholar] [CrossRef] [PubMed]



| Type of Disease | Front-line Treatment | Alternatives |
|---|---|---|
| Congenital toxoplasmosis | Pyrimethamine + Sulfadiazine + Folinic acid | Spiramycin for prophilaxis |
| Cerebral toxoplasmosis | Co-trimoxazole or Atovaquone | |
| Retinocoroiditis | Prednisone as coadjuvant; Clindamycin; Co-trimoxazole; Atovaquone |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secrieru, A.; Costa, I.C.C.; O'Neill, P.M.; Cristiano, M.L.S. Antimalarial Agents as Therapeutic Tools Against Toxoplasmosis—A Short Bridge between Two Distant Illnesses. Molecules 2020, 25, 1574. https://doi.org/10.3390/molecules25071574
Secrieru A, Costa ICC, O'Neill PM, Cristiano MLS. Antimalarial Agents as Therapeutic Tools Against Toxoplasmosis—A Short Bridge between Two Distant Illnesses. Molecules. 2020; 25(7):1574. https://doi.org/10.3390/molecules25071574
Chicago/Turabian StyleSecrieru, Alina, Inês C. C. Costa, Paul M. O'Neill, and Maria L. S. Cristiano. 2020. "Antimalarial Agents as Therapeutic Tools Against Toxoplasmosis—A Short Bridge between Two Distant Illnesses" Molecules 25, no. 7: 1574. https://doi.org/10.3390/molecules25071574
APA StyleSecrieru, A., Costa, I. C. C., O'Neill, P. M., & Cristiano, M. L. S. (2020). Antimalarial Agents as Therapeutic Tools Against Toxoplasmosis—A Short Bridge between Two Distant Illnesses. Molecules, 25(7), 1574. https://doi.org/10.3390/molecules25071574







