A Simple Iron-Catalyst for Alkenylation of Ketones Using Primary Alcohols
Abstract
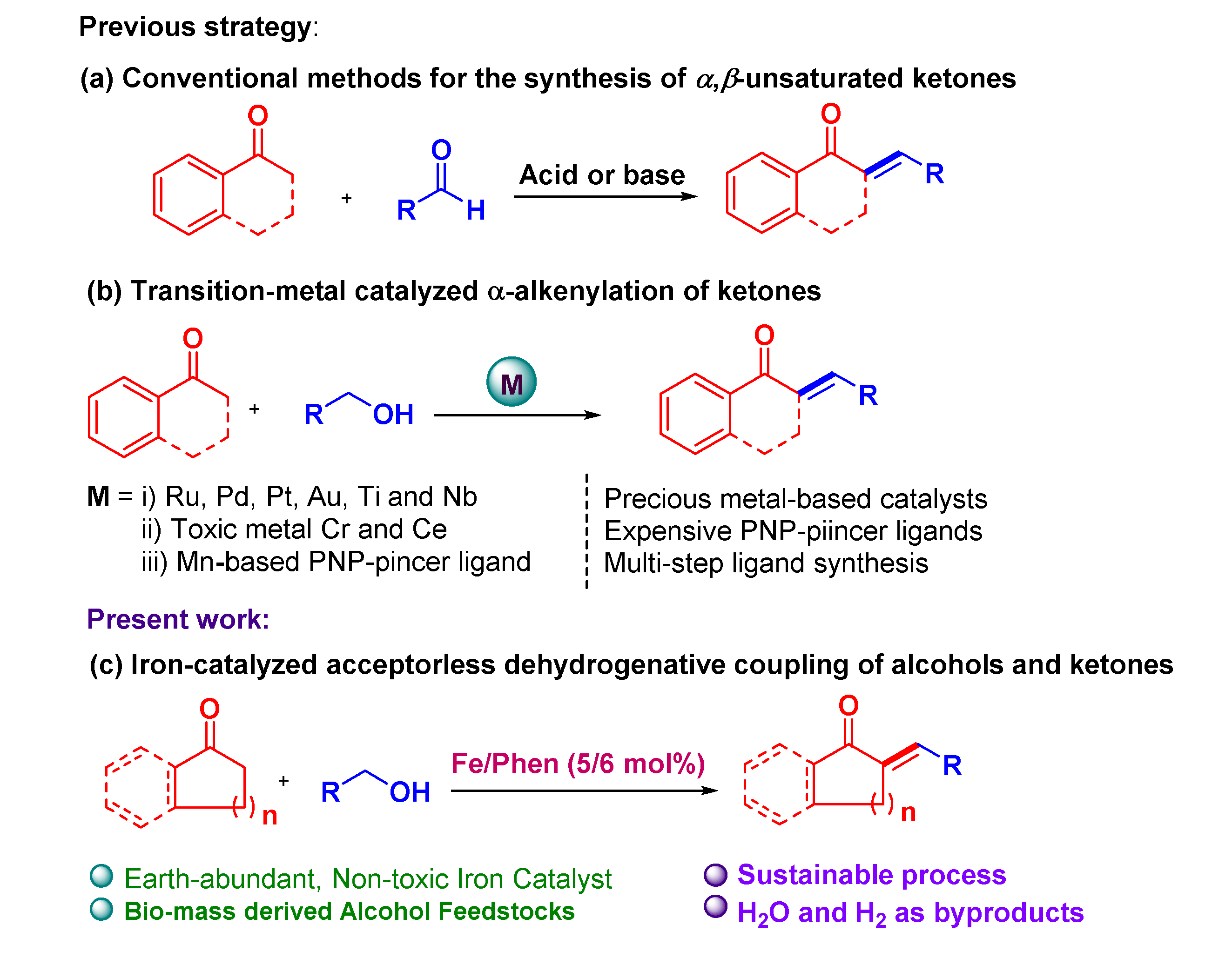
1. Introduction
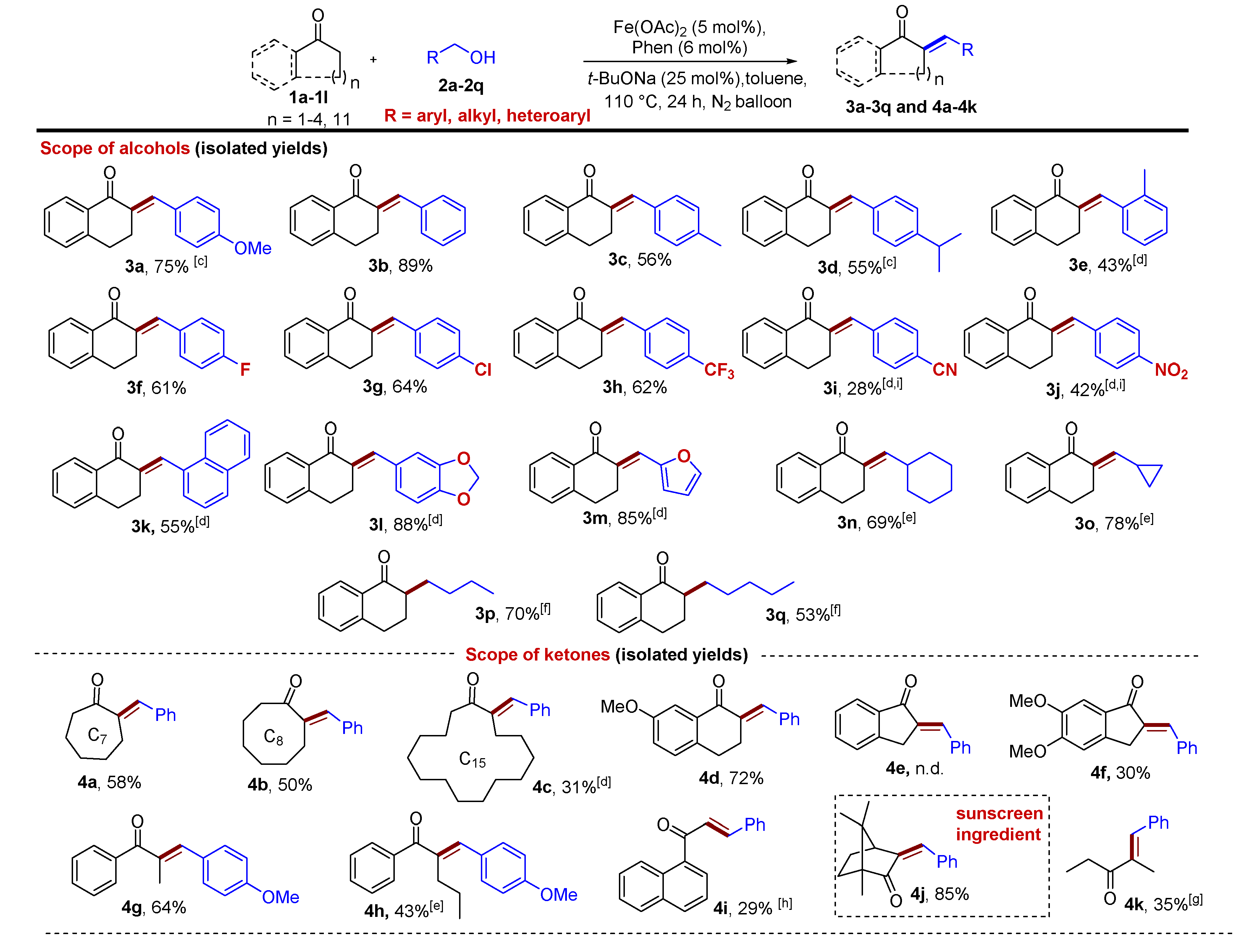
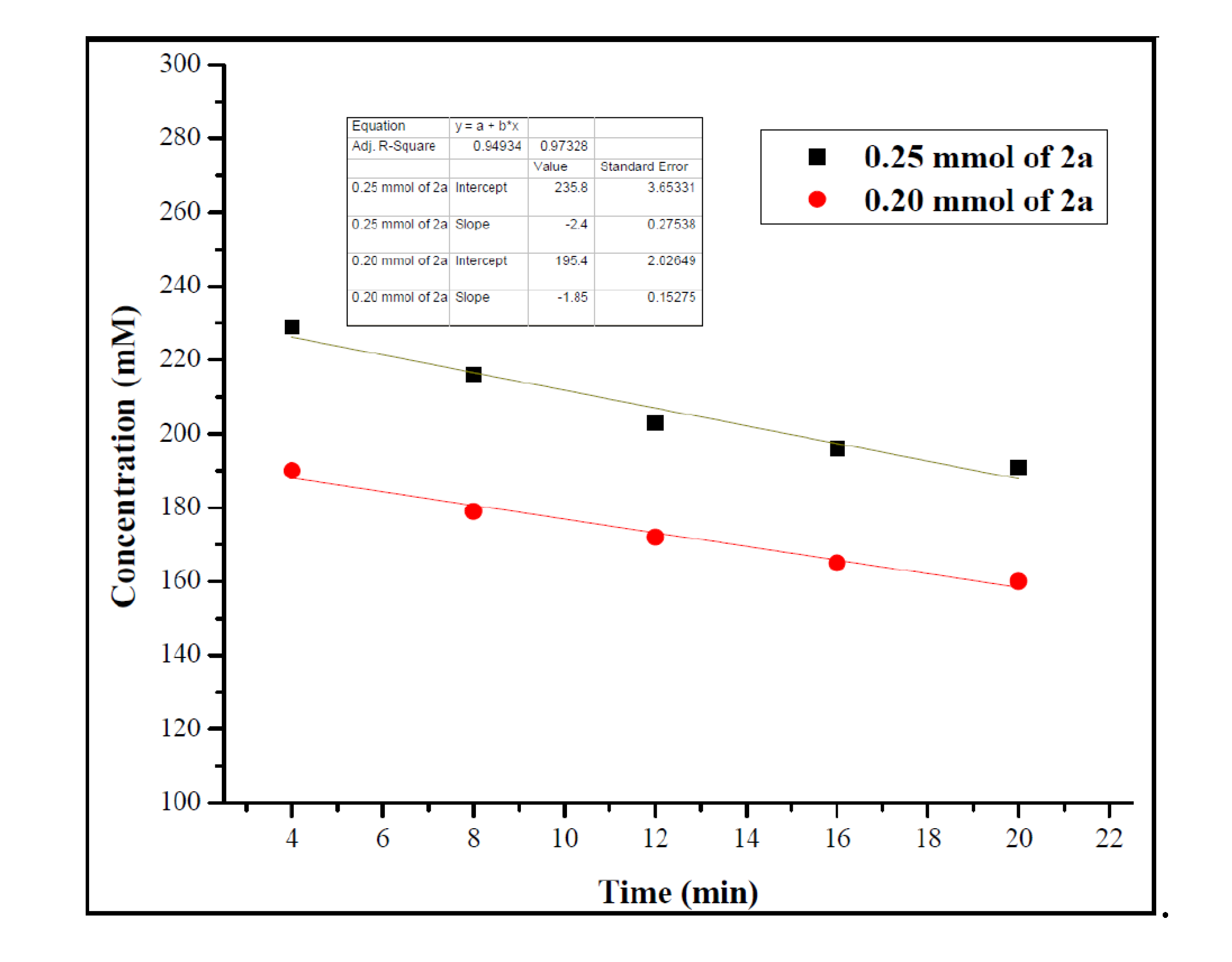
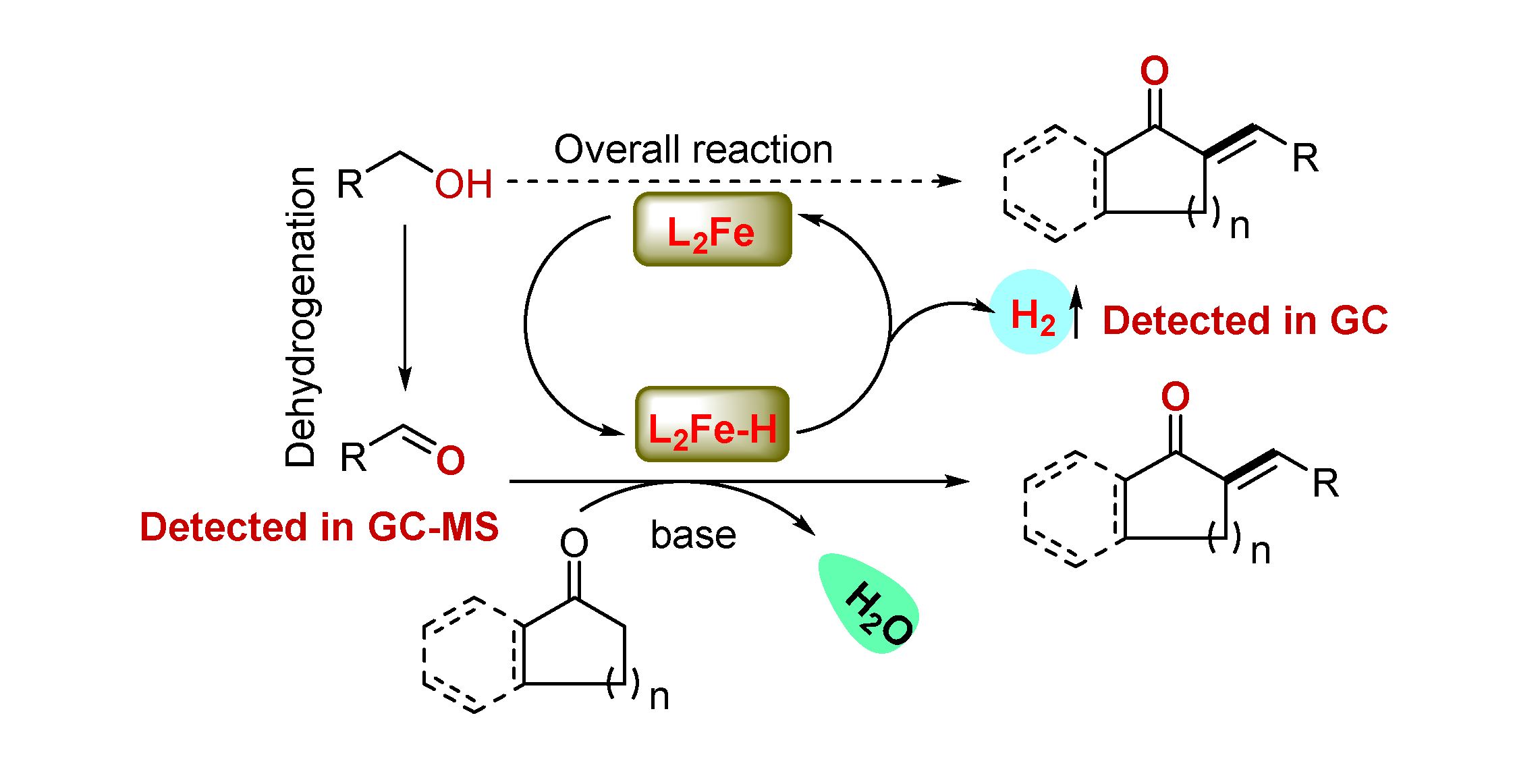
2. Results and Discussions
3. Materials and Methods
3.1. General Experimental Details
3.2. General Procedure for Iron-Catalyzed Alkenylation of Ketones with Alcohols:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S.; Velty, A. Activated Hydrotalcites as Catalysts for the Synthesis of Chalcones of Pharmaceutical Interest. J. Catal. 2004, 221, 474–482. [Google Scholar] [CrossRef]
- Li, R.; Kenyon, G.L.; Cohen, F.E.; Chen, X.; Gong, B.; Dominguez, J.N.; Davidson, E.; Kurzban, G.; Miller, R.E.; Nuzman, E.O. Vitro Antimalarial Activity of Chalcones and Their Derivatives. J. Med. Chem. 1995, 38, 5031–5037. [Google Scholar] [CrossRef]
- Dhar, D.N. Chemistry of Chalcones and Related Compounds; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Harbone, J.B.; Mabry, T.J. The Flavonoids: Advances in Research; Chapman & Hall: New York, NY, USA, 1982. [Google Scholar]
- Micheli, F.; Degiorgis, F.; Feriani, A.; Paio, A.; Pozzan, A.; Zarantonello, P.; Seneci, P.A. Combinatorial Approach to [1,5]- Benzothiazepine Derivatives as Potential Antibacterial Agents. J. Comb. Chem. 2001, 3, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Sinisterra, J.V.; Garcia-Raso, J.V.; Cabello, J.A.; Marinas, J.M. An Improved Procedure for the Claisen-Schmidt Reaction. Synthesis 1984, 1984, 502–504. [Google Scholar] [CrossRef]
- Crabtree, R.H. Homogeneous Transition Metal Catalysis of Acceptorless Dehydrogenative Alcohol Oxidation: Applications in Hydrogen Storage and to Heterocycle Synthesis. Chem. Rev. 2017, 117, 9228–9246. [Google Scholar] [CrossRef]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. 3d-Metal Catalyzed N- and C--Alkylation Reactions via Borrowing Hydrogen or Hydrogen Autotransfer. Chem. Rev. 2019, 119, 2524–2549. [Google Scholar] [CrossRef]
- Yang, Q.; Wanga, Q.; Yu, Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. [Google Scholar] [CrossRef]
- Reed-Berendt, B.G.; Polidano, K.; Morrill, L.C. Recent Advances in Homogeneous Borrowing Hydrogen Catalysis using Earth-abundant First Row Transition Metals. Org. Biomol. Chem. 2019, 17, 1595–1607. [Google Scholar] [CrossRef]
- Barta, K.; Ford, P.C. Catalytic Conversion of Nonfood Woody Biomass Solids to Organic Liquids. Acc. Chem. Res. 2014, 47, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Vispute, T.P.; Zhang, H.; Sanna, A.; Xiao, R.; Huber, G.W. Renewable Chemical Commodity Feedstocks from Integrated Catalytic Processing of Pyrolysis Oils. Science 2010, 330, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Ramón, D.J.; Yus, M. Easy α-Alkylation of Ketones with Alcohols Through a Hydrogen Autotransfer Process Catalyzed by RuCl2(DMSO)4. Tetrahedron 2006, 62, 8988–9001. [Google Scholar] [CrossRef]
- Kwon, M.S.; Kim, N.; Seo, S.H.; Park, I.S.; Cheedrala, R.K.; Park, J. Recyclable Palladium Catalyst for Highly Selective α-Alkylation of Ketones with Alcohols. Angew. Chem. 2005, 117, 7073–7075. [Google Scholar] [CrossRef]
- Jana, S.K.; Kubota, Y.; Tatsumi, T. Selective α-Alkylation of Ketones with Alcohols Catalyzed by Highly Active Mesoporous Pd/MgO-Al2O3 Type Basic Solid Derived from Pd-Supported Mg Al-Hydrotalcite Stud. Surf. Sci. Catal. 2007, 165, 701–704. [Google Scholar]
- Chaudhari, C.; Siddiki, S.M.A.H.; Kon, K.; Tomita, A.; Tai, Y.; Shimizu, K. C-3 Alkylation of Oxindole with Alcohols by Pt/CeO2 Catalyst in Additive-Free Conditions. Catal. Sci. Technol. 2014, 4, 1064–1069. [Google Scholar] [CrossRef]
- Kim, S.; Bae, S.W.; Lee, J.S.; Park, J. Recyclable Gold Nanoparticle Catalyst for the Aerobic Alcohol Oxidation and C−C Bond Forming Reaction Between Primary Alcohols and Ketones Under Ambient Conditions. Tetrahedron 2009, 65, 1461–1466. [Google Scholar] [CrossRef]
- Fischer, A.; Makowski, P.; Müller, J.-O.; Antonietti, M.; Thomas, A.; Goettmann, F. High Surface Area TiO2 and TiN as Catalysts for the C−C Coupling of Alcohols and Ketones. Chem Sus Chem 2008, 1, 444–449. [Google Scholar] [CrossRef]
- Yao, W.; Makowski, P.; Giordano, C.; Goettmann, F. Synthesis of Early-Transition-Metal Carbide and Nitride Nanoparticles Through the Urea Route anTheir Use as Alkylation Catalysts. Chem.-Eur. J. 2009, 15, 11999–12004. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D. Novel and Efficient One Pot Condensation Reactions between Ketones and Aromatic Alcohols in the Presence of CrO3 Producing α, β-Unsaturated Carbonyl Compounds. Chin. J. Chem. 2011, 29, 2086–2090. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wang, M.; Lu, J.; Zhang, C.; Li, L.; Jiang, J.; Wang, F. The Cascade Synthesis of α, β-Unsaturated Ketones via Oxidative C−C Coupling of Ketones and Primary Alcohols Over a Ceria Catalyst. Catal. Sci. Technol. 2016, 6, 1693–1700. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Ma, J.; Yu, X.; Wang, Y.; Li, J. Preparation of a Bi2WO6 catalyst and its catalytic performance in an alpha alkylation reaction under visible light irradiation. Mol. Catal. 2019, 466, 157–166. [Google Scholar] [CrossRef]
- Zou, H.; Daib, J.; Wang, R. Encapsulating mesoporous metal nanoparticles: Towards a highly active and stable nanoreactor for oxidative coupling reactions in water. Chem. Commun. 2019, 55, 5898–5901. [Google Scholar] [CrossRef] [PubMed]
- Gawali, S.S.; Pandia, B.K.; Gunanathan, C. Manganese (I)-Catalyzed α--Alkenylation of Ketones Using Primary Alcohols. Org. Lett. 2019, 21, 3842–3847. [Google Scholar] [CrossRef]
- Das, J.; Singh, K.; Vellakkaran, M.; Banerjee, D. Nickel-Catalyzed Hydrogen-Borrowing Strategy for α-Alkylation of Ketones with Alcohols: A New Route to Branched gem-Bis(alkyl) Ketones. Org. Lett. 2018, 20, 5587–5591. [Google Scholar] [CrossRef]
- Das, J.; Vellakkaran, M.; Banerjee, D. Nickel-Catalyzed Alkylation of Ketone Enolates: Synthesis of Monoselective Linear Ketones. J. Org. Chem. 2019, 84, 769–779. [Google Scholar] [CrossRef]
- Das, J.; Vellakkaran, M.; Banerjee, D. Nickel-catalysed direct α-olefination of alkyl substituted N-heteroarenes with alcohols. Chem. Commun. 2019, 55, 7530–7533. [Google Scholar] [CrossRef]
- Kabadwal, L.M.; Das, J.; Banerjee, D. Mn(II)-catalysed alkylation of methylene ketones with alcohols: Direct access to functionalised branched products. Chem. Commun. 2018, 54, 14069–14072. [Google Scholar] [CrossRef]
- Das, J.; Vellakkaran, M.; Sk, M.; Banerjee, D. Iron-Catalyzed Coupling of Methyl N-Heteroarenes with Primary Alcohols: Direct Access to E-Selective Olefins. Org. Lett. 2019, 21, 7514–7518. [Google Scholar] [CrossRef]
- Alanthadka, A.; Bera, S.; Banerjee, D. Iron-Catalyzed Ligand Free α-Alkylation of Methylene Ketones and β-Alkylation of Secondary Alcohols Using Primary Alcohols. J. Org. Chem. 2019, 84, 11676–11686. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.H. Exploiting Metal−Ligand Bifunctional Reactions in the Design of Iron Asymmetric Hydrogenation Catalysts. Acc. Chem. Res. 2015, 48, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Chirik, P.J. Iron- and Cobalt-Catalyzed Alkene Hydrogenation: Catalysis with Both Redox-Active and Strong Field Ligands. Acc. Chem. Res. 2015, 48, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Knolker, H.J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Putten, R.V.; Hensen, E.J.M.; Pidko, E.A. Catalytic (de)hydrogenation promoted by non-precious metals–Co, Fe and Mn: Recent advances in an emerging field. Chem. Soc. Rev. 2018, 47, 1459–1483. [Google Scholar] [CrossRef]
- Wei, D.; Darcel, C. Iron Catalysis in Reduction and Hydrometalation Reactions. Chem. Rev. 2019, 119, 2550–2610. [Google Scholar] [CrossRef]
- Balaraman, E.; Nandakumar, A.; Jaiswalab, G.; Sahoo, M.K. Iron-catalyzed dehydrogenation reactions and their applications in sustainable energy and catalysis. Catal. Sci. Technol. 2017, 7, 3177–3195. [Google Scholar] [CrossRef]
- Wei, D.; Netkaew, C.; Darcel, C. Multi-Step Reactions Involving Iron-Catalysed Reduction and Hydrogen Borrowing Reactions. Eur. J. Inorg. Chem. 2019, 2471–2487. [Google Scholar] [CrossRef]
- Kessler, S.N.; Bäckvall, J.E. Iron-Catalyzed Cross-Coupling of Propargyl Carboxylates and Grignard Reagents: Synthesis of Substituted Allenes. Angew. Chem. Int. Ed. 2016, 55, 3734–3738. [Google Scholar] [CrossRef]
- Fürstner, A.; Leitner, A.; Mendez, M.; Krause, H. Iron-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc. 2002, 124, 13856–13863. [Google Scholar] [CrossRef]
- Sui-Seng, C.; Freutel, F.; Lough, A.J.; Morris, R.H. Highly Efficient Catalyst Systems Using Iron Complexes with a Tetradentate PNNP Ligand for the Asymmetric Hydrogenation of Polar Bonds. Angew. Chem. Int. Ed. 2008, 47, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Lough, A.J.; Li, Y.F.; Morris, R.H. Amine (imine)-diphosphine Iron Catalysts for Asymmetric Transfer Hydrogenation of Ketones and Imines. Science 2013, 342, 1080–1083. [Google Scholar] [CrossRef]
- Kessler, S.N.; Hundemer, F.; Bäckvall, J.E. A Synthesis of Substituted α-Allenols via Iron-Catalyzed Cross-Coupling of Propargyl Carboxylates with Grignard Reagents. 2016, 6, 7448−7451. ACS Catal. 2016, 6, 7448–7451. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Feringa, B.L.; Barta, K. Iron Catalysed Direct Alkylation of Amines with Alcohols. Nat. Commun. 2014, 5, 5602. [Google Scholar] [CrossRef]
- Polidano, K.; Allen, B.D.W.; Williams, J.M.J.; Morrill, L.C. Iron- Catalyzed Methylation Using the Borrowing Hydrogen Approach. ACS Catal. 2018, 8, 6440–6445. [Google Scholar] [CrossRef]
- Brown, T.J.; Cumbes, M.; Diorazio, L.J.; Clarkson, G.J.; Wills, M. Use of (Cyclopentadienone)iron Tricarbonyl Complexes for C−N Bond Formation Reactions between Amines and Alcohols. J. Org. Chem. 2017, 82, 10489–10503. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lagaditis, P.O.; Förster, M.; Bielinski, A.E.; Hazari, N.; Holthausen, M.C.; Jones, W.D.; Schneider, S. Well-Defined Iron Catalysts for the Acceptorless Reversible dehydrogenation-Hydrogenation of Alcohols and Ketones. ACS Catal. 2014, 4, 3994–4003. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Enthaler, S.; Junge, K.; Beller, M. Iron-Catalyzed Enantioselective Hydrosilylation of Ketones. Angew. Chem. Int. Ed. 2008, 47, 2497–2501. [Google Scholar] [CrossRef]
- Jiang, F.; Bézier, D.; Sortais, J.-B.; Darcel, C. N-Heterocyclic Carbene Piano-Stool Iron Complexes as Efficient Catalysts for Hydrosilylation of Carbonyl Derivatives. Adv. Synth. Catal. 2011, 353, 239–244. [Google Scholar] [CrossRef]
- Tondreau, A.M.; Atienza, C.C.H.; Weller, K.J.; Nye, S.A.; Lewis, K.M.; Delis, J.G.; Chirik, P.J. Iron Catalysts for Selective Anti-Markovnikov Alkene Hydrosilylation Using Tertiary Silanes. Science 2012, 335, 567–570. [Google Scholar] [CrossRef]
- Gorgas, N.; Alves, L.G.; Stoger, B.; Martins, A.M.; Veiros, L.F.; Kirchner, K. Stable, Yet Highly Reactive Nonclassical Iron (II) Polyhydride Pincer Complexes: Z-Selective Dimerization and Hydroboration of Terminal Alkynes. J. Am. Chem. Soc. 2017, 139, 8130–8133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Song, H.; Zhuang, X.; Tung, C.-H.; Wang, W. Iron-Catalyzed 1,2-Selective Hydroboration of N-Heteroarenes. J. Am. Chem. Soc. 2017, 139, 17775–17778. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, L.; Seck, C.; Mbaye, M.D.; Gaillard, S.; Renaud, J.L. Iron-Catalyzed Tandem Three-Component Alkylation: Access to α-Methylated Substituted Ketones. Org. Lett. 2019, 21, 3057–3061. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Sortais, J.B.; Beller, M.; Darcel, C. Iron-Catalyzed α-Alkylation of Ketones with Alcohols. Angew. Chem. Int. Ed. 2015, 54, 14483–14486. [Google Scholar] [CrossRef] [PubMed]
- Seck, C.; Mbaye, M.D.; Coufourier, S.; Lator, A.; Lohier, J.-F.; Poater, A.; Ward, T.R.; Gaillard, S.; Renaud, J.-L. Alkylation of Ketones Catalyzed by Bifunctional Iron Complexes: From Mechanistic Understanding to Application. ChemCatChem 2017, 9, 4410–4416. [Google Scholar] [CrossRef]
- Polidano, K.; Williams, M.J.J.; Morrill, L.C. Iron-Catalyzed Borrowing Hydrogen β—C (sp3) -Methylation of Alcohols. ACS Catal. 2019, 9, 8575–8580. [Google Scholar] [CrossRef]
- Dambatta, M.B.; Polidano, K.; Northey, A.D.; Williams, M.J.J.; Morrill, L.C. Iron-Catalyzed Borrowing Hydrogen C-Alkylation of Oxindoles with Alcohols. ChemSusChem 2019, 12, 2345–2349. [Google Scholar] [CrossRef]
- Ceylan, M.; Kocyigit, U.M.; Usta, N.C.; Gürbüzlü, B.; Temel, Y.; Alwasel, S.H.; Gülçin, Y. Synthesis, carbonic anhydrase I and II isoenzymes inhibition properties, and antibacterial activities of novel tetralone-based 1,4-benzothiazepine derivatives. J. Biochem. Mol. Toxicol. 2016, 31, 1–11. [Google Scholar] [CrossRef]
- Feng, L.; Lanfranchi, D.A.; Cotos, L.; Rodo, E.C.; Ehrhardt, K.; Alice-Anne Goetz, A.A.; Zimmermann, H.; Fenaille, F.; Blandin, S.A.; Charvet, D.E. Synthesis of plasmodione metabolites and 13C-enriched plasmodione as chemical tools for drug metabolism investigation. Org. Biomol. Chem. 2018, 16, 2647–2665. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Horn, H.; Eppner, B.; Kuehmstedt, H. Synthesis of 2-(3- and 4-amidinobenzylidene) Derivatives of 1-indanone, 1-tetralone and benzosuberone. Pharmazie 1979, 34, 56. [Google Scholar]
- Trost, B.M.; Stambuli, J.P.; Silverman, S.M.; Schworer, U. Palladium-Catalyzed Asymmetric [3 + 2] Trimethylenemethane Cycloaddition Reactions. J. Am. Chem. Soc. 2006, 128, 13328–13329. [Google Scholar] [CrossRef]
- Yokota, M.; Fujita, D.; Ichikawa, J. Activation of 1,1-Difluoro-1-alkenes with a Transition-Metal Complex: Palladium(II)-Catalyzed Friedel Crafts Type Cyclization of 4,4-(Difluorohomoallyl)arenes. Org. Lett. 2007, 9, 4639–4642. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Alvaro, M.; Garciaa, H. Claisen–Schmidt Condensation Catalyzed by Metal-Organic Frameworks. Adv. Synth. Catal. 2010, 352, 711–717. [Google Scholar] [CrossRef]
- Chu, G.H.; Li, P.K. Synthesis of Naphthalenic Melatonin Receptor Ligands. Synth. Commun. 2001, 31, 621–629. [Google Scholar] [CrossRef]
- Lantaño, B.; Aguirre, J.M.; Drago, E.V.; Bollini, M.; Faba, D.J.; Mufato, J.D. Synthesis of Benzylidenecycloalkan-1-ones and 1,5-diketones under Claisen–Schmidt Reaction: Influence of the Temperature and Electronic Nature of Arylaldehydes. Synth. Commun. 2017, 47, 2202–2214. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Cao, Y.; He, L. Catalytic Efficient Nazarov Reaction of Unactivated Aryl Vinyl Ketones via a Bidentate Diiron Lewis Acid Activation Strategy. Adv. Synth. Catal. 2017, 359, 3325–3331. [Google Scholar] [CrossRef]
- Rahman, A.F.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α, α′-bis-(Substituted-benzylidene) cycloalkanones and α, α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571–583. [Google Scholar] [CrossRef]
- Bechara, W.S.; Pelletier, G.; Charette, A.B. Chemoselective Synthesis of Ketones and Ketimines by Addition of Organometallic Reagents to Secondary Amides. Nat. Chem. 2012, 4, 228–234. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Entry | Deviations from the above | Conv. 3a (%) [b] | 3a/3a’ |
|---|---|---|---|
| 1 | - | 77(75) | >25:1 |
| 2 | Fe(acac)3 | 68 | 17:1 |
| 3 | Fe2(CO)9 | 64 | 16:1 |
| 4 | L2 instead of L1 | 68 | 17:1 |
| 5 | L3 instead of L1 | 54 | >10:1 |
| 6 | L4 instead of L1 | 67 | >11:1 |
| 7 | L5 instead of L1 | 70 | 4:1 |
| 8 | L6 instead of L1 | 60 | 2:1 |
| 9 | t-BuOK | 58 | 2:1 |
| 10 | Na2CO3, K2CO3, Cs2CO3, | 12–30 | - |
| 11 | p-xylene, 1,4-dioxane, t-amyl-alcohol | 25–45 | >10 |
| 12 | Fe(OAc)2 (2.5 mol%), L1 (3.0 mol%) | 53 | >26:1 |
| 13 | Reaction at 80 °C | 58 | - |
| 14 | 9 h reaction time | 62 | >31:1 |
| 15 | no Fe(OAc)2, no ligand | 20 | - |
| 16 | No base | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sk, M.; Kumar, A.; Das, J.; Banerjee, D. A Simple Iron-Catalyst for Alkenylation of Ketones Using Primary Alcohols. Molecules 2020, 25, 1590. https://doi.org/10.3390/molecules25071590
Sk M, Kumar A, Das J, Banerjee D. A Simple Iron-Catalyst for Alkenylation of Ketones Using Primary Alcohols. Molecules. 2020; 25(7):1590. https://doi.org/10.3390/molecules25071590
Chicago/Turabian StyleSk, Motahar, Ashish Kumar, Jagadish Das, and Debasis Banerjee. 2020. "A Simple Iron-Catalyst for Alkenylation of Ketones Using Primary Alcohols" Molecules 25, no. 7: 1590. https://doi.org/10.3390/molecules25071590
APA StyleSk, M., Kumar, A., Das, J., & Banerjee, D. (2020). A Simple Iron-Catalyst for Alkenylation of Ketones Using Primary Alcohols. Molecules, 25(7), 1590. https://doi.org/10.3390/molecules25071590






