Abstract
The artificial production of Ophiocordyceps sinensis mycelia and fruiting bodies and the Chinese cordyceps has been established. However, the volatile components from these O. sinensis products are not fully identified. An efficient, convenient, and widely used approach based on headspace solid-phase microextraction (HS-SPME) combined with comprehensive two-dimensional gas chromatography and quadrupole time-of-flight mass spectrometry (GC×GC-QTOFMS) was developed for the extraction and the analysis of volatile compounds from three categories of 16 products, including O. sinensis fungus, Thitarodes hosts of O. sinensis, and the Chinese cordyceps. A total of 120 volatile components including 36 alkanes, 25 terpenes, 17 aromatic hydrocarbons, 10 ketones, 5 olefines, 5 alcohols, 3 phenols, and 19 other compounds were identified. The contents of these components varied greatly among the products but alkanes, especially 2,5,6-trimethyldecane, 2,3-dimethylundecane and 2,2,4,4-tetramethyloctane, are the dominant compounds in general. Three categories of volatile compounds were confirmed by partial least squares-discriminant analysis (PLS-DA). This study provided an ideal method for characterizing and distinguishing different O. sinensis and insect hosts-based products.
1. Introduction
The Chinese cordyceps, a parasitic Ophiocordyceps sinensis fungus-Thitarodes/Hepialus caterpillar complex endemic only at an elevation of 3000–5000 m in the Tibetan plateau, is a valuable health food and medicinal herb [1]. Modern pharmacological studies indicate that the Chinese cordyceps is good for human circulatory, immune, hematogenic, cardiovascular, respiratory, and glandular systems [2,3,4]. Due to the limited distribution, high cost, and over exploitation, the natural Chinese cordyceps is extremely expensive and not satisfactory for market demand [5]. Therefore, artificial cultivation is needed to satisfy the natural resource protection and human comsumption.
The artificial cultivation of O. sinensis fruiting bodies on rice media [6] and host caterpillar Thitarodes spp. [7,8] has been established. Mycelial products of O. sinensis fungus have also been manufactured by fermentation technology [9]. More excitingly, the success of cultivation on a large scale has been achieved recently in China [10,11].
Several main bioactive compounds were detected in natural and cultured Chinese cordyceps and fermented fungal products, such as nucleosides (adenosine and inosine), carbohydrates (mannitol, trehalose, and polysaccharides), sterols (ergosterol), and sphingolipids, etc. [12,13,14,15]. Some free fatty acids and sterols in natural and cultured Chinese cordyceps were determined by one-step derivatization and GC/MS [16].
From the mycelia of O. sinensis cultured with solid-state media and submerged fermentation, 51 volatile compounds were identified, and there is a great difference in the numbers of compounds in the two mycelia, but phenols, acids, and alkanes were the major classes of compounds, while butylated hydroxytoluene was the most abundant volatile compound in both mycelia [17]. The volatile components in several commercial fermentation products from mycelial strains isolated from natural Chinese cordyceps were also analyzed, and 5,6-Dihydro-6-pentyl-2H-pyran-2-one (massoia lactone) was discovered as the dominant component in the essential oils of Jinshuibao capsule volatiles, and fatty acids including palmitic acid (C16:0) and linoleic acid (C18:2) were also found to be major volatile compositions of the fermentation products [18]. It seems that products from different cultivation methods may exhibit different volatile components. However, a comprehensive volatile profiling from natural and artificially cultivated Chinese cordyceps is unknown.
The Green Analytical Chemistry technique of headspace solid-phase microextraction (HS-SPME) coupled with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC-TOFMS) proves to be a sensitive, accurate, efficient, and convenient approach for volatile compounds analysis [19,20,21,22,23,24]. HS-SPME can extract chemicals directly from sample headspace for volatile compounds analysis. It is well suitable for volatile sampling by the advantages of ease of automation, solvent-free procedure, high preconcentration capacity, little manipulation of the sample, and high cost-efficiency [19,20,21,24,25]. GC×GC is a powerful technique with high resolution and enhanced sensitivity for separating and analyzing complex samples [19,20,21,24,25,26,27]. In addition, with the strengths in accurate mass measurements and good sensitivity in full-scan acquisition mode of TOFMS, GC×GC-TOFMS becomes an increasingly popular analytical technique for characterization of the chemical compositions of biological samples [19,20,21,24,27]. Recently, the combination of HS-SPME and GC×GC-TOFMS has been applied to volatile analysis in many fields [19,20,21,24]. However, there is no report of the simultaneous analysis of volatile components in different O. sinensis and insect hosts-based products by this method.
In the present work, HS-SPME and GC×GC-QTOFMS were employed to analyze volatile compounds from three categories of samples, including O. sinensis fungus, insect hosts of O. sinensis, and the Chinese cordyceps. Qualitative analysis was performed by comparing the mass spectra with the library and confirmed by their retention indices and fragmentation patterns. In addition, the three categories (O. sinensis fungus, Thitarodes hosts of O. sinensis, and the Chinese cordyceps) of samples were comparatively analyzed and differentiated using this method combined with multivariate partial least squares-discriminant analysis (PLS-DA).
2. Results and Discussion
2.1. Comparison of 1-DGC and GC×GC
Using the technique of HS-SPME combined with GC×GC-QTOFMS, unknown analytes can be identified and quantified in one GC injection. To compare the techniques of GC-MS and GC×GC-MS, a quality control sample (mix of each collected sample) was analyzed by both techniques under the same chromatographic conditions as described by Xiang et al. [19]. The result shows that the number of detected peaks as well as the chromatographic response in the GC×GC-MS chromatogram significantly increase compared to that of the GC-MS (Figure 1). With the high chromatographic resolution, many overlapped peaks by 1-DGC were resolved by GC×GC. In the quality control sample, the higher resolving power of GC×GC is visibly demonstrated by the constituent D-limonene (peak 3), which appears as a single overlapped peak from 1D-GC (Figure 1A) and can be separated into seven individual peaks by GC×GC (Figure 1B), namely 2,5,9-trimethyldecane, 2,2,4,4-tetramethyloctane, o-cymene, 2,4-dimethyl-2,3-heptadien-5-yne, 3-octene-2-one, and 2,3-dihydropyran-6-one (peaks 1, 2, 4, 5, 6, 7, respectively); these compounds are separated only in the second dimension. The results revealed that the volatile components of O. sinensis and insect host products were complex and required GC×GC for complete characterization; GC×GC-MS has a superior sensitivity and resolution, providing an efficient and convenient approach for studying the volatile compounds of these products. Furthermore, the present method is automated and meets the requirement of the principles of green analytical chemistry, such as solvent-free sampling, small amounts of reagents, hermetic sealing of analytical process, reduced waste, and less time consumption [22,23].
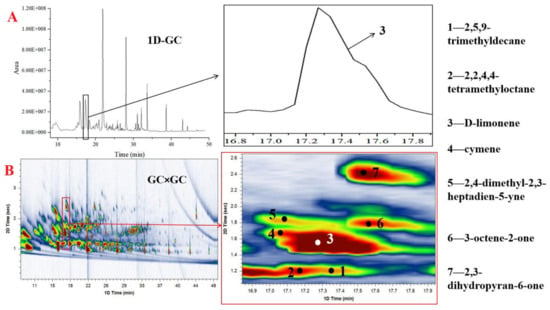
Figure 1.
Comparison of an expanded region of the 1D GC (A) and the same region from GC × GC (B) chromatograms of volatile components from a quality control sample. Compounds identification: (1) 2,5,9-trimethyldecane; (2) 2,2,4,4-tetramethyloctane; (3) d-limonene; (4) o-cymene; (5) 2,4-dimethyl-2,3-heptadien-5-yne; (6) 3-octene-2-one; (7) 2,3-dihydropyran-6-one.
2.2. Identification of Volatile Components
Component identification was achieved by matching the QTOFMS spectral with a commercial mass spectral library (NIST 17), with a minimum match factor of 800. The current quantitative method was consistent with similarly reported references [25,26], and the qualitative data of volatile components in different analyzed products (Table 1) with their peak area percentages are presented in Table 2. A total of 120 volatile compounds were detected in all samples with various concentration levels. A total of 107, 101, 71, 70, 89, 89, 113, 103, 105, 107, 102, 99, 40, 45, 82, and 69 compounds (Figure 2A) were identified in the products of W, A, A0, A1, A2, A3, FB, FC20d, FC40d, FC60d, FC80d, FC100d, Tx-LU, Tx-LI, Tx-PU, and Tx-PI (Table 1), accounting for 95.70%, 88.14%, 91.96%, 89.50%, 90.59%, 90.73%, 93.42%, 91.08%, 89.84%, 87.04%, 91.54%, 91.45%, 87.10%, 90.15%, 87.63%, and 91.81% of the total peaks areas, respectively. The identified compounds included 36 alkanes, 25 terpenes, 17 aromatic hydrocarbons, 10 ketones, 5 olefines, 5 alcohols, 3 phenols, and 19 other compounds. The volatile compound amounts showed great variation in different samples and ranged from 40 compounds in Tx-LU to 113 compounds in FB. In general, natural and mature artificial Chinese cordyceps (fungus–insect complexes), fruiting bodies, and fermented products had more volatile compounds than insect larvae, insect pupae and immature artificial Chinese cordyceps.

Table 1.
Information.

Table 2.
Volatile components identified in different products.
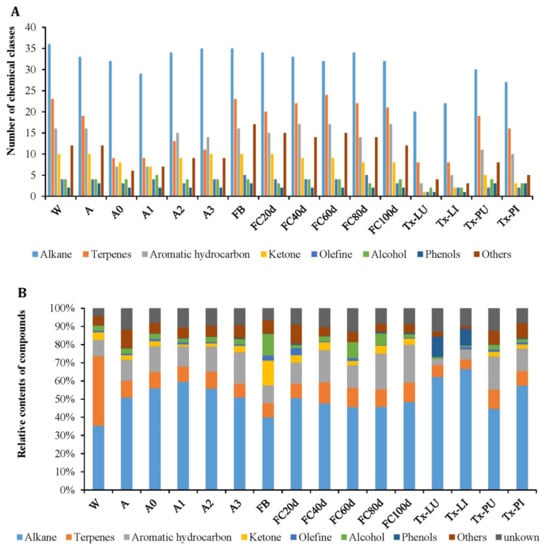
Figure 2.
The number (A) and relative content (B) of volatile compounds of each class in different products. Note: For descriptions of W, A, A0, A1, A2, A3, FB, FC20d, FC40d, FC60d, FC80d, FC100d, Tx-LU, Tx-LI, Tx-PU, and Tx-PI, please refer to Table 1).
Twenty-four volatile compounds were identified in all the 16 samples, including 4-carene (C10), 3-carene (C18), 2,2,4,4-tetramethyloctane (24), 2-propyltoluene (C29), 2,4,6-trimethyldecane (C31), 2,6-dimethyl-6-trifluoroacetoxyoctane (C34), linalool (C41), 2-nonen-1-ol (C42), 6-ethyl-2-methyloctane (C44), (+)-α-terpineol (C61), 2,6-dimethylundecane (C65), 2,8-dimethylundecane (C68), 4-methyldodecane (C73), 4,7-dimethylindan (C75), 2,3-dimethylundecane (C76), 2,4-dimethyldodecane (C77), 2,6,11-trimethyldodecane(C78), 4,6-dimethyldodecane (C84), 2,3,5,8-tetramethyldecane (C86), farnesane (C94), tetradecane (C97), seychellene (C108), 2,6-di-tert-butyl-4-methyl-p-quinol (C109), and hexadecane (C118). The mutual volatile compounds accounted for 40.19%, 41.24%, 51.44%, 48.59%, 50.58%, 41.86%, 38.44%, 48.04%, 48.31%, 47.03%, 44.37%, 48.49%, 64.94%, 68.73%, 49.13%, and 40.87% of the total volatile compounds in W, A, A0, A1, A2, A3, FB, FC20d, FC40d, FC60d, FC80d, FC100d, Tx-LU, Tx-LI, Tx-PU, and Tx-PI, respectively. With the method of simultaneous distillation-extraction (SDE) and GC-MS, 17 and 42 volatile compounds were identified in the mycelia of O. sinensis from solid-state media and submerged fermentation, respectively [17]; in Bailing capsule and Zhiling capsule, the commercial fermentation products of O. sinensis mycelia, 39 and 56 volatile compounds were identified, respectively [18]. While by the technique of HS-SPME combined with GC×GC-QTOFMS in this study, 99–107 volatile compounds were identified from the fermentation cultures of O. sinensis mycelia, indicating the superior sensitivity and resolution of the present method.
2.3. Major Compounds in Different Products
The numbers and percentage contents of volatile compounds in samples are of marked differences. Alkanes are the dominant volatile compounds in all samples. Alkane is also the class with the largest number in all samples (Figure 2). The top five compounds in the concentration of each sample are shown in Table 3.

Table 3.
Top five compounds in concentration from different products.
2,5,6-trimethyldecane (C19) is the most abundant compound in artificial cultivated Chinese cordyceps (A, A0, A1, A2, A3) and insect pupae (Tx-PU and Tx-PI), and it is also the major compound in W, FB, and FC40d, FC80d, and FC100d (Table 3). This compound is so far detected from beneficial plants such as Irish York cabbage [28], stevia Stevia rebaudiana leaves [29], an aquatic perennial herb Limnophila indica extract [30], plant-based food such as chestnut and jujube honey [31], and from exhaled breath in both children with allergic asthma and control [32]. 5,6-Dihydro-6-pentyl-2H-pyran-2-one (massoia lactone) is discovered as the dominant volatile component in a fermented mycelial product of Paecilomyces hepiali fungus [18]. 2,5,6-trimethyldecane is the first reported dominant volatile compound in O. sinensis-based products in the present study. Moreover, it seemed interesting that uninfected insect pupae also contained high concentrations of this compound. Its characteristics and possible pharmacological functions need further study.
2,3-dimethylundecane (C76) is another major component presented in 13 samples accounting for >5% of the total peak areas; however, the contents in samples of W, Tx-LU, and Tx-LI were lower, accounting for 2.97%, 1.25%, and 0.91%, respectively (Table 2 and Table 3). This compound was found from the essential oil of a small glabrous, perennial herb Viola serpens [33] and from the odors emitted from the dung of free-ranging white rhinos for differentiating sex [34].
A high content of 2,2,4,4-tetramethyloctane (C24) was found in the two larval samples of Tx-LU and Tx-LI, accounting for 56.02% and 61.87% of the total peak areas, although it is not reported from other insects. 2,2,4,4-tetramethyloctane is also the major compound of all the liquid fermentation samples (FC20d, FC40d, FC60d, FC80d, and FC100d). It was reported also in aged vinegar as an aroma compound [35], common wasp Vespula vulgaris colonies [36], Manchego and Gouda cheeses [37], Allium macrostemon flowers and aerial parts [38], the seeds and leaves of Synsepalum dulcificum [39], green teas [40], dry-cured meat products [41], and the stem of Guanyin tea [42]. It appears that this volatile mainly acts as an aroma compound from the plants and foods.
2,4-di-tert-butyl-6-methylphenol (C116) is the second principal component in the two larval samples of Tx-LU and Tx-LI, accounting for 11.27% and 9.14% of the total peak areas, but it accounts for little or no proportion in other samples. This volatile is detected from the essential oil in eaglewood [43] and entomopathogenic Metarhizium anisopliae fungus cultures [44].
The most abundant volatile compound was butylated hydroxytoluene, and the major classes compounds were phenols, acids, and alkanes in the mycelia of O. sinensis cultured by solid-state media and submerged fermentation [17]. 5,6-Dihydro-6-pentyl-2H-pyran-2-one (massoia lactone) was the dominant component in Jinshuibao capsule (Paecilomyces hepiali) volatiles, and fatty acids including palmitic acid (C16:0) and linoleic acid (C18:2) were also found to be major volatile compositions in the commercial fermentation products of Bailing capsule (O. sinensis), Zhiling capsule (Mortierella SP), Ningxinbao capsule (Cephalosporium sinensis), and Xinganbao capsule (Gliocladium roseum) [18]. In the present study, volatile compounds of alkanes are the most abundant all products, although there are differences among the volatile compound profiles of O. sinensis fungus, Thitarodes hosts of O. sinensis, and the natural and aritificial-producing Chinese cordyceps, even between the natural and artificial-producing Chinese cordyceps. It appeared that O. sinensis-based products from different culture conditions exhibit quite different metabolites.
The fermented products of O. sinensis mycelia are claimed to be used as sustainable substitutes for natural Chinese cordyceps [45]. However, from the view of the differences in volatile compounds, it seems that the fermented products are not the same as the natural and artificial Chinese cordyceps.
2.4. Multivariate PLS-DA Analysis
PLS-DA was performed to evaluate the variations among the volatile compound profiles obtained from GC×GC-QTOFMS data for different products. The PLS-DA scores plot shows clear classification of the three groups: fruiting bodies and fermented cultures of O. sinenis fungus, T. xiaojinensis insects, and insect–fungus complexes (Figure 3A). PLS (Partial least square) component 1 (PLS 1) and PLS component 2 (PLS 2) explained 21.5% and 19.0% of the variance, respectively, and hence together, they explained 40.05% of the total variance. The parameters of the cross-validation modeling for the fifth PLS component were R2X = 0.73, R2Y = 0.992, and Q2Y = 0.896, showing high levels of an explained variance and predictability. A permutation test involving 200 iterations was also conducted to validate the model, which yielded R2 = 0.770 and Q2 = −0.480.
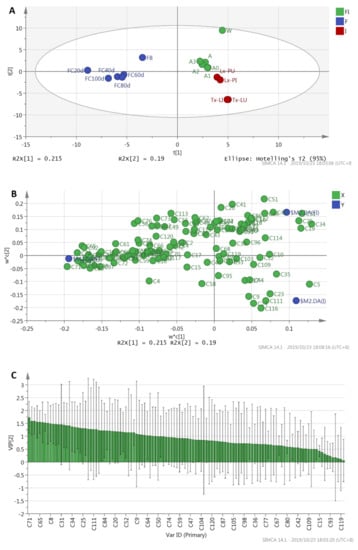
Figure 3.
Partial least squares-discriminant analysis (PLS-DA) analysis of 16 products. (A): Scores plot, (B): variable importance projection (VIP) score plot, (C): loadings plot. F: Fungus of Ophiocordyceps sinensis; I: Larvae or pupae of Thitarodes xiaojinensis; FI: Fungus–insect complexes.
To explain the relationships between variables and products, loading scatter plots were performed (Figure 3B). As shown in the loadings plot PLS-DA model (Figure 3B), X-variables situated in the vicinity of the dummy Y-variables had the highest discriminatory power among the groups and had higher VIP (variable importance projection) values, thus contributing more to the differences of different groups. The VIP values of each compound were calculated (Figure 3C). The compounds with larger VIP values represent higher contributions to the discrimination of different groups. In the study, volatile components with VIP values > 1 and p < 0.05 were considered as representative differential compounds. A total of 28 differential volatile compounds were identified, although there were 48 volatile compounds with VIP values > 1. It showed that the majority of variables gave a not significant contribution to the model. The 28 differential volatile compounds included thieno [2,3-c] pyridine (C71), 2,6,11-trimethyldodecane (C78), 2,3,4-trimethyldodecane (C79), 4,7-dimethylindan (C75), 2,6-dimethylundecane (C65), farnesane (C94), 2,3-dimethyldodecane (C92), [but-2-en-2-yl] benzene (C51), 2-methylcyclopentanone (C8), 2-undecanone (C83), 2,8-dimethylundecane (C69), α-methyl-1H-indene-1-methanol acetate (C88), 2,6-dimethylheptadecane (C108), 1,2-dimethoxyethylbenzene (C38), 2,6-dimethyl-6-trifluoroacetoxyoctane (C34), modephene (C97), 4’-methylpropiophenone (C53), 2,6-di-tert-butyl-4-methyl-p-quinol (C110), o-xylene (C5), 3-methyl-undecane (C56), tridecane (C84), 2,5,6-trimethyldecane (C19), sabinene (C13), dodecane (C61), β-sesquiphellandrene (C113), 2,3-dimethyldecane (C52), 3,4-dimethylcumene (C63), and 4,7-dimethylundecane (C64). Among them, the first eight volatiles including thieno [2,3-c] pyridine (C71), 2,6,11-trimethyldodecane (C78), 2,3,4-trimethyldodecane (C79), 4,7-dimethylindan (C75), 2,6-dimethylundecane (C65), farnesane (C94), 2,3-dimethyldodecane (C92), and [but-2-en-2-yl] benzene (C51) showed higher discriminatory potential with VIP values greater than 1.5.
3. Materials and Methods
3.1. Chemicals
All solvents used were chromatographic grade. Phenylethyl acetate (internal standard) with purity greater than 99.0% was purchased from Sigma-Aldrich-Fluka (Buchs, Switzerland). The internal standard with a concentration of 22.9 µg/mL was prepared in acetonitrile. A standard series of n-alkanes (C8–C25) were provided by Dr. Ehrensorfer (Augsburg, Germany). Methanol (chromatographic grade purity) and acetonitrile (chromatographic grade purity) were purchased from Merck (LiChrosolv, Germany). All chemicals were stored at 4 °C until use. The SPME holder for manual sampling and fibers of 65 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 1 cm of length) were purchased from Supelco (Aldrich, Bellefonte, PA, USA).
3.2. Samples
The samples for GC-MS analysis are listed in Table 1. Natural Chinese cordyceps were collected from Kangding, Sichuan Province, China. The KD1223 strain of O. sinensis fungus isolated from the fruiting bodies of natural Chinese cordyceps was cultured in a 100 rpm shaker with potato dextrose liquid medium supplemented with 10% peptone (PPD) at 13 °C. The fungus was identified by molecular method using the internal transcribed spacer (ITS; ITS1-5.8S-ITS2) of nuclear ribosomal DNA amplification as described before. The identified O. sinensis strain was preserved at −80 °C at the Guangdong Institute of Applied Biological Resources, Guangzhou, China.
Artificial cultivation of fruiting bodies on rice media [6] or whole Chinese cordyceps by challenging T. xiaojinensis larvae with O. sinensis fungus [7,8,11] were established in low altitude Guangzhou, with mimicking environmental conditions. The insect species was identified using a molecular method by the amplification of the Cytochrome b sequences with the primers CB1 (TATGTACTACCATGAGGACAAATATC) and CB2 (ATTACACCTCCTAATTTATTAGGAAT) [46], as described previously [47,48]. The mummified cadavers with mycelia but without fruiting body (before stroma development), and the insect larvae and pupae with or without the injected blastospores (9 months for larvae or 9 months for pupae) were also used for the analysis. The existence of blastospores in the live larvae and pupae was confirmed by hemolymph microscopic examination.
A total of 50 individuals of each sample (for natural and artificial Chinese cordyceps, mummified cadavers, live larvae and pupae with or without blastospores), 30 g of fresh artificial fruiting bodies, and three flasks of fermentation cultures (150 mL/flask) were sampled. Samples were frozen at −80 °C overnight and lyophilized for 48–72 h by vacuum-freeze dryer (Alpha 1-2 LD plus, Marin Christ Gefriertrocknungsanlagen, Osterode, Germany) to consistent weight. The dried samples were grinded at 1000 rpm for 3 min by a multifunctional high-throughput tissue ball mill (GT100, Beijing Grinder Instrument Co., Ltd., Beijing, China) and stored at −80 °C. A quality control (QC) sample was prepared by mixing each collected sample in equal quantities and used for analytical method establishment and methodology examination.
3.3. GC×GC-QTOFMS Analysis for Volatile Components
The analysis of volatile composition and analytical method validation were referenced by the method of previous reports [19,21,24]. The volatile constituents of O. sinensis and host insects were analyzed by comprehensive two-dimensional gas chromatography (7890B-SSM1800, Agilent Technologies, Santa Clara, CA, USA and J&X Technologies, Shanghai, China) coupled with a high-resolution quadrupole time-of-flight mass spectrometry (QTOFMS) (7250, Agilent Technologies). First, 100 mg of samples were accurately weighed into a 20 mL vial, and then the SPME fiber that was equilibrated at 270 °C for 30 min in an autosampler (PAL RSI 120, CTC Technologies, Alexandria, VA, USA) was exposed to the headspace of the bottle for 20 min at 60 °C. Then, the SPME fiber was introduced into the GC splitless injector and kept there for 3.0 min to allow thermal desorption of the analytes. All samples were conducted in triplicate to check the repeatability and reliability of the method development. Reproducibility is expressed as the relative standard deviation (RSD). To compare the techniques of GC-MS and GC×GC-MS, a quality control sample (mix of each collected sample) was analyzed by both techniques under the same chromatographic conditions. The analytical system was equipped with simultaneous 1DGC and GC×GC in one instrument, which can conduct both techniques at the same time without any change of columns. The samples were introduced by a splitless injector (SSL) system equipped with an autosampler. Peak separation was performed on a weak-polar column HP-5 MS (5% phenyl-95% dimethylpolysiloxane, 30 m × 250 μm, 0.25 μm) in the first dimension and a more polar column DB-17 MS (50% phenyl-50% dimethylpolysiloxane, 1.2 m × 180 μm, 0.18 μm) in the second dimension (both from Agilent Technologies, USA).
The 1DGC and GC×GC conditions were the same. The GC injector was kept at 250 °C in splitless mode. The carrier gas was helium with a flow rate of 1.0 mL/min for the first dimensional column. The initial oven temperature was 50 °C; it was held for 3 min and then ramped at a rate of 4 °C/min to 230 °C and held for 1 min. For the GC×GC system, the carrier gas was helium with a flow rate of 3.14 mL/min for second dimensional column, and the cold zone temperature of modulator was set at −50 °C. The temperatures of the entry hot zone and exit hot zone were +30 and +120 °C relative to oven temperatures, respectively, with a cap temperature of 320 °C for both hot zones. The modulation period was 4 s.
The MS transfer line temperature was kept at 280 °C, and the ion source temperature was kept at 200 °C. Electron impact ionization was 70 eV. Data were collected as a mass range of 50–500 m/z at a sampling rate of 50 scan/s, and a solvent delay of 8 min was used.
3.4. Data Analysis
Qualitative and semi-quantitative methods primarily were referenced with similar reports [26,27]. Compound identification was based on mass spectra comparison with NIST 17 library (NIST/EPA/NIH 2017) with the minimum requirements of match factor above 800. Further confirmation was carried out using one-dimensional retention index (RI) and accurate mass, as described in many previous studies [49]. In order to compare the reference RI values with experimental RI values obtained in this work, a standard mixture of n-alkanes (C8–C25) was injected (0.5 μL) in the GC×GC-QTOFMS system under the same conditions used for the samples. The semi-quantitative method was performed based on peak area normalization. The 1-DGC data were processed using Agilent Mass Hunter Qualitative Analysis Navigator B.08.00. The GC×GC data were analyzed by a dedicated GC×GC data processing software Canvas (V1.4.0, J & X Technologies).
To visualize the clustering among categories and identify the differentially changed components responsible for the separation, supervised partial least squares discriminant analysis (PLS-DA) and variable importance in projection (VIP) score were carried out using SIMCA 14.1 software (Umetrics, Umea, Sweden). A data set consisting of a 16 × 120 matrix was conducted by PLS-DA. The rows represent the samples analyzed and the columns represent the relative contents of the volatile metabolites determined by GC×GC-QTOFMS. All variables were scaled with unit variance (UV) prior to PLS-DA. To gain the chemical markers for discrimination of the three groups in the PLS-DA model, VIP values were calculated and inspected for identified volatile compounds. Generally, VIP values > 1 and p < 0.05 are considered as significant contributors to the model [40,50,51]. In this study, seven-fold cross-validation and 200 response permutation testing (RPT) methods were used to investigate the quality of the model.
4. Conclusions
This study presents the volatile metabolite profiles by HS-SPME-GC×GC-QTOFMS from O. sinensis fungus and insect host-based products. A total of 120 volatile compounds including 36 alkanes, 25 terpenes, 17 aromatic hydrocarbons, 10 ketones, 5 olefines, 5 alcohols, 3 phenols, and 19 other compounds were identified. There are great differences in the volatile compounds among the three categories of O. sinensis fungus, Thitarodes hosts of O. sinensis, and the Chinese cordyceps. In general, natural and mature artificial Chinese cordyceps (fungus–insect complexes), fruiting bodies, and fermented products had more volatile compounds than insect larvae, insect pupae, and immature artificial Chinese cordyceps. Twenty-four volatile compounds were identified in all the 16 samples, including 4-carene (C10), 3-carene (C18), 2,2,4,4-tetramethyloctane (24), 2-propyltoluene (C29), 2,4,6-trimethyldecane (C31), 2,6-dimethyl-6-trifluoroacetoxyoctane (C34), linalool (C41), 2-nonen-1-ol (C42), 6-ethyl-2-methyloctane (C44), (+)-α-terpineol (C61), 2,6-dimethylundecane (C65), 2,8-dimethylundecane (C68), 4-methyldodecane (C73), 4,7-dimethylindan (C75), 2,3-dimethylundecane (C76), 2,4-dimethyldodecane (C77), 2,6,11-trimethyldodecane(C78), 4,6-dimethyldodecane (C84), 2,3,5,8-tetramethyldecane (C86), farnesane (C94), tetradecane (C97), seychellene (C108), 2,6-di-tert-butyl-4-methyl-p-quinol (C109), and hexadecane (C118). Alkanes are the dominant volatile compounds in all products. 2,5,6-trimethyldecane and 2,6,7-trimethyldecane are the major volatile compounds in all products except the larval ones, while 2,2,4,4-tetramethyloctane dominates in the larval products. From the view of the differences in volatile compounds, it seems that the fermented products are not the same as the natural and artificial Chinese cordyceps. Based on the volatile compounds, three classes (O. sinensis fungus, Thitarodes insect, and fungus–insect complexe) were confirmed by partial least squares-discriminant analysis (PLS-DA). Thieno [2,3-c] pyridine, 2,6,11-trimethyldodecane, 2,3,4-trimethyldodecane, 4,7-dimethylindan, 2,6-dimethylundecane, farnesane, 2,3-dimethyldodecane, and [but-2-en-2-yl] benzene are potential discriminatory compounds. The present results suggested that HS-SPME-GC×GC-QTOFMS combined with multivariate data analysis is an ideal method for analyzing and distinguishing different O. sinensis and insect hosts-based products. The information provided in this study is of importance for the further identification of bioactive components and for proposals of possible mechanisms to obtain those bioactive compounds in a different form than the traditional fungus-insect interaction.
Author Contributions
R.H. and X.Q. conceived and designed the experiments; L.C. and X.Q. prepared experimental samples; X.Q. performed the experiments and analyzed the data; X.Q. and R.H. wrote the manuscript. All the authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Guangzhou Science and Technology Projects (201803010087; 201604020030), GDAS Special Project of Science and Technology Development (2018GDASCX-0107), and Open Project of Guangdong Key Laboratory of Animal Conservation and Resource Utilization (GIABR-KF201703).
Acknowledgments
The authors are grateful to Professor Zhangmin Xiang from Guangdong Institute of Analysis, Guangzhou, China for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, S.P.; Yang, F.Q.; Tsim, K.W. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Gong, Z.H.; Su, Y.; Lin, J.; Tang, K.X. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, M.G.; Giridhar, P.; Sankar, K.U.; Manohar, B. Bioactive principles from Cordyceps sinensis: A potent food supplement–A review. J. Funct. Food 2013, 5, 1013–1030. [Google Scholar] [CrossRef]
- Yue, K.; Ye, M.; Zhou, Z.J.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. J. Pharm. Pharmacol. 2013, 65, 474–493. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.L.; Jiao, L.; Jiang, Y.; Li, H.; Jiang, S.P.; Lhosumtseiring, N.; Fu, S.Z.; Dong, C.H.; Zhan, Y.; et al. A survey of the geographic distribution of Ophiocordyceps sinensis. J. Microbiol. 2011, 49, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ye, Y.; Han, R. Fruiting body production of the medicinal Chinese caterpillar mushroom, Ophiocordyceps sinensis (Ascomycetes), in artificial medium. Int. J. Med. Mushrooms 2015, 17, 1017–1022. [Google Scholar] [CrossRef]
- Cao, L.; Han, R.C. Artificial Cultivation of Host Insects of Ophiocordyceps sinensis in Low Altitude Areas. Chinese Patent No. ZL201410413333.7, 20 August 2014. [Google Scholar]
- Tao, Z.; Cao, L.; Zhang, Y.; Ye, Y.; Han, R. Laboratory rearing of Thitarodes armoricanus and Thitarodes jianchuanensis (Lepidoptera: Hepialidae), hosts of the Chinese medicinal fungus Ophiocordyceps sinensis (Hypocreales: Ophiocordycipitaceae). J. Econ. Entomol. 2016, 109, 176–781. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Food 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Li, W.; Li, Q.; Qian, Z.; Liu, X.; Dong, C. A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2019, 39, 181–191. [Google Scholar] [CrossRef]
- Liu, G.; Han, R.; Cao, L. Artificial cultivation of the Chinese cordyceps from injected ghost moth larvae. Environ. Entomol. 2019, 48, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Zhao, S.Y.; Wang, J.H.; Kuang, H.C.; Liu, X. Distribution of nucleosides and nucleobases in edible fungi. J. Agric. Food Chem. 2008, 56, 809–915. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, J.; Wang, L.Y.; Li, S.P. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.H.; Wang, W.; Zhang, H.Y.; Zhang, X.L.; Han, C.C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid.-Based Complement. Altern. Med. 2015, 2015, 575063. [Google Scholar]
- Mi, J.; Han, Y.; Xu, Y.; Kou, J.; Li, W.J.; Wang, J.R.; Jiang, Z.H. Deep profiling of immunosuppressive glycosphingolipids and sphingomyelins in wild Cordyceps. J. Agric. Food. Chem. 2018, 66, 8991–8998. [Google Scholar] [CrossRef]
- Yang, F.Q.; Feng, K.; Zhao, J.; Li, S.P. Analysis of sterols and fatty acids in natural and cultured Cordyceps by one-step derivatization followed with gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 1172–1178. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Fan, M. Analysis of volatile compounds of mycelia of Hirsutella sinensis, the anamorph of Ophiocordyceps sinensis. Appl. Mech. Mater. 2012, 140, 253–257. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Mi, J.; Zhang, M.; Wang, Y.; Jiang, Z.; Hu, P. GC-MS profiling of volatile components in different fermentation products of Cordyceps sinensis mycelia. Molecules 2017, 22, 1800. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, X.; Zhao, Z.; Xiao, X.; Guo, P.; Song, H.; Yang, X.; Huang, M. Analysis of volatile components in Dalbergia cochinchinensis Pierre by a comprehensive two-dimensional gas chromatography with mass spectrometry method using a solid-state modulator. J. Sep. Sci. 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, W.J.; Li, H.D.; Mao, J.S.; Guo, C.Y.; Ding, R.Y.; Wang, Y.; Fang, L.P.; Chen, Z.L.; Yang, G.S. Analysis of volatile compounds in pears by HS-SPME-GC×GC-TOFMS. Molecules 2019, 24, 1795. [Google Scholar] [CrossRef]
- Qian, C.Y.; Quan, W.X.; Xiang, Z.M.; Li, C.C. Characterization of volatile compounds in four different Rhododendron Flowers by GC×GC-QTOFMS. Molecules 2019, 24, 3327. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Konieczka, P.; Migaszewski, Z.M.; Namiesnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Cai, K.; Liang, G.; Zhou, S.; Ge, Y.; Zhang, J.; Geng, Z. Analysis of volatile flavour components in flue-cured tobacco by headspace solid-phase microextraction combined with GC×GC-TOFMS. Anal. Methods 2014, 6, 3300–3308. [Google Scholar] [CrossRef]
- Wajs, A.; Pranovich, A.; Reunanen, M.; Willfor, S.; Holmbom, B. Headspace-SPME analysis of the sapwood and heartwood of Picea abies, Pinus sylvestris and Larix decidua. J. Essent. Oil. 2007, 19, 125–133. [Google Scholar] [CrossRef]
- Hantao, L.W.; Toledo, B.R.; Ribeiro, F.A.; Pizetta, M.; Pierozzi, C.G.; Furtado, E.L.; Augustoa, F. Comprehensive two-dimensional gas chromatography combined to multivariate data analysis for detection of disease-resistant clones of Eucalyptus. Talanta 2013, 116, 1079–1084. [Google Scholar] [CrossRef]
- Tajuddin, S.N.; Muhamad, N.S.; Yarmob, M.A.; Yusoff, M.M. Characterization of the chemical constituents of agarwood oils from malaysia by comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. Mendeleev Commun. 2013, 23, 51–52. [Google Scholar] [CrossRef]
- Lonchamp, J.; Barry-Ryan, C.; Devereux, M. Identification of volatile quality markers of ready-to-use lettuce and cabbage. Food Res. Int. 2009, 42, 1077–1086. [Google Scholar] [CrossRef]
- Gasmalla, M.A.A.; Tessema, H.A.; Alahmed, K.; Hua, X.; Liao, X.; Yang, R. Effect of different drying techniques on the volatile compounds, morphological characteristics and thermal stability of Stevia rebaudiana Bertoni leaf. Trop. J. Pharm. Res. 2017, 16, 1399–1406. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Kumar, R.; Prakash, O.; Srivastava, R.M.; Pant, A.K. GC-MS analysis of the hexane extract of Limnophila indica (L.) Druce, its total phenolics, in-vitro antioxidant, anti-inflammatory and antifeeding activity against Spilosoma obliqua. J. Entomol. Zool. Stud. 2019, 7, 970–975. [Google Scholar]
- Dekebo, A.; Kwon, S.Y.; Kim, D.H.; Jung, C. Volatiles analysis of honey by gas chromatography-mass spectrometry (GC-MS): Comparison of SPME volatiles extraction methods. J. Apiculture 2018, 33, 117–128. [Google Scholar] [CrossRef]
- Caldeira, M.; Barros, A.S.; Bilelo, M.J.; Parada, A.; Câmara, J.S.; Rocha, S.M. Profiling allergic asthma volatile metabolic patterns using a headspace-solid phase microextraction/gas chromatography based methodology. J. Chromatogr. A 2011, 1218, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Kohli, G.; Prasad, K.; Bisht, G.; Punetha, V.D.; Pandey, H.K. Chemical composition of the essential oil of Viola serpens from Bageshwar (Shama), Uttarakhad, India. J. Med. Plants Res. 2017, 11, 513–517. [Google Scholar]
- Marneweck, C.; Jürgens, A.; Shrader, A.M. Dung odours signal sex, age, territorial and oestrous state in white rhinos. Proc. R. Soc. 2017, 284, 20162376. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, P.; Liu, X.; Luo, L.; Lin, W. Bacterial dynamics and metabolite changes in solid-state acetic acid fermentation of Shanxi aged vinegar. Appl. Microbiol. Biotechnol. 2016, 100, 4395–4411. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.C.; Oi, C.A.; Vollet-Neto, A.; Wenseleers, T. Intraspecific worker parasitism in the common wasp, Vespula vulgaris. Anim. Behav. 2016, 113, 79–85. [Google Scholar] [CrossRef]
- Fernández, M.; Hospital, X.F.; Arias, K.; Hierro, E. Application of pulsed light to sliced cheese: Effect on Listeria inactivation, sensory quality and volatile profile. Food Bioprocess Technol. 2016, 9, 1335–1344. [Google Scholar] [CrossRef]
- Yang, S. Study on the Volatile Components of Thirteen Medicinal Plants by SHS/GC-MS. Master’s Thesis, Northwest University, Xian, China, 2018. [Google Scholar]
- Jian, H.J.; Qiao, F. Analysis of nutritional and volatile components in seeds and leaves of Synsepalum dulcificum. Stor. Proc. 2018, 18, 149–155. [Google Scholar]
- Yang, Y.; Zhang, M.; Yin, H.; Deng, Y.; Jiang, Y.; Yuan, H.; Dong, C.; Li, J.; Hua, J.; Wang, J. Rapid profiling of volatile compounds in green teas using Micro-Chamber/Thermal Extractor combined with thermal desorption coupled to gas chromatography-mass spectrometry followed by multivariate statistical analysis. LWT-Food Sci. Technol. 2018, 96, 42–50. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Method. 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Wang, W.; Huang, X.; Lin, Y.; Tang, R.; Guo, Y. Analysis of volatile compounds in Guanyin tea stem. Chinese J. Trop. Crop. 2019, 40, 965–972. [Google Scholar]
- He, Z.; Fan, Y.; Xie, X.; Yuan, G. GC-MS analysis of fragrance constituents from offcuts of eaglewood and Chinese Eaglewood in Guangdong. Adv. Mater. Res. 2012, 550–553, 1904–1907. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Lei, Y.Y. Differential fluctuation in virulence and VOC profiles among different cultures of entomopathogenic fungi. J. Invertebr. Pathol. 2010, 104, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, E.; Wang, C.; Li, Y.; Liu, X. Ophiocordyceps sinensis, the flagship fungus of China: Terminology, life strategy and ecology. Mycology 2012, 3, 2–10. [Google Scholar]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Quan, Q.M.; Chen, L.L.; Wang, X.; Li, S.; Yang, X.L.; Zhu, Y.G.; Wang, M.; Cheng, Z. Genetic diversity and distribution patterns of host insects of caterpillar fungus Ophiocordyceps sinensis in the Qinghai-Tibet plateau. PLoS ONE 2014, 9, e92293. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, S.; Li, Y.L.; Ma, S.L.; Wang, C.S.; Xiang, M.C.; Liu, X.Z. Phylogeography and evolution of a fungal-insect association on the Tibetan plateau. Mol. Ecol. 2014, 23, 5337–5355. [Google Scholar] [CrossRef]
- Silva, É.A.S.; Saboia, G.; Jorge, N.C.; Hoffmann, C.; Dos Santos Isaias, R.M.; Soares, G.L.G.; Zini, C.A. Development of a HS-SPME-GC/MS protocol assisted by chemometric tools to study herbivore-induced volatiles in Myrcia splendens. Talanta 2017, 175, 9–20. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic profiling of pitaya (Hylocereus polyrhizus) during fruit development and maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef]
- Yan, Y.; Chena, S.; Niea, Y.; Xua, Y. Characterization of volatile sulfur compounds in soy sauce aroma type Baijiu and changes during fermentation by GC×GC-TOFMS, organoleptic impact evaluation, and multivariate data analysis. Food Res. Int. 2020, 131, 109043. [Google Scholar] [CrossRef]
Sample Availability: Samples of the different Ophiocordyceps sinensis and insect host products in this study are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).