Analysis of the Gas Phase Acidity of Substituted Benzoic Acids Using Density Functional Concepts
Abstract
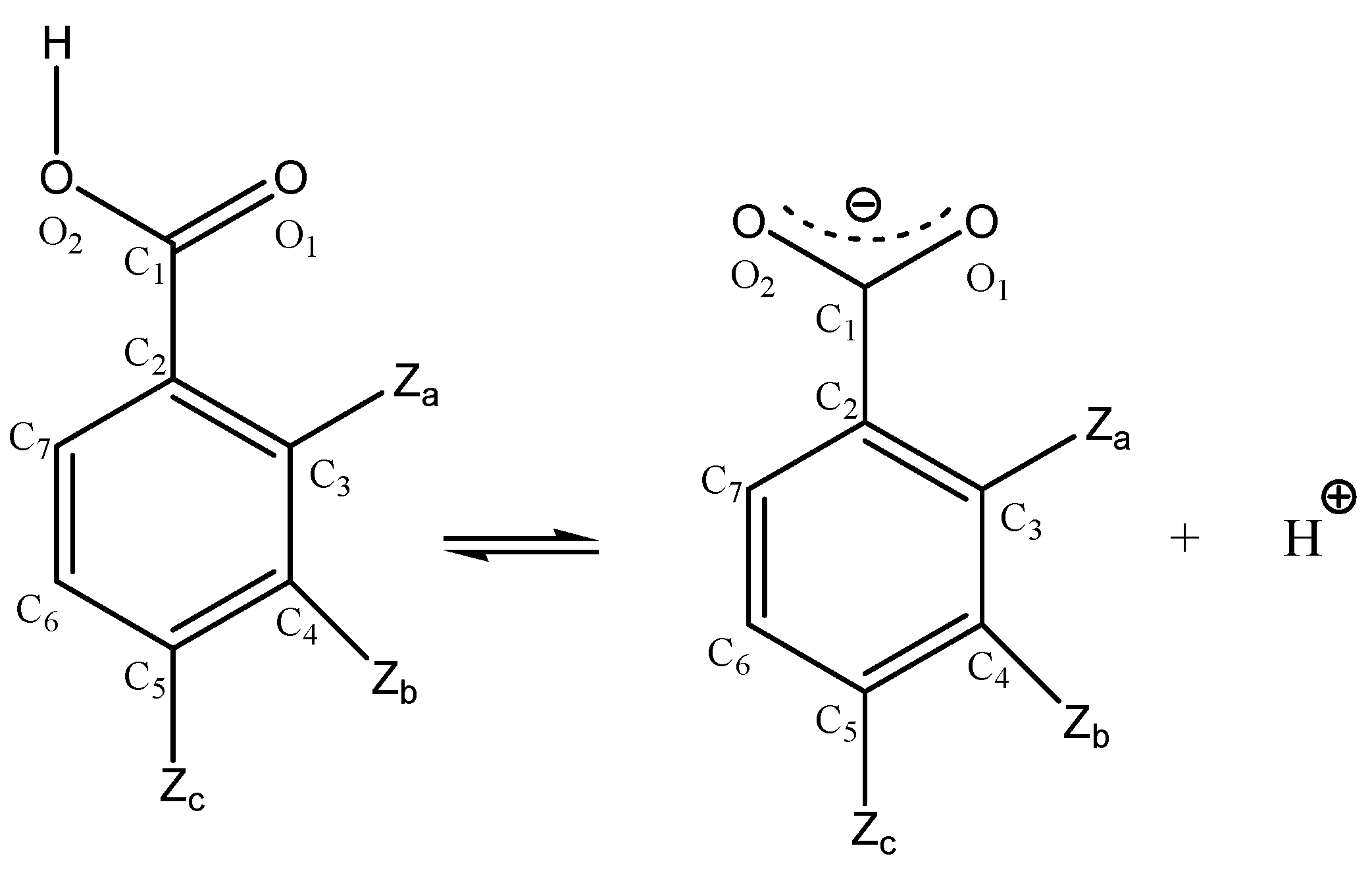
1. Introduction
2. Results and Discussion
3. Methodology and Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammett, L.P. Physical Organic Chemistry, 2nd ed.; McGraw-Hill: New York, NY, USA, 1970; pp. 146–177. ISBN 978-0070259058. [Google Scholar]
- Exner, O.; Böhm, S. Background of the Hammett Equation As Observed for Isolated Molecules: Meta- and Para-Substituted Benzoic Acids. J. Org. Chem. 2002, 67, 6320–6327. [Google Scholar] [CrossRef]
- Böhm, S.; Fiedller, P.; Exner, O. Analysis of the ortho effect: Acidity of 2-substituted benzoic acids. New J. Chem. 2004, 28, 67–74. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Stepurko, E.N.; Zherikova, K.V. Benzoic acid derivatives: Evaluation of thermochemical properties with complementary experimental and computational method. Thermochim. Acta 2015, 622, 18–30. [Google Scholar] [CrossRef]
- Taft, R.W.; Topsom, R.D. The Nature and Analysis of substitutent electronic effects Prog. Phys. Org. Chem. 1987, 16, 1–83. [Google Scholar] [CrossRef]
- Taft, R.W.; Koppel, I.A.; Topsom, R.D.; Anvia, F. Acidities of OH compounds, including alcohols, phenol, carboxylic acids, and mineral acids. J. Am. Chem. Soc. 1990, 112, 2047–2052. [Google Scholar] [CrossRef]
- Abboud, J.-L.M.; Catalán, J.; Elguero, J.; Taft, W. Polarizability effects on the aqueous solution basicity of substituted pyridines. J. Org. Chem. 1987, 53, 1137–1140. [Google Scholar] [CrossRef]
- Roithova, J.; Exner, O. Protonation of alkylpyridines: Polarizability and steric effects in the base and in the cation. J. Phys. Org. Chem. 2001, 14, 752–758. [Google Scholar] [CrossRef]
- Kulhánek, J.; Decouzon, M.; Gal, J.-F.; Maria, P.-C.; Fiedler, P.; Jiménez, P.; Roux, M.-V.; Exner, O. Steric effect and steric hindrance to resonance in tert-butylbenzoic acids in the gas phase and the solution. Eur. J. Org. Chem. 1999, 1999, 1589–1594. [Google Scholar] [CrossRef]
- De Proft, F.; Langenaeker, W.; Geerlings, P. Ab initio determination of substituent constants in a Density Functional Theory formalism: Calculation of intrinsic group electronegativity, hardness and softness. J. Phys. Chem. 1993, 97, 1826–1831. [Google Scholar] [CrossRef]
- De Proft, F.; Amira, S.; Choho, K.; Geerlings, P. Quantum Chemical Study of the acidity of substituted acetic acids with density functional theory based descriptors. J. Phys. Chem. 1994, 98, 5227–5233. [Google Scholar] [CrossRef]
- Gupta, K.; Giria, S.; Chattaraj, P.K. Acidity of meta- and para-substituted aromatic acids: A conceptual DFT study. New, J. Chem. 2008, 32, 1945–1952. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, L.; Liu, W.; Liu, S.; Liu, S. Modeling Molecular Acidity with Electronic Properties and Hammett Constants for Substituted Benzoic Acids. J. Phys. Chem. A 2011, 115, 14697–14707. [Google Scholar] [CrossRef] [PubMed]
- Vianello, R.; Maksic, Z.B. Gas-phase acidity of para-substituted benzoic acids—A triadic analysis of substituent effects. J. Phys. Org. Chem. 2005, 18, 699–705. [Google Scholar] [CrossRef]
- Hollingsworth, C.A.; Seybold, P.G.; Hadad, C.M. Substituent Effects on the Electronic Structure and pKa of Benzoic Acid. Int. J. Quantum Chem. 2002, 90, 1396–1403. [Google Scholar] [CrossRef]
- Romero María de, L.; Méndez, F. Is the Hydrogen Atomic Charge Representative of the Acidity of Parasubstituted Phenols? J. Phys. Chem. A 2003, 107, 4526–4530. [Google Scholar] [CrossRef]
- Méndez, F.; Romero, M.L.; De Proft, F.; Geerlings, P. The basicity of p-substituted phenolates and the elimination-substitution ratio in p-nitrophenethyl bromide: A HSAB theoretical study. J. Org. Chem. 1998, 63, 5774–5778. [Google Scholar] [CrossRef]
- Romero, M.L.; Méndez, F. The local HSAB principle and the bond dissociation energy of p-substituted phenols. J. Phys. Chem. A 2003, 107, 5874–5875. [Google Scholar] [CrossRef]
- Ramírez, E.R.; García-Martínez, C.; Méndez, F. Influence of fluorine atoms and aromatic rings on the acidity of ethanol. J. Phys. Chem. A 2009, 113, 10753–10758. [Google Scholar] [CrossRef]
- Ramírez, E.R.; García-Martínez, C.; Méndez, F. Understanding the Nucleophilic Character and Stability of the Carbanions and Alkoxides of 1-(9-Anthryl)ethanol and Derivatives. Molecules 2013, 18, 10254–10265. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989; pp. 47–67, 978-0195092769. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Méndez, F.; Gázquez, J.L. Chemical reactivity of enolate ions: The local hard and soft acids and bases principle viewpoint. J. Am. Chem. Soc. 1994, 116, 9298–9301. [Google Scholar] [CrossRef]
- Vela, A.; Gázquez, J.L. A relationship between the static dipole polarizability, the global softness, and the fukui function. J. Am. Chem. Soc. 1990, 12, 1490–1492. [Google Scholar] [CrossRef]
- Roy, R.; Chandra, A.K.; Pal, S. Correlation of Polarizability, Hardness, and Electronegativity: Polyatomic Molecules. J. Phys. Chem. 1994, 98, 10447–10450. [Google Scholar] [CrossRef]
- Boisdenghien, Z.; Fias, S.; Da Pieve, F.; De Proft, F.; Geerlings, P. The polarizability of atoms and molecules: A comparison between a conceptual density functional theory approach and time-dependent density functional theory. Mol. Phys. 2015, 113, 1890–1898. [Google Scholar] [CrossRef]
- Komorowski, L.; Lipinski, J.; Szarek, P.; Ordon, P. Polarization justified Fukui functions: The theory and applications for molecules. J. Chem. Phys. 2011, 135, 014109. [Google Scholar] [CrossRef]
- Krishtal, A.; Senet, P.; Van Alsenoy, C. Local softness, softness dipole and polarizabilities of functional groups: Application to the side chains of the twenty amino acids. J. Chem. Phys. 2009, 131, 044312. [Google Scholar] [CrossRef]
- López, P.; Méndez, F. Fukui function as a descriptor of the imidazolium protonated cation resonance hybrid structure. Org. Lett. 2004, 6, 1781–1783. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- NIST Chemistry Web Book. Available online: http://webbook.nist.gov/chemistry/ (accessed on 12 May 2019).
- Yang, W.; Mortier, W.J. The use of global and local molecular parameters for the analysis of the gas phase basicity of amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theoret. Chim. Acta (Berl.) 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Levine, I. Quantum Chemistry, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001; pp. 154–196, 414–415, 0136855121. [Google Scholar]
- Laaksonen, L. gOpenMol, Center for Scientific Computing: Espoo, Finland.
- Domingo, L.R.; Pérez, P.; Sáezc, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr function. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Lambrinidis, G.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Biological Evaluation and In Silico Study of Benzoic Acid Derivatives from Bjerkandera adusta Targeting Proteostasis Network Modules. Molecules 2020, 25, 666. [Google Scholar] [CrossRef] [PubMed]




| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| a |
331.09 (332.46 ± 2.01) |
330.21 (332.46 ± 2.01) |
331.03 (330.31 ± 2.01) |
325.99 (-------) |
321.86 (324.33 ± 2.01) |
319.06 (-----) |
| b |
332.76 (333.65 ± 2.01) |
332.47 (332.46 ± 2.01) |
333.62 (334.61 ± 2.01) |
324.34 (325.29 ± 2.01) |
320.51 (322.18 ± 2.01) |
318.19 (-----) |
| c |
333.35 (333.89 ± 2.01) |
333.97 (333.89 ± 2.01) |
336.24 (336.28 ± 2.01) |
323.61 (325.29 ± 2.01) |
319.13 (320.98 ± 2.01) |
316.87 (-----) |
| Fragment | Position | R2 |
|---|---|---|
| Z | o | 0.1974 |
| m | 0.0004 | |
| p | 0.0493 | |
| C6H4 | o | 0.5038 |
| m | 0.855 | |
| p | 0.8021 | |
| ZC6H4 | o | 0.0011 |
| m | 0.4267 | |
| p | 0.227 | |
| ZC6H4COO | o | 0.0089 |
| m | 0.31 | |
| p | 0.0026 | |
| ZC6H4COOH | o | 0.0034 |
| m | 0.2933 | |
| p | 0.0008 | |
| qH | o | 0.9239 |
| m | 0.9938 | |
| p | 0.9903 |
| Fragment | Position | R2 |
|---|---|---|
| Z | o | 0.7333 |
| m | 0.5409 | |
| p | 0.0125 | |
| C6H4 | o | 0.4642 |
| m | 0.6352 | |
| p | 0.6654 | |
| ZC6H4 | o | 0.9415 |
| m | 0.8498 | |
| p | 0.8699 | |
| ZC6H4COO | o | 0.8422 |
| m | 0.920 | |
| p | 0.9746 | |
| ZC6H4COOH | o | 0.8266 |
| m | 0.9206 | |
| p | 0.9711 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| a | 18.79% (81.21%) | 31.26% (68.74%) | 43.31% (56.69%) | 19.78% (80.22%) | 22.69% (77.31%) | 18.20% (81.80%) |
| b | 15.51% (84.49%) | 25.80% (74.20%) | 35.76% (64.24%) | 16.33% (83.67%) | 18.74% (81.26%) | 15.03% (84.97%) |
| c | 13.71% (86.29%) | 22.82% (77.18%) | 31.61% (68.39%) | 14.44% (85.56%) | 16.57% (83.43%) | 13.29% (86.71%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amador-Balderas, J.A.; Martínez-Sánchez, M.-A.; Ramírez, R.E.; Méndez, F.; Meléndez, F.J. Analysis of the Gas Phase Acidity of Substituted Benzoic Acids Using Density Functional Concepts. Molecules 2020, 25, 1631. https://doi.org/10.3390/molecules25071631
Amador-Balderas JA, Martínez-Sánchez M-A, Ramírez RE, Méndez F, Meléndez FJ. Analysis of the Gas Phase Acidity of Substituted Benzoic Acids Using Density Functional Concepts. Molecules. 2020; 25(7):1631. https://doi.org/10.3390/molecules25071631
Chicago/Turabian StyleAmador-Balderas, Jorge A., Michael-Adán Martínez-Sánchez, Ramsés E. Ramírez, Francisco Méndez, and Francisco J. Meléndez. 2020. "Analysis of the Gas Phase Acidity of Substituted Benzoic Acids Using Density Functional Concepts" Molecules 25, no. 7: 1631. https://doi.org/10.3390/molecules25071631
APA StyleAmador-Balderas, J. A., Martínez-Sánchez, M.-A., Ramírez, R. E., Méndez, F., & Meléndez, F. J. (2020). Analysis of the Gas Phase Acidity of Substituted Benzoic Acids Using Density Functional Concepts. Molecules, 25(7), 1631. https://doi.org/10.3390/molecules25071631






