Encapsulation for Cancer Therapy
Abstract
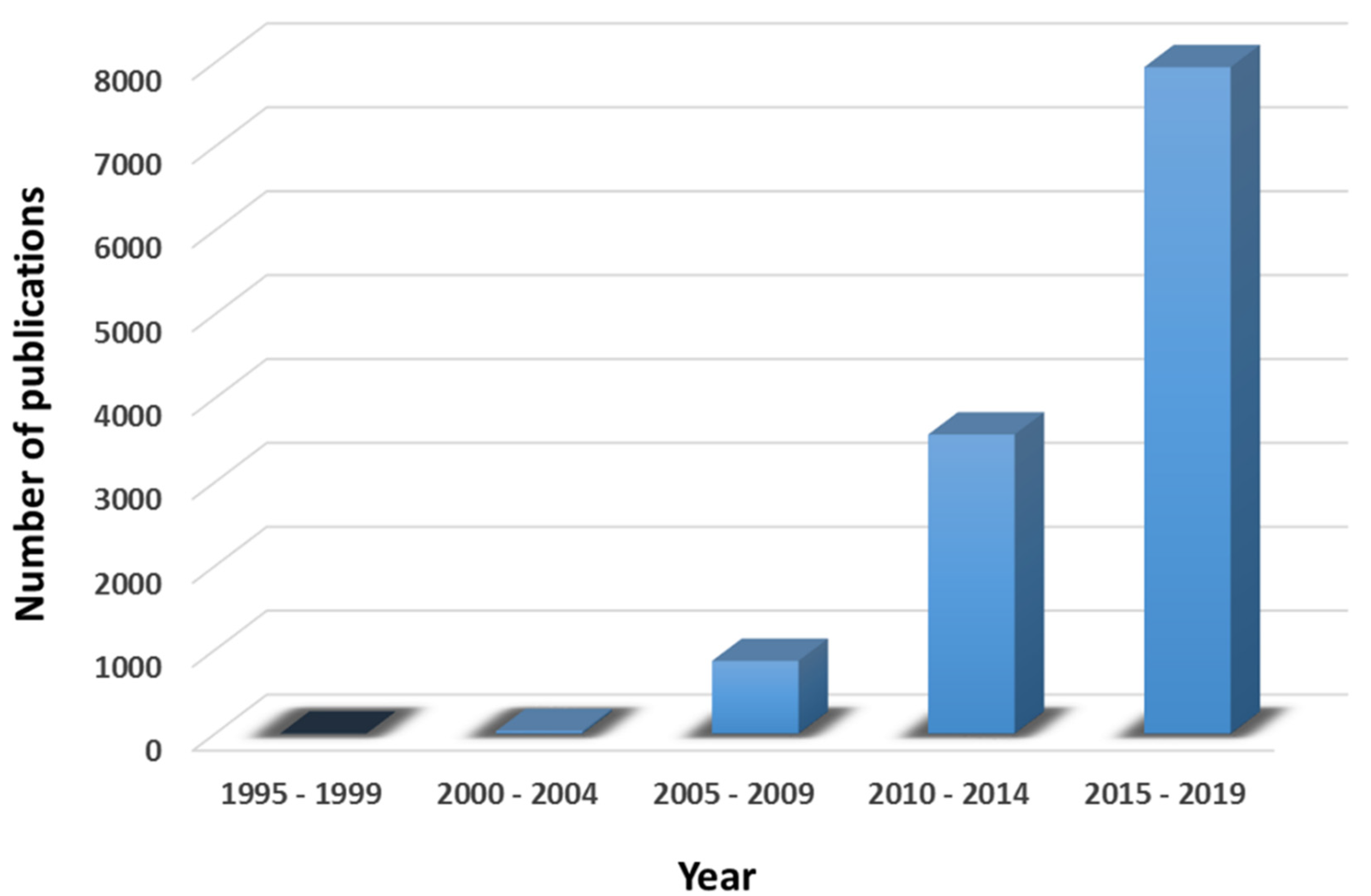
:1. Introduction
2. Benefits of the Encapsulation of Therapeutic Agents in Nanocapsules
- NCs have the ability to target and enter into selective tissue at molecular level.
- NCs provide large surface area.
- NCs provide high absorption rate.
- Increased cellular uptake and drug localization.
- Accurate and targeted drug delivery to cancerous cell without interactions with healthy cells.
- Lower dosage required due to the encapsulation of drugs or small molecules.
- Improved uptake of poorly soluble drugs.
- Decrease in medicinal toxicity.
- Greater precision in delivering drugs to tiny areas within the body.
- Decrease in drug resistance.
- Nanoencapsulation of the drugs minimizes or suppresses the resistance arising from the physiological barriers in the body.
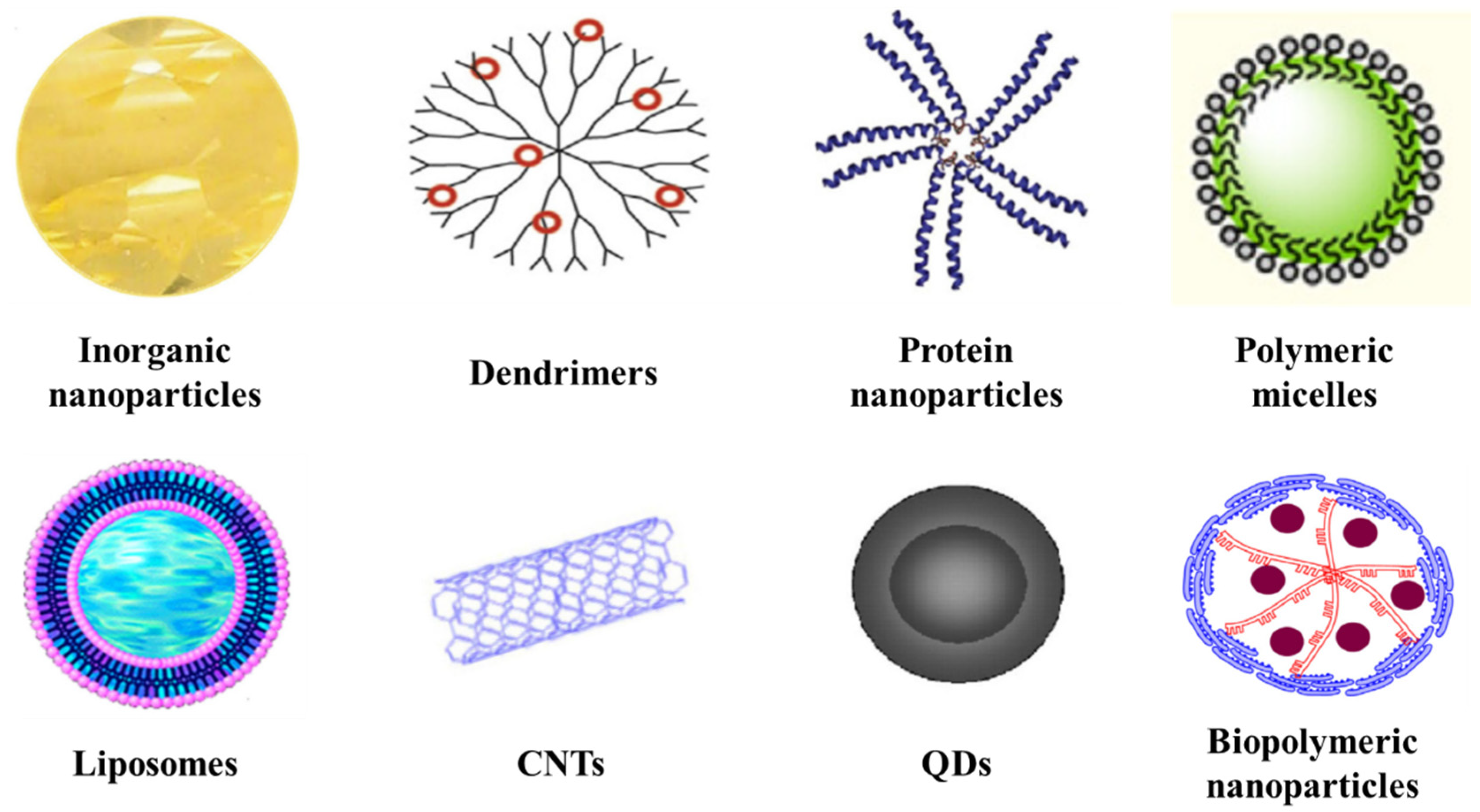
3. Nanobased Drug Delivery Systems
- The size of the nanomaterial.
- The biocompatibility and biodegradability of the nanosystem.
- The desired drug release profile.
- The toxicity and antigenicity of the encapsulated drug.
- The properties of the entrapped medicine into the nanomaterial (for example drug stability or drug solubility in water or other solvents).
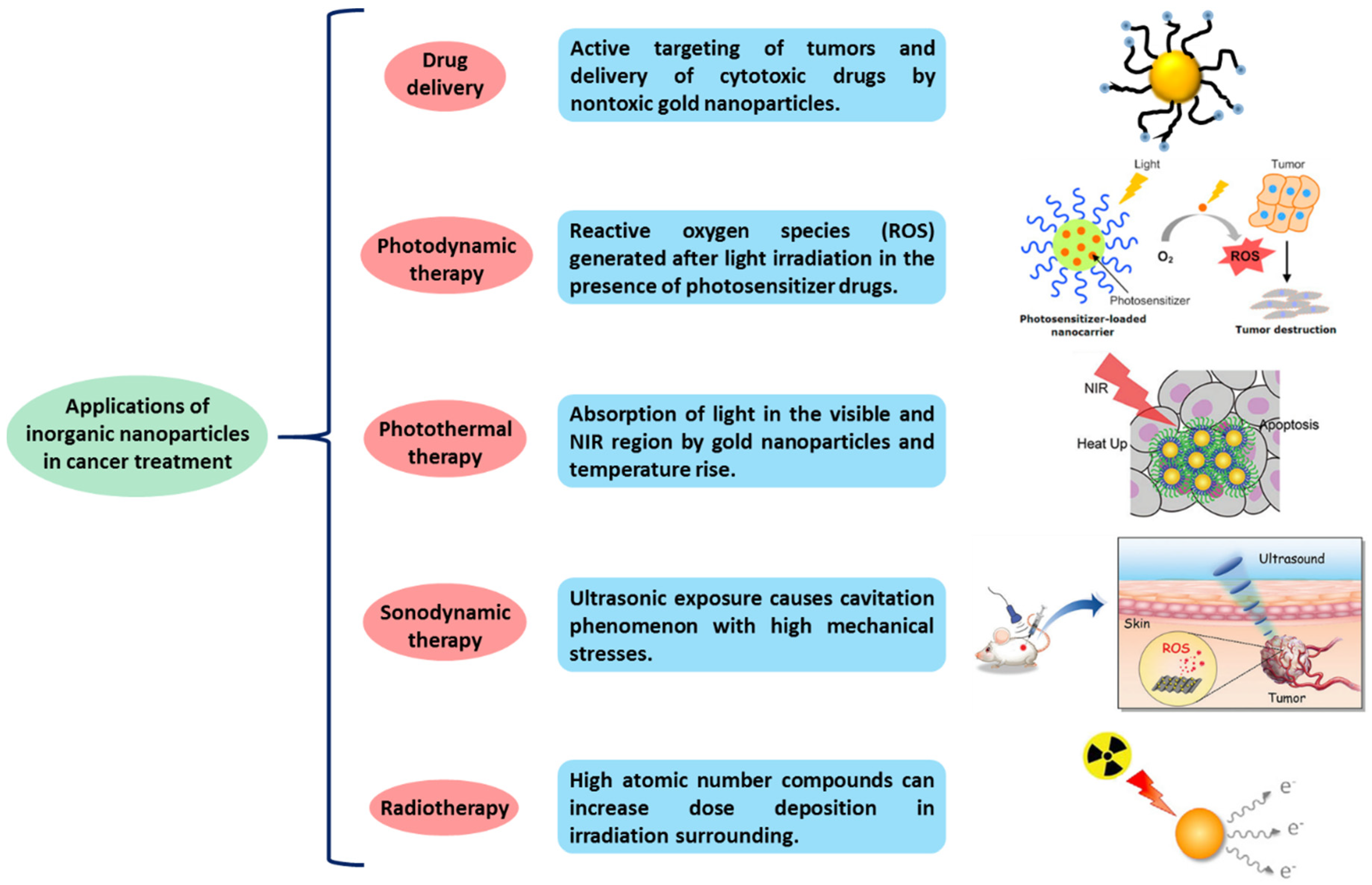
3.1. Inorganic Nanoparticles
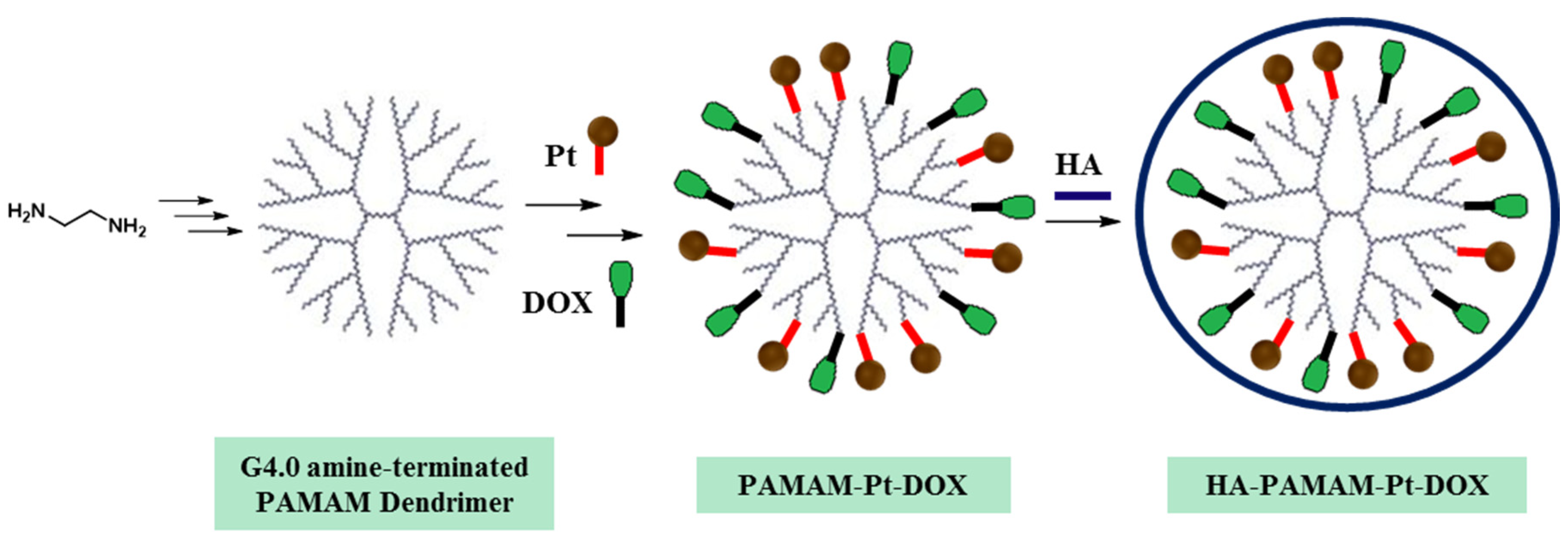
3.2. Dendrimers
- The internal core.
- Branches: The interior layers or also called generations composed of repeating units.
- Surface moieties: Outer part, which involves the peripheral end groups of the most external generation.
- Divergent approach: Dendrimer grows from a multifunctional core molecule towards periphery.
- Convergent approach: The dendrimer formation starts from the peripheral end and it progresses towards the core.
- Double stage convergent approach: The building blocks are synthesized by divergent method followed by convergent dendrimer assembly.
- Cisplatin (Pt): an anticancer drug used in chemotherapy to treat diverse types of cancer like testicular cancer, ovarian cancer, cervical cancer, breast cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer and brain tumors.
- Doxorubicin (DOX): an anticancer drug from the anthracycline family. Applied in the treatment of distinct human tumors (bladder, stomach, ovaries, lung, and thyroid among others).
3.3. Protein Nanoparticles
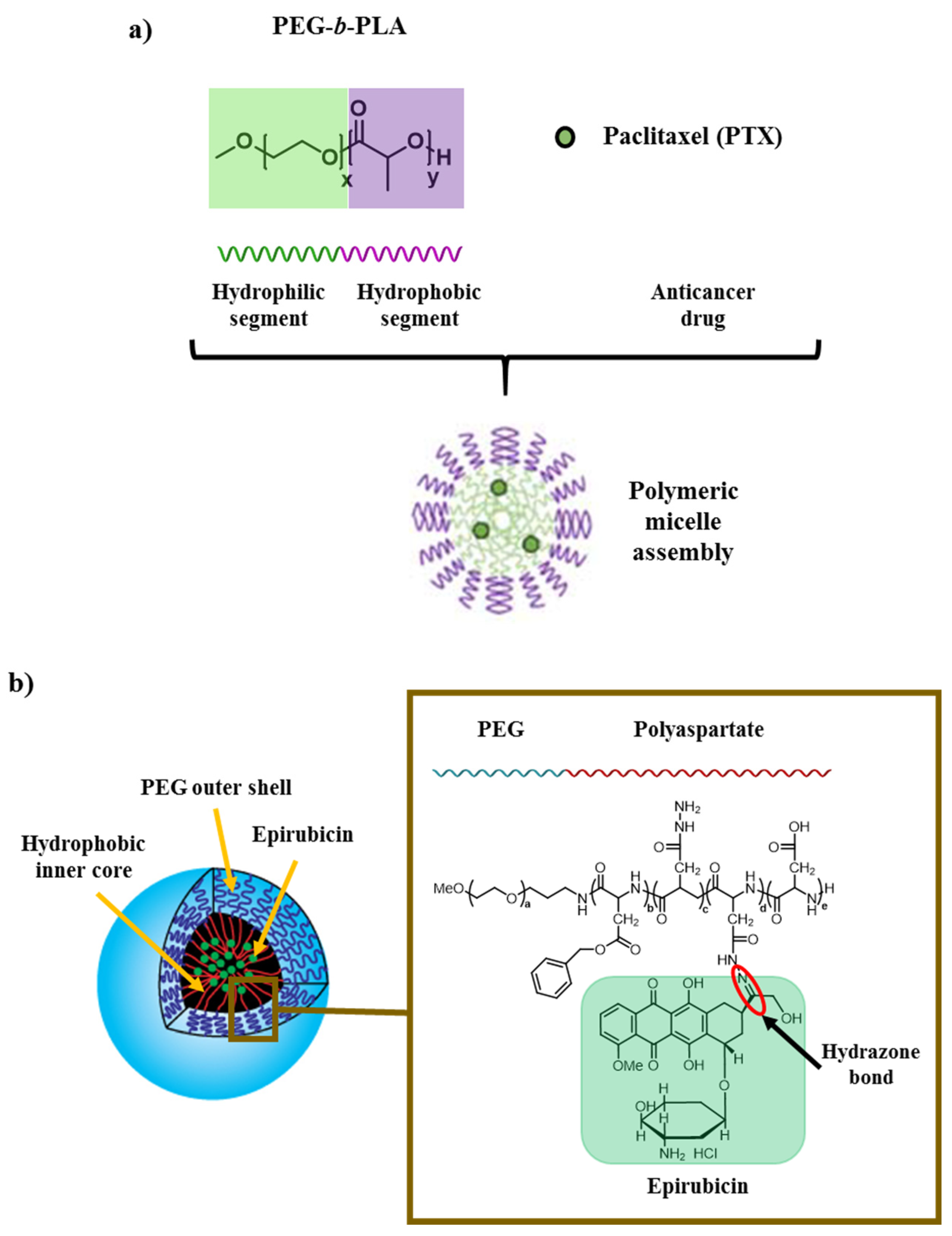
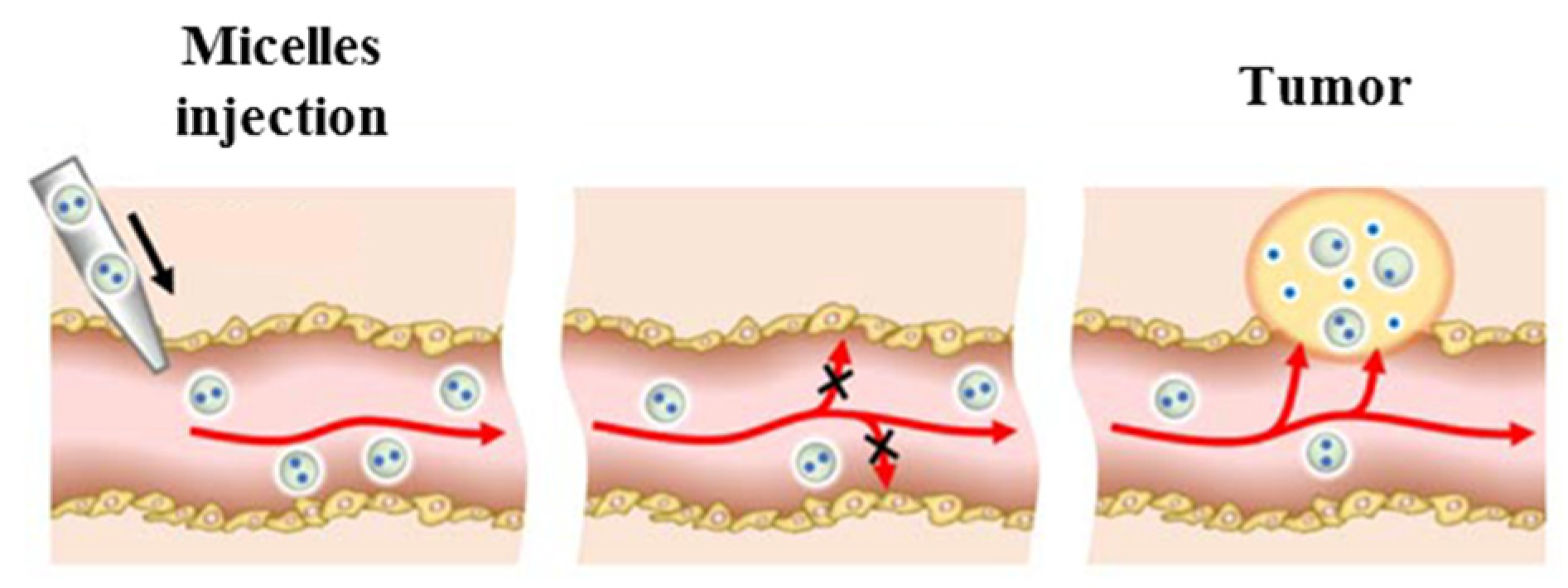
3.4. Polymeric Micelles
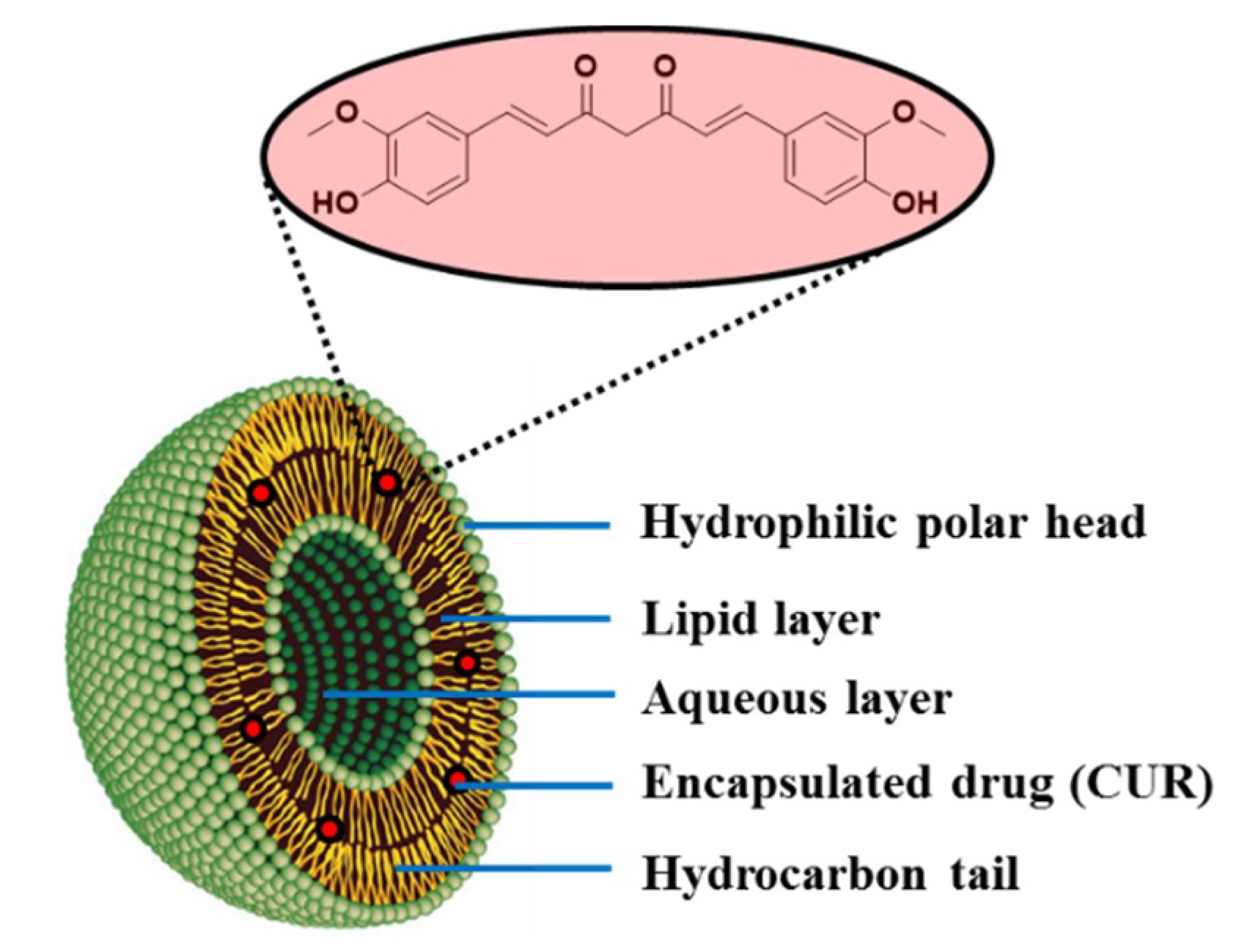
3.5. Liposomes
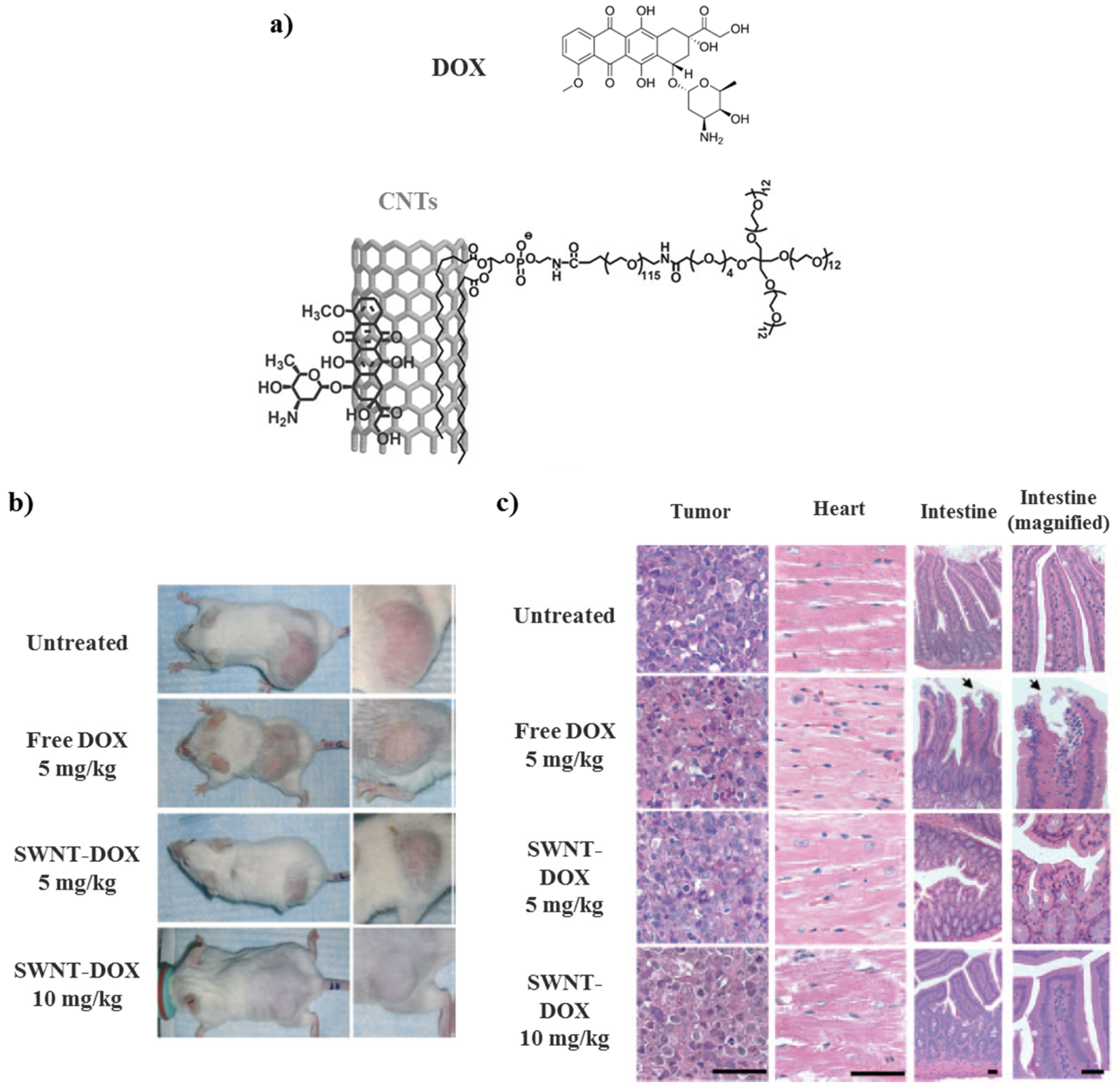
3.6. Carbon Nanotubes (CNTs)
3.7. Quantum Dots (QDs)
- Low toxicity compared to inorganic nanoparticles.
- Strong fluorescence intensity compared to organic fluorophores in biomedical imaging.
- Improved aqueous solubility upon surface modification.
3.8. Biopolymeric Nanoparticles
- Higher plants: Starch, cellulose, guar gum, gum arabic.
- Animals: Chitin, chitosan, glycosaminoglycans, hyaluronic acid.
- Microorganisms: Dextran, gellan gum, xanthan gum, bacterial cellulose.
- Algae: Alginate, galactans, carrageenan.
3.9. Overview
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wiwanitkit, V. Cancer nanotherapy: Concept for design of new drug. J. Med. Hypotheses Ideas 2013, 7, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Costa, J. Cancer. Available online: https://www.britannica.com/science/cancer-disease (accessed on 2 January 2020).
- Afshar, M.; Madani, S.; Tarazoj, A.A.; Papi, S.H.; Otroshi, O.; Gandomani, H.S.; Rahimi, A.; Salehiniya, H. Physical Activity and Types of Cancer. WCRJ 2018, 5, 1–11. [Google Scholar]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. World Cancer Report 2014; IARC: Lyon, France, 2014. [Google Scholar]
- Tsuruo, T.; Naito, M.; Tomida, A.; Fujita, N.; Mashima, T.; Sakamoto, H.; Haga, N. Molecular targeting therapy of cancer: Drug resistance, apoptosis and survival signal. Cancer Sci. 2003, 94, 15–21. [Google Scholar] [CrossRef]
- Campbell, P.J.; Getz, G.; Korbel, J.O.; Stuart, J.M.; Jennings, J.L.; Stein, L.D.; Perry, M.D.; Nahal-Bose, H.K.; Ouellette, B.F.F.; Li, C.H.; et al. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nanotoday 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Pandit, A.; Zeugolis, D.I. Twenty-five years of nano-bio-materials: Have we revolutionized healthcare? Nanomed. UK 2016, 11, 985–987. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.; Fernandes, A.R.; Baptista, P.V. Nanoparticles as Delivery Systems in Cancer Therapy: Focus on Gold Nanoparticles and Drugs. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S., Ranjan, S., Dasgupta, N., Kumar, R., Thomas, S., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2019; pp. 257–295. [Google Scholar]
- Freitas, R.A. Nanomedicine, Volume I: Basic Capabilities, 1st ed.; Landes Bioscience: Austin, TX, USA, 1999. [Google Scholar]
- Kawasaki, E.S.; Player, A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomed. Nanotechnol. 2005, 1, 101–109. [Google Scholar] [CrossRef]
- Bae, K.H.; Chung, H.J.; Park, T.G. Nanomaterials for Cancer Therapy and Imaging. Mol. Cells 2011, 31, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Surface Engineering of Iron Oxide Nanoparticles for Targeted Cancer Therapy. Acc. Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.K.; Ho, D. Cancer Nanomedicine: From Drug Delivery to Imaging. Sci. Transl. Med. 2013, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Tar. 2018, 3, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, D. Polymers and Polymer Nanocomposites for Cancer Therapy. Appl. Sci.-Basel 2019, 9, 3899. [Google Scholar] [CrossRef] [Green Version]
- Lim, Z.Z.J.; Li, J.E.J.; Ng, C.T.; Yung, L.Y.L.; Bay, B.H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Vlăsceanu, G.M.; Marin, Ş.; Ţiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M.; Andronescu, E. Silver nanoparticles in cancer therapy. In Nanobiomaterials in Cancer Therapy: Applications of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew: Oxford, UK, 2016; pp. 29–56. [Google Scholar]
- Martinkova, P.; Brtnicky, M.; Kynicky, J.; Pohanka, M. Iron Oxide Nanoparticles: Innovative Tool in Cancer Diagnosis and Therapy. Adv. Healthc. Mater. 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Tan, W.; Wang, K.; He, X.; Zhao, X.J.; Drake, T.; Wang, L.; Bagwe, R.P. Bionanotechnology based on silica nanoparticles. Med. Res. Rev. 2004, 24, 621–638. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Lu, L.; Qiao, Y.; Ravi, S.; Salatan, F.; Melancon, M.P. Stimuli-Responsive Gold Nanoparticles for Cancer Diagnosis and Therapy. J. Funct. Biomater. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.J. Gold Nanoparticles as a Delivery Vehicle in Biomedical Applications. In Gold Nanoparticles: Properties, Characterization and Fabrication; Chow, P.E., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 225–243. [Google Scholar]
- Khlebtsov, N.G.; Dykman, L.A. Optical properties and biomedical applications of plasmonic nanoparticles. J. Quant. Spectrosc. Radiat. Trans. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Liang, H.-P.; Wan, L.-J.; Bai, C.-L.; Jiang, L. Gold Hollow Nanospheres: Tunable Surface Plasmon Resonance Controlled by Interior-Cavity Sizes. J. Phys. Chem. B 2005, 109, 7795–7800. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Black, K.C.L.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.-Y.; Li, M.; Kim, P.; et al. Comparison Study of Gold Nanohexapods, Nanorods, and Nanocages for Photothermal Cancer Treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef]
- Liu, M.Z.; Guyot-Sionnest, P. Mechanism of Silver(I)-Assisted Growth of Gold Nanorods and Bipyramids. J. Phys. Chem. B 2005, 109, 22192–22200. [Google Scholar] [CrossRef]
- Wang, H.; Brandl, D.W.; Le, F.; Nordlander, P.; Halas, N.J. Nanorice: A Hybrid Plasmonic Nanostructure. Nano Lett. 2006, 6, 827–832. [Google Scholar] [CrossRef]
- Ye, J.; Van Dorpe, P.; Van Roy, W.; Borghs, G.; Maes, G. Fabrication, Characterization, and Optical Properties of Gold Nanobowl Submonolayer Structures. Langmuir 2009, 25, 1822–1827. [Google Scholar] [CrossRef]
- Sanchez-Gaytan, B.L.; Park, S.-J. Spiky Gold Nanoshells. Langmuir 2010, 26, 19170–19174. [Google Scholar] [CrossRef]
- Nehl, C.L.; Liao, H.; Hafner, J.H. Optical Properties of Star-Shaped Gold Nanoparticles. Nano Lett. 2006, 6, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.H.; Zhang, L.M.; Chen, Q.T.; Zhang, Y.; Zhang, Z.J. Synthesis of a novel magnetic drug delivery system composed of doxorubicin-conjugated Fe3O4 nanoparticle cores and a PEG-functionalized porous silica shell. Chem. Commun. 2010, 46, 8633–8635. [Google Scholar] [CrossRef] [Green Version]
- Barreto, J.A.; O’Malley, W.; Kubeil, M.; Graham, B.; Stephan, H.; Spiccia, L. Nanomaterials: Applications in Cancer Imaging and Therapy. Adv. Mater. 2011, 23, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-N.; Zhang, C.-Q.; Wang, W.; Wang, P.C.; Zhou, J.-P.; Liang, X.-J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar] [PubMed]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: Advances, challenges, and outlook. Int. J. Nanomed. 2017, 12, 87–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, H.; Chen, W.; Xu, Z.P.; Qian, G.; An, J.; Zhang, H. Shape-Controlled Hollow Mesoporous Silica Nanoparticles with Multifunctional Capping for In Vitro Cancer Treatment. Chem. Eur. J. 2017, 23, 10878–10885. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Lim, J.Z.Z.; Ng, C.-T.; Li, J.J.; Yung, L.Y.L.; Bay, B.-H. Silver Nanoparticles in Cancer: Therapeutic Efficacy and Toxicity. Curr. Med. Chem. 2013, 20, 772–781. [Google Scholar]
- Fahimirada, S.; Ajalloueianb, F.; Ghorbanpourc, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotox. Environ. Safe 2019, 168, 260–278. [Google Scholar] [CrossRef]
- Barabadi, H.; Kamali, K.D.; Shoushtari, F.J.; Tajani, B.; Mahjoub, M.A.; Alizadeh, A.; Saravanan, M. Emerging Theranostic Silver and Gold Nanomaterials to Combat Prostate Cancer: A Systematic Review. J. Clust. Sci. 2019, 30, 1375–1382. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vogtle, F. Cascade-chain-like and nonskid-chain-like syntheses of molecular cavity topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Hirao, A.; Yoo, H.S. Dendrimer-like star-branched polymers: Novel structurally well-defined hyperbranched polymers. Polym. J. 2011, 43, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, S.; Das, M.K. Dendrimers and their Applications as Novel Drug Delivery Carriers. J. Appl. Pharm. Sci. 2013, 3, 142–149. [Google Scholar]
- Morgan, M.T.; Nakanishi, Y.; Kroll, D.J.; Griset, A.P.; Carnahan, M.A.; Wathier, M.; Oberlies, N.H.; Manikumar, G.; Wani, M.C.; Grinstaff, M.W. Dendrimer-encapsulated camptothecins: Increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res. 2006, 66, 11913–11921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzyniak, W.; Schmidt, J.; Glac, W.; Berkholz, J.; Steinemann, G.; Hoffmann, B.; Ermilov, E.A.; Gürek, A.G.; Ahsen, V.; Nitzsche, B.; et al. Novel zinc phthalocyanine as a promising photosensitizer for photodynamic treatment of esophageal cancer. Int. J. Oncol. 2017, 50, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zheng, K.; Xuan, G.; Huang, M.; Xue, J. Novel pH-sensitive zinc phthalocyanine assembled with albumin for tumor targeting and treatment. Int. J. Nanomed. 2018, 13, 7681–7695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug Delivery Systems for Phthalocyanines for Photodynamic Therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crampton, H.L.; Simanek, E.E. Dendrimers as drug delivery vehicles: Non-covalent interactions of bioactive compounds with dendrimers. Polym. Int. 2007, 56, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1–21. [Google Scholar]
- Guo, X.L.; Kang, X.X.; Wang, Y.Q.; Zhang, X.J.; Li, C.J.; Liu, Y.; Du, L.B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019, 84, 367–377. [Google Scholar] [CrossRef]
- Bassas-Galia, M.; Follonier, S.; Pusnik, M.; Zinn, M. Natural polymers: A source of inspiration. In Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Perale, G., Hilborn, J., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 31–64. [Google Scholar]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Biomed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gulfam, M.; Kim, J.E.; Lee, J.M.; Ku, B.; Chung, B.H.; Chung, B.G. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir 2012, 28, 8216–8223. [Google Scholar] [CrossRef]
- Seib, F.P.; Jones, G.T.; Rnjak-Kovacina, J.; Lin, Y.; Kaplan, D.L. pH-Dependent anticancer drug release from silk nanoparticles. Adv. Healthc. Mater. 2013, 2, 1606–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskin, D.; Tezcaner, A. Micelles as Delivery System for Cancer Treatment. Curr. Pharm. Des. 2017, 23, 5230–5241. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016, 10, 348–359. [Google Scholar] [CrossRef]
- Takahashi, A.; Yamamoto, Y.; Yasunaga, M.; Koga, Y.; Kuroda, J.; Takigahira, M.; Harada, M.; Saito, H.; Hayashi, T.; Kato, Y.; et al. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013, 104, 920–925. [Google Scholar] [CrossRef]
- Lee, S.C.; Huh, K.M.; Lee, J.; Cho, Y.W.; Galinsky, R.E.; Park, K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: In vitro and in vivo characterization. Biomacromolecules 2007, 8, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Kim, S. Liposomes as carriers of cancer chemotherapy. Current status and future prospects. Drugs 1993, 46, 618–638. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.R.; Yadav, J.D.; Vaidya, K.A. Liposomes: A novel drug delivery system. Int. J. Curr. Pharm. Res. 2011, 3, 10–18. [Google Scholar]
- Bangham, A.D.; Horne, R.W. Action of saponin on biological cell membranes. Nature 1962, 196, 952–953. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-targeted liposome design: Challenges and fundamental considerations. Trends Biotechnol. 2014, 32, 32–45. [Google Scholar] [CrossRef]
- Kale, M.; Suruse, P.; Singh, R.; Malhotra, G.; Raut, P. Effect of size reduction techniques on doxorubicin hydrochloride loaded liposomes. Int. J. Biol. Pharm. Res. 2012, 3, 308–316. [Google Scholar]
- Xiong, X.B.; Huang, Y.; Lu, W.L.; Zhang, X.; Zhang, H.; Nagai, T.; Zhang, Q. Enhanced intracellular delivery and improved antitumor efficacy of doxorubicin by sterically stabilized liposomes modified with a synthetic RGD mimetic. J. Control. Release 2005, 107, 262–275. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC Adv. 2017, 7, 25978–25986. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Gu, L.; Meziani, M.J.; Wang, X.; Luo, P.G.; Veca, L.M.; Cao, L.; Sun, Y.P. Advances in Bioapplications of Carbon Nanotubes. Adv. Mater. 2009, 21, 139–152. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Liu, Z. Carbon nanotubes in biology and medicine: An overview. Chin. Sci. Bull. 2012, 57, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, P.; Marches, R.; Zimmerman, N.S.; Swafford, A.D.E.; Bajaj, P.; Musselman, I.H.; Pantano, P.; Draper, R.K.; Vitetta, E.S. Thermal ablation of tumor cells with anti body-functionalized single-walled carbon nanotubes. Proc. Natl. Acad. Sci. USA 2008, 105, 8697–8702. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.T.; Welsher, K.; Tabakman, S.M.; Sherlock, S.P.; Wang, H.; Luong, R.; Dai, H. High Performance In Vivo Near-IR (>1 μm) Imaging and Photothermal Cancer Therapy with Carbon Nanotubes. Nano Res. 2010, 3, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wieckowski, S.; Pastorin, G.; Benincasa, M.; Klumpp, C.; Briand, J.P.; Gennaro, R.; Prato, M.; Bianco, A. Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew. Chem. Int. Ed. 2005, 44, 6358–6362. [Google Scholar] [CrossRef]
- Bhirde, A.A.; Patel, V.; Gavard, J.; Zhang, G.; Sousa, A.A.; Masedunskas, A.; Leapman, R.D.; Weigert, R.; Gutkind, J.S.; Rusling, J.F. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 2009, 3, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Fan, A.C.; Rakhra, K.; Sherlock, S.; Goodwin, A.; Chen, X.; Yang, Q.; Felsher, D.W.; Dai, H. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew. Chem. Int. Ed. 2009, 48, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.E.; Fong, C.Y.; Pickett, W.E. Quantum confinement in CdSe nanocrystallites. J. Non Cryst. Solids 2002, 299, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Tomasulo, M.; Yildiz, I.; Raymo, F.M. pH-sensitive quantum dots. J. Phys. Chem. B 2006, 110, 3853–3855. [Google Scholar] [CrossRef]
- Ekimov, A.I.; Efros, A.L.; Onushchenko, A.A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- Shi, Y.; Pramanik, A.; Tchounwou, C.; Pedraza, F.; Crouch, R.A.; Chawa, S.R.; Vangara, A.; Sinha, S.S.; Jones, S.; Sardar, D.; et al. Multifunctional biocompatible graphene oxide quantum dots decorated magnetic nanoplatform for efficient capture and two-photon imaging of rare tumor cells. ACS Appl. Mater. Inter. 2015, 7, 10935–10943. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Cai, W.B.; Shin, D.W.; Chen, K.; Gheysens, O.; Cao, Q.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef]
- Xu, G.; Zeng, S.; Zhang, B.; Swihart, M.T.; Yong, K.T.; Prasad, P.N. New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine. Chem. Rev. 2016, 116, 12234–12327. [Google Scholar] [CrossRef]
- Zheng, F.F.; Zhang, P.H.; Xi, Y.; Chen, J.J.; Li, L.L.; Zhu, J.J. Aptamer/Graphene Quantum Dots Nanocomposite Capped Fluorescent Mesoporous Silica Nanoparticles for Intracellular Drug Delivery and Real-Time Monitoring of Drug Release. Anal. Chem. 2015, 87, 11739–11745. [Google Scholar] [CrossRef]
- Huang, C.L.; Huang, C.C.; Mai, F.D.; Yen, C.L.; Tzing, S.H.; Hsieh, H.T.; Ling, Y.C.; Chang, J.Y. Application of paramagnetic graphene quantum dots as a platform for simultaneous dual-modality bioimaging and tumor-targeted drug delivery. J. Mater. Chem. B 2015, 3, 651–664. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots−Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Inter. 2016, 8, 22442–22450. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Batool, A.; Noreen, S.; Maqbool, I.; Rehman, F.; Kashif, P.M.; Tahir, N.; Raza, A. Novel nanoparticulate systems for lung cancer therapy: An updated review. J. Drug Target. 2017, 25, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [Green Version]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohyd. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef]
- Ntoutoume, G.M.A.N.; Granet, R.; Mbakidi, J.P.; Bregier, F.; Leger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V.; et al. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorg. Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef]
- Kathle, P.K.; Gautam, N.; Kesavan, K. Tamoxifen citrate loaded chitosan-gellan nanocapsules for breast cancer therapy: Development, characterisation and in-vitro cell viability study. J. Microencapsul. 2018, 35, 292–300. [Google Scholar] [CrossRef]
- Raţă, D.M.; Chailan, J.F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan: Poly(N-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—A promising alternative for the lung cancer treatment. J. Nanopart. Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [Green Version]
- Grazú, V.; Moros, M.; Sánchez-Espinel, C. Nanocarriers as Nanomedicines: Design Concepts and Recent Advances. In Nanobiotechnology—Inorganic Nanoparticles vs. Organic Nanoparticles; De La Fuente, J.M., Grazú, V., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2012; pp. 337–440. [Google Scholar]





| Nanostructure | Advantages | Drawbacks |
|---|---|---|
| Inorganic nanoparticles | Facile synthesis Easy surface functionalization Good stability Versatility Exceptional optical and electronic properties | Non-biodegradable Toxicity Coating required |
| Dendrimers | Synthesis of well-defined structures High chemical and biological stability Efficacy, purity and long shelf life High surface area, loading capacity and targeting Biodegradable and biocompatible | Complex synthetic route Low yield and difficulties in obtaining higher generations |
| Protein nanoparticles | Low toxicity Biodegradability Good mechanical properties Versatility | Chemical modifications of their surface are usually required to yield stimulus-responsive nanomedicines Low drug loading efficiency |
| Polymeric micelles | Efficient carrier system for hydrophilic drugs Biodegradable and biocompatible Self-assembling Potential targeting Functional modification Low toxicity | Short circulation times in blood Specific cytotoxicity Need of surface modifications |
| Liposomes | Amphiphilic structures Easy surface functionalization Biocompatibility | Conventional liposomes: Instability Insufficient drug loading Faster drug release Shorter circulation times in blood |
| CNTs | Quasi 1D nanostructure Easy surface functionalization Exceptional surface area and cell membrane penetrability Efficient loading Remarkable optical and electronic properties | Poor solubility in many solvents including water Low biodegradability Toxicity |
| QDs | Good solubility in water after surface modification Strong fluorescence intensity | Non-biodegradable Citotoxicity to lung cells Induction of oxidative stress |
| Biopolymeric nanoparticles | Isolated from different natural resources (abundance) Excellent geometrical dimensions High specific surface area Good mechanical and barrier properties Lack of toxicity Biodegradable and biocompatible | Hydrophobic materials Poor encapsulation efficiency of medicines Resistance against enzymatic degradation |
| Type of Nanostructure | Anticancer Agent | Applications in Cancer Therapy | Reference |
|---|---|---|---|
| F3O4 nanoparticles | DOX | Controlled drug delivery system/Magnetic carrier | [37] |
| Hollow MSNs | DOX | Controlled drug delivery system under the application of an external stimuli (pH) | [41] |
| (PGLSA)-COONa) dendrimer | 10HCPT | Controlled drug delivery system of hydrophobic drugs | [48] |
| Dendritic phthalocyanine systems | Zinc phthalocyanine | Controlled drug delivery system by photochemical activation | [52] |
| PAMAM dendrimer coated with Hyaluronic acid | Pt and DOX | Controlled drug delivery system under the application of an external stimuli (pH) | [54] |
| Gliadin and Gliadin-gelatin nanoparticles | Cyclophosphamide | Controlled release of anticancer drug in breast cancer cells | [57] |
| Silk nanoparticles | DOX | Controlled drug delivery system under the application of an external stimuli (pH) | [58] |
| PEG-Polyaspartate micelle | Epirubicin | Controlled drug delivery system under the application of an external stimuli (pH) | [62] |
| PEG-b-PLA micelle | PTX | Controlled drug delivery system of hydrophobic drugs | [63] |
| Liposome | DOX | Controlled drug delivery system of toxic drugs | [73] |
| Liposome | CUR | Controlled drug delivery system | [76] |
| PEG-SWNTs | PTX | Controlled drug delivery system | [85] |
| SWNTs | Pt | Targeted drug delivery system | [87] |
| PEG-SWNTs | DOX | Controlled drug delivery system | [88] |
| Graphene oxide QDs covered with luminescent magnetic nanoplatform | - | Human cancer imaging applications | [92] |
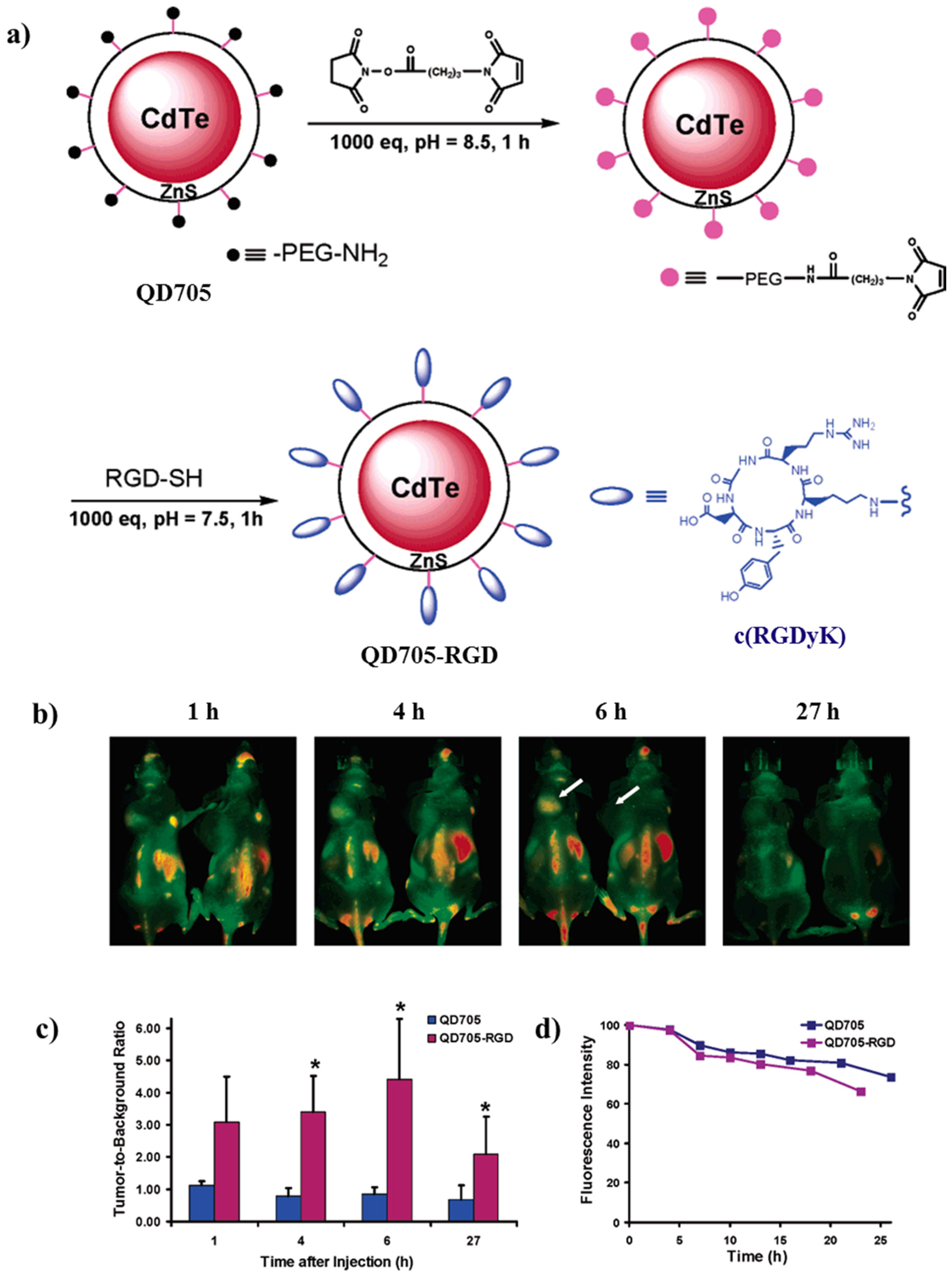
| Tripeptide-tagged (arginine-glycine-aspartic) CdTe/ZnS QDs | - | Targeted near-infrared imaging of tumors Cancer detection and management including imaging-guided surgery | [95] |
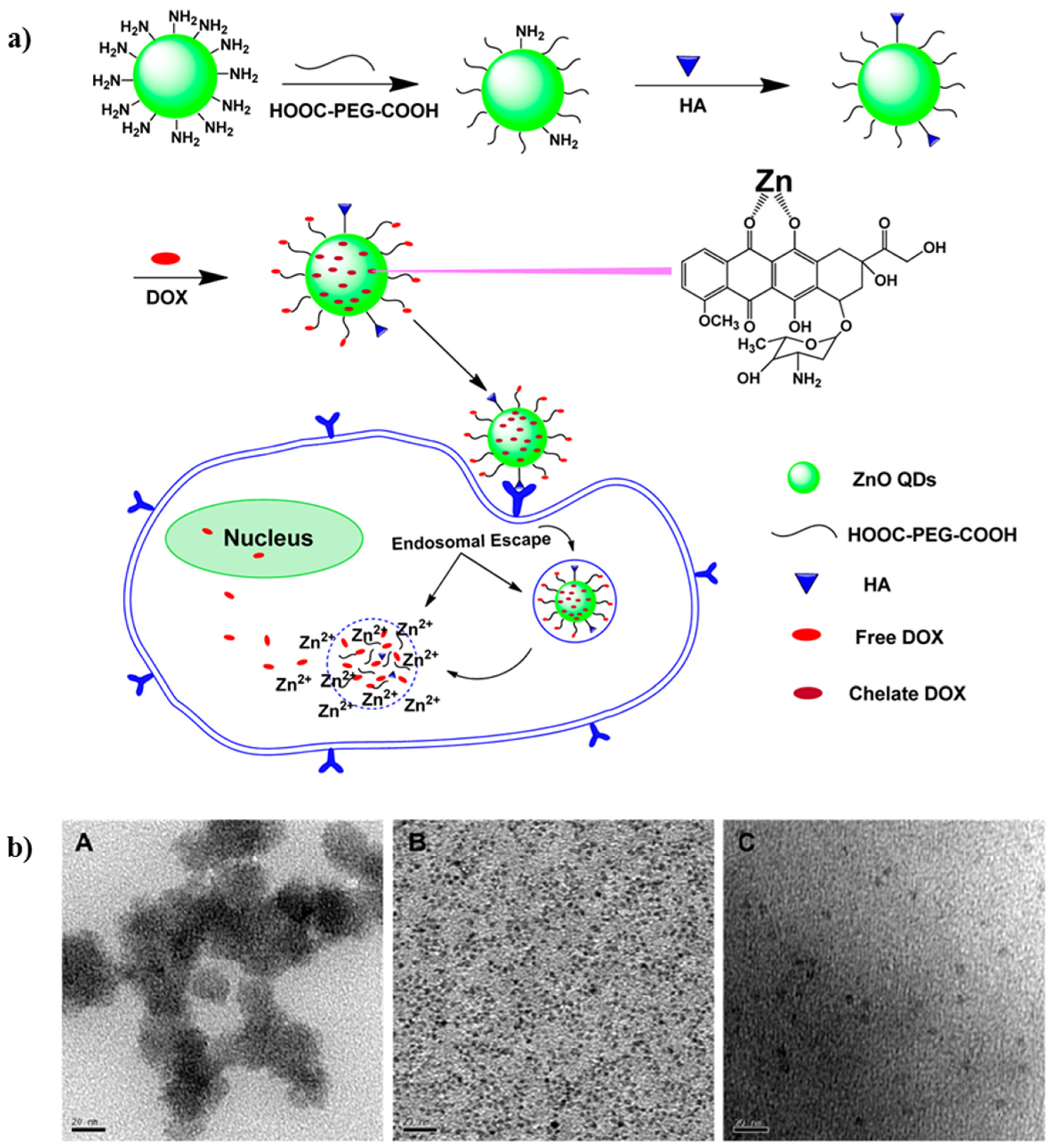
| ZnO QDs | DOX | Controlled drug delivery system under the application of an external stimuli (pH) | [99] |
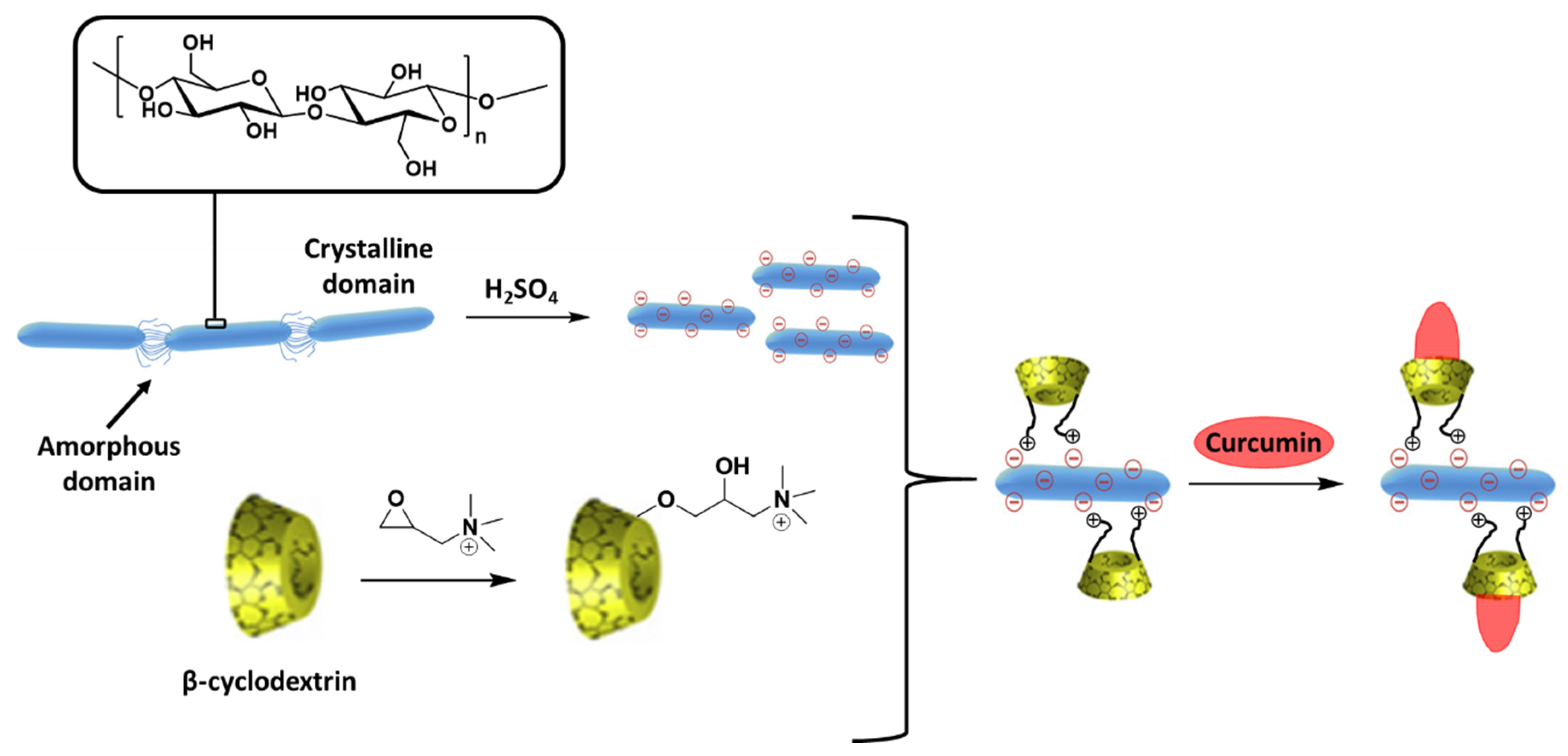
| β-cyclodextrin attached to cellulose nanocrystals | CUR | Targeted and controlled drug delivery system | [104] |
| Chitosan-gellan gum | Tamoxifen citrate | Controlled drug delivery system | [105] |
| Chitosan-poly(N-vinylpyrrolidone-alt-itaconic anhydride) | 5-FU | Controlled drug delivery system under the application of an external stimuli (pH) | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605. https://doi.org/10.3390/molecules25071605
Montané X, Bajek A, Roszkowski K, Montornés JM, Giamberini M, Roszkowski S, Kowalczyk O, Garcia-Valls R, Tylkowski B. Encapsulation for Cancer Therapy. Molecules. 2020; 25(7):1605. https://doi.org/10.3390/molecules25071605
Chicago/Turabian StyleMontané, Xavier, Anna Bajek, Krzysztof Roszkowski, Josep M. Montornés, Marta Giamberini, Szymon Roszkowski, Oliwia Kowalczyk, Ricard Garcia-Valls, and Bartosz Tylkowski. 2020. "Encapsulation for Cancer Therapy" Molecules 25, no. 7: 1605. https://doi.org/10.3390/molecules25071605
APA StyleMontané, X., Bajek, A., Roszkowski, K., Montornés, J. M., Giamberini, M., Roszkowski, S., Kowalczyk, O., Garcia-Valls, R., & Tylkowski, B. (2020). Encapsulation for Cancer Therapy. Molecules, 25(7), 1605. https://doi.org/10.3390/molecules25071605












