Synthesis, Characterization, and Histological Evaluation of Chitosan-Ruta Graveolens Essential Oil Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Characterization
2.2. Physical-Chemical Characterization of the Film-Forming CS+RGEO Emulsions
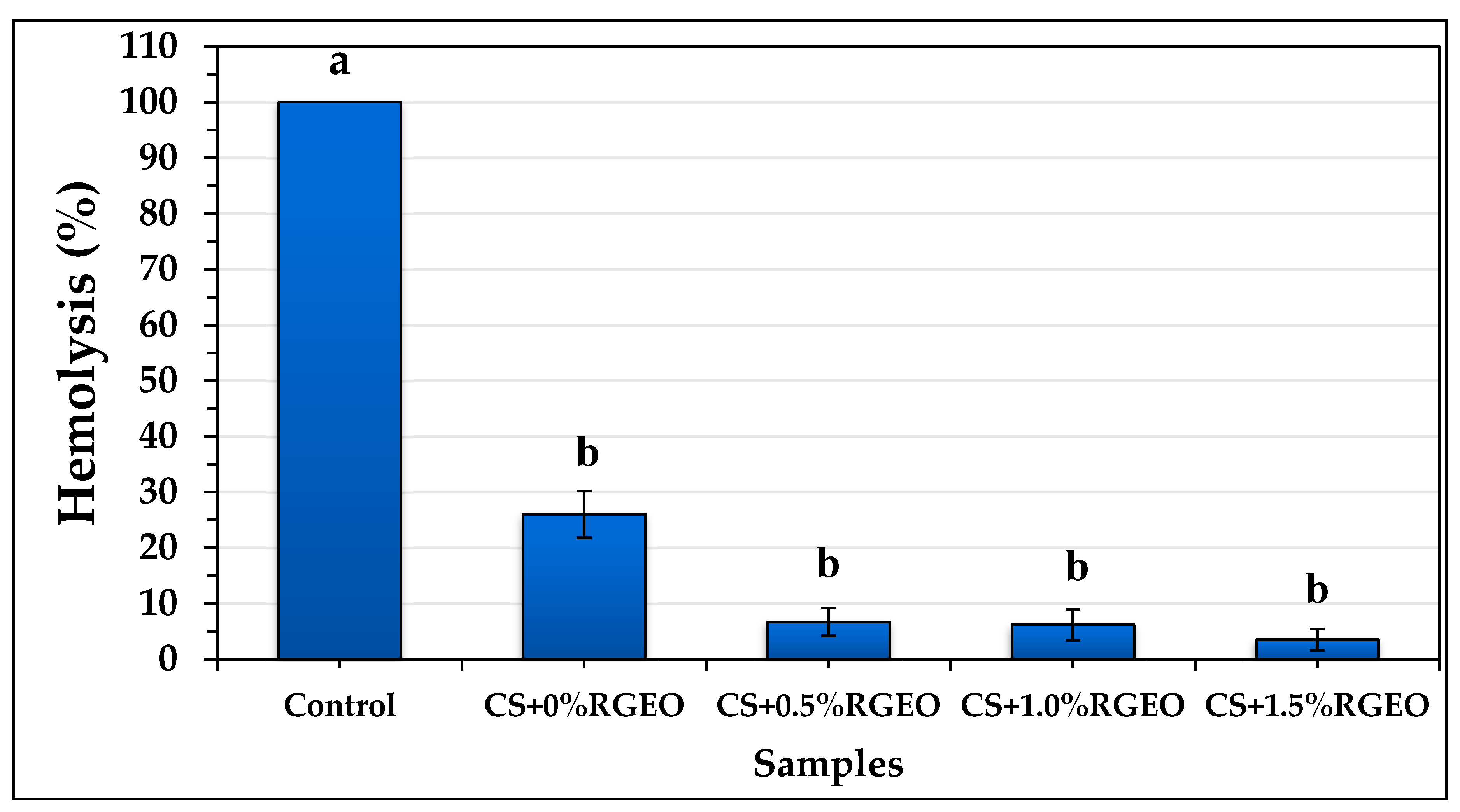
2.3. Hemolysis Assay of the Essential Oil and Coatings of CS+RGEO.
2.4. Physical-Chemical Properties of CS+RGEO Films
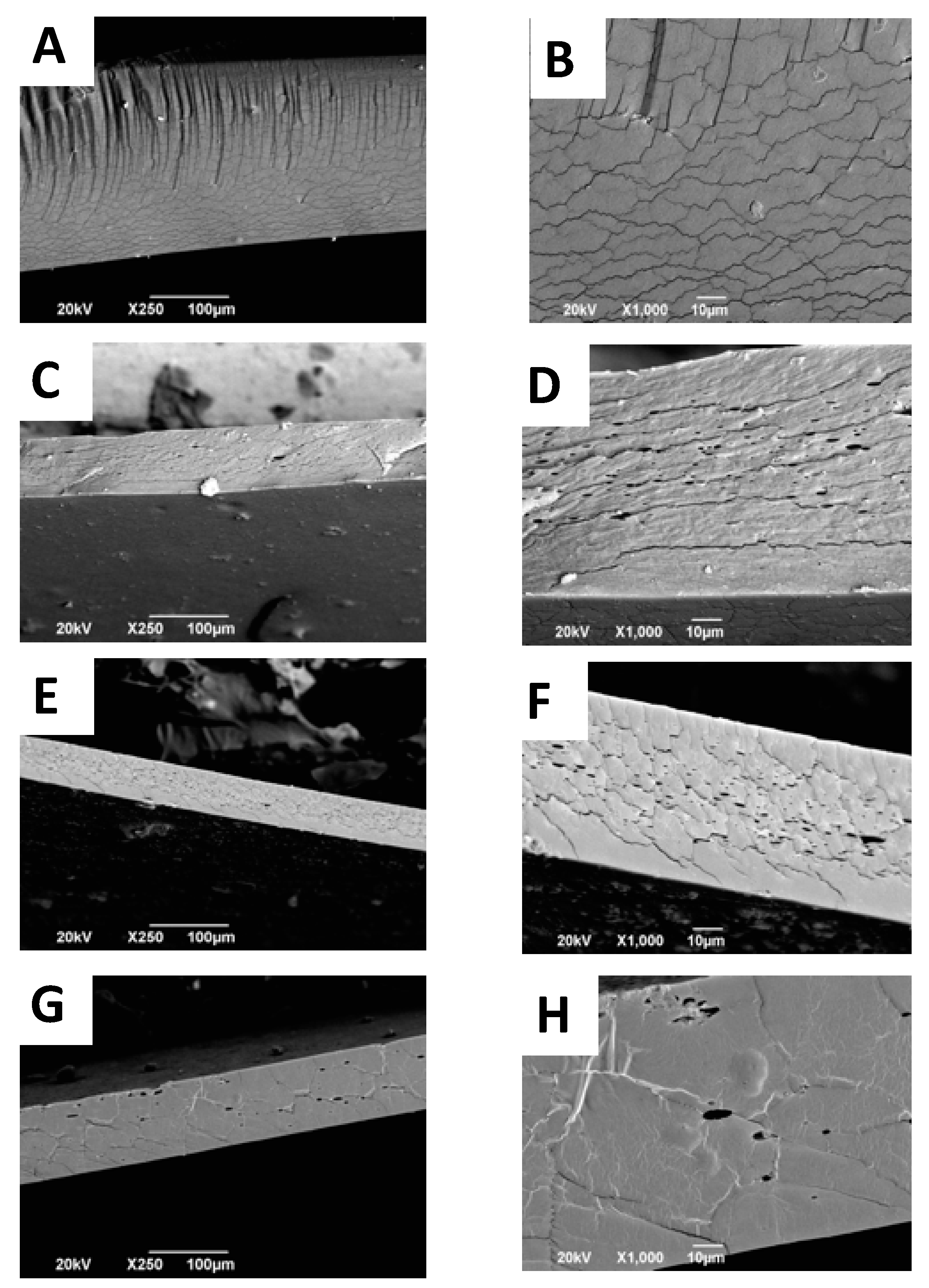
2.5. Scanning Electron Microscope (SEM) Analysis of CS+RGEO Films
Color Analysis of CS+RGEO Films
2.6. FTIR Analysis of CS+RGEO Films
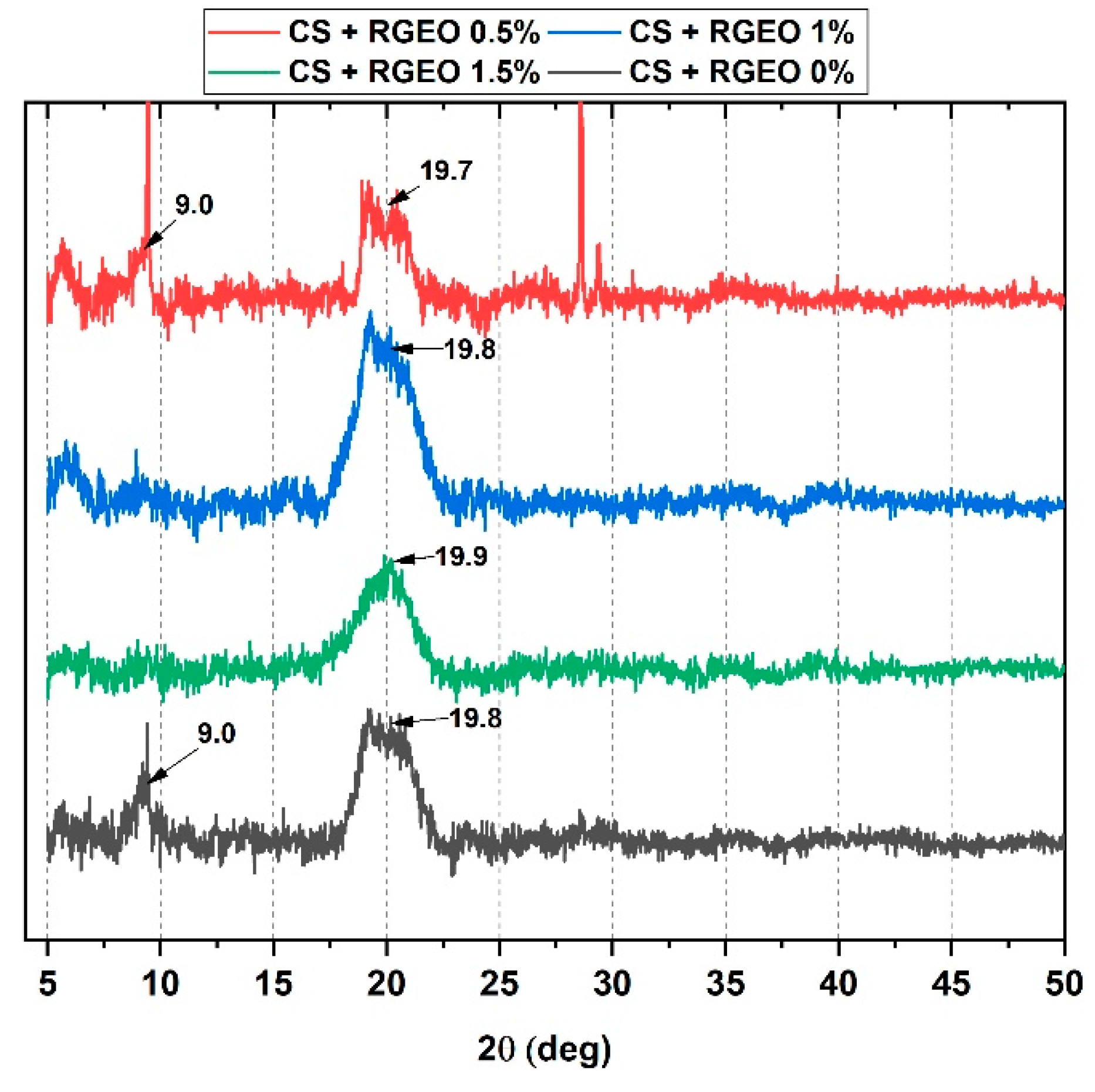
2.7. X-Ray Diffractometry (XRD) Analysis of CS+RGEO Films
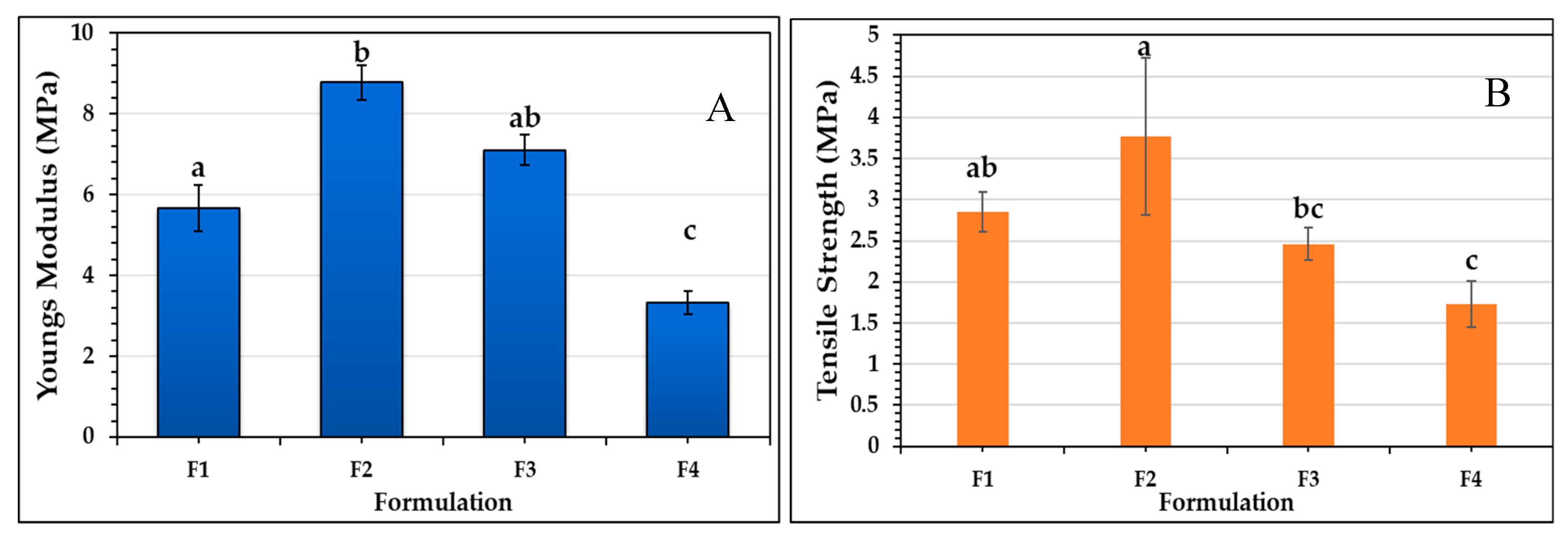
2.8. Mechanical Properties of the Films
2.9. Thermal Analysis of CS+RGEO Films
Thermogravimetric Analysis (TGA) of the Films
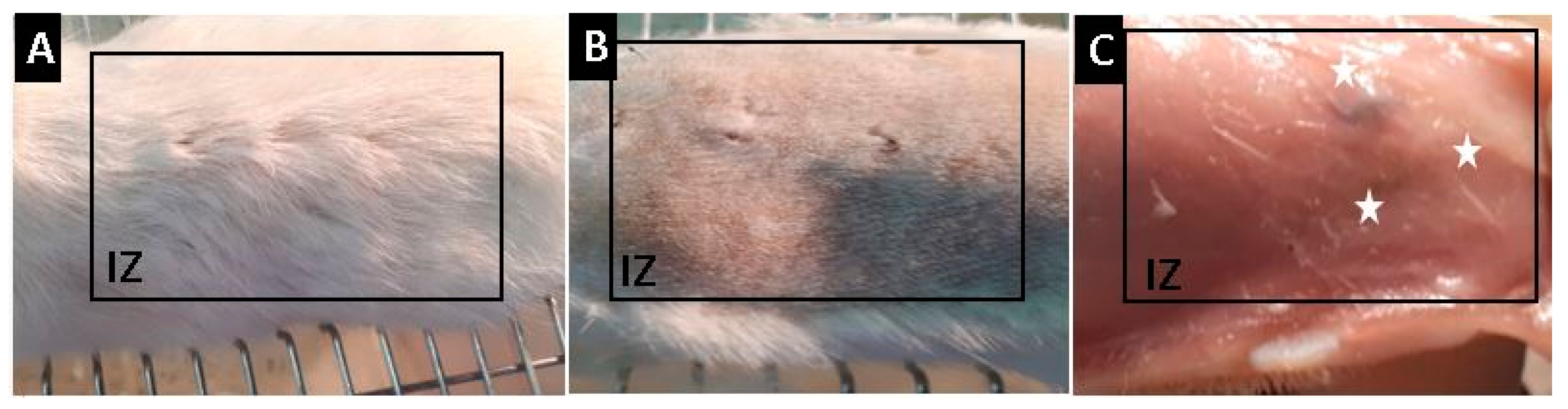
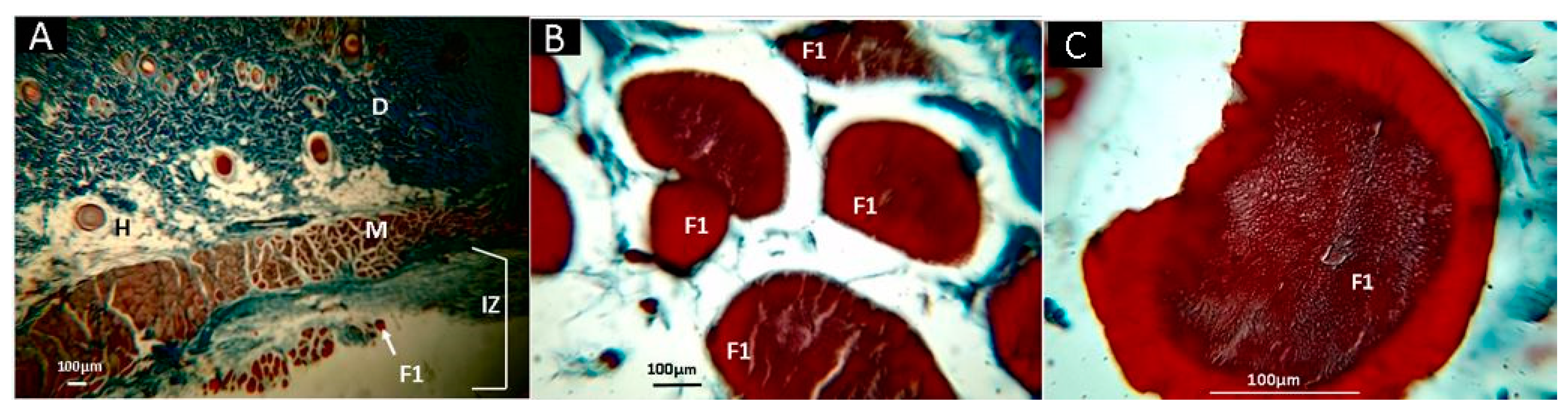
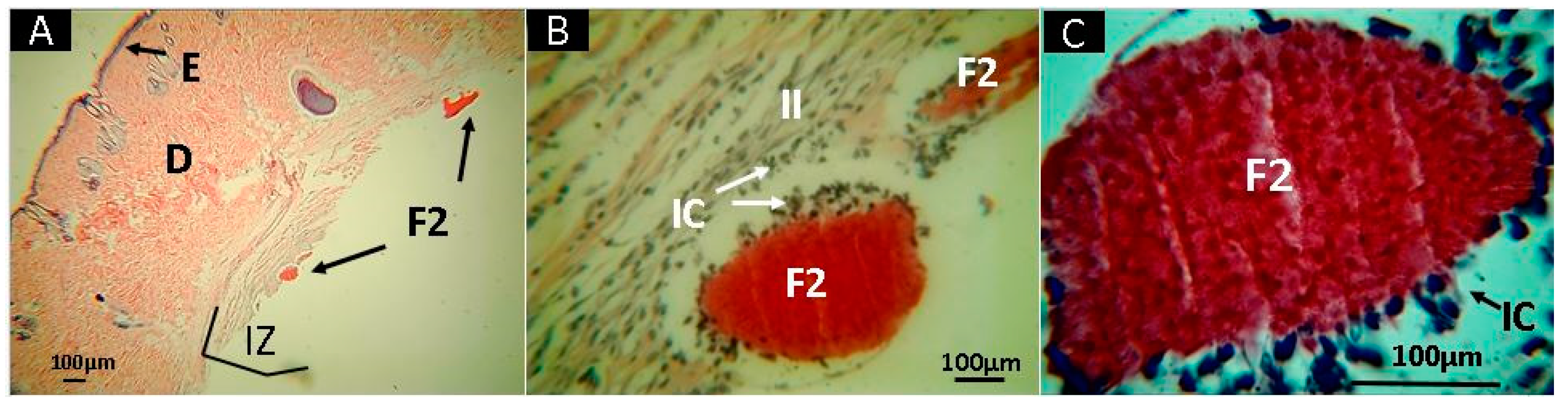
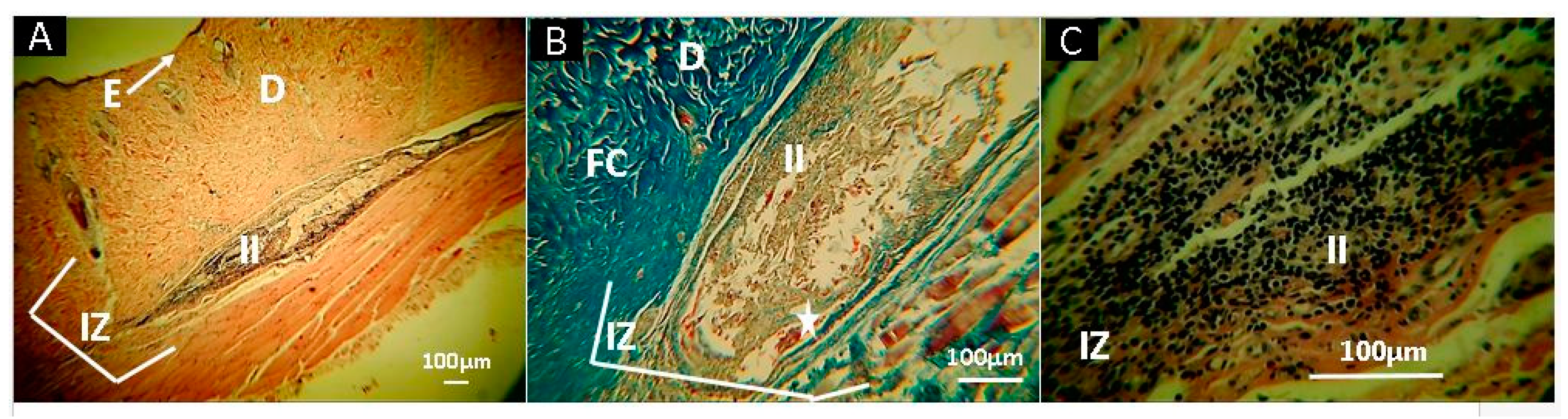
2.10. In Vivo Studies
3. Materials and Methods
3.1. Composition of Essential Oil of Ruta graveolens
3.2. Emulsion Preparation and Characterization
3.2.1. Droplet Size
3.2.2. Viscosity Measurements
3.2.3. Total Solid Content
3.3. Hemolysis Assay
3.4. Physicochemical Characterization of CS+RGEO Films
3.4.1. Film Characterization
3.4.2. Film Solubility in Water
3.4.3. The Moisture Content of CS+RGEO Films
3.4.4. Water Vapor Permeability
3.4.5. Color Analysis of CS+RGEO Films
3.4.6. Fourier-Transform Infrared Spectroscopy (FTIR)
3.4.7. X-Ray Diffraction (XRD)
3.4.8. Scanning Electron Microscopy (SEM)
3.4.9. Mechanical Studies
3.4.10. Thermal Studies
3.5. In Vivo Studies
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, W.; Zhang, Z.; Jiang, X. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv. Healthc. Mater. 2019, 8, 1801433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Hsu, S.; Hung, K.-C.; Chen, C.-W. Biodegradable polymer scaffolds. J. Mater. Chem. B 2016, 4, 7493–7505. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Chouhan, D.; Mandal, B.B. 3D functional scaffolds for skin tissue engineering. In Functional 3D tissue engineering scaffolds; Elsevier: Cambridge, MA, USA, 2018; pp. 345–365. [Google Scholar]
- Singh, M.R.; Patel, S.; Singh, D. Natural polymer-based hydrogels as scaffolds for tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Cambridge, MA, USA, 2016; pp. 231–260. [Google Scholar]
- Ahmed, S.; Annu, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Gopal, S.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz-Bortuzzo, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int. J. Biol. Macromol. 2018, 114, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 42001. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolym. Orig. Res. Biomol. 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in biomedical engineering: A critical review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Senel, S.; Aksoy, E.A.; Akca, G. Application of Chitosan Based Scaffolds for Drug Delivery and Tissue. Mar. Biomater. Tissue Eng. Appl. 2019, 14, 157. [Google Scholar]
- Yu, S.; Ma, P.; Cong, H.; Jiang, G. Preparation and Performances of Warp-Knitted Hernia Repair Mesh Fabricated with Chitosan Fiber. Polymers 2019, 11, 595. [Google Scholar] [CrossRef] [Green Version]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer–Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G. Culture of organized cell communities. Adv. Drug Deliv. Rev. 1998, 33, 15–30. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Bhattarai, N.; Yi, H.K.; Hwang, P.H.; Cha, D., II; Kim, H.Y. Novel biodegradable electrospun membrane: Scaffold for tissue engineering. Biomaterials 2004, 25, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical properties, cytocompatibility and manufacturability of chitosan: PEGDA hybrid-gel scaffolds by stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Ma, Y.; Tan, H.; Jia, Y.; Zou, S.; Guo, S.; Zhao, M.; Huang, H.; Ling, Z.; Chen, Y. Covalent and injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Mater. Sci. Eng. C 2017, 71, 67–74. [Google Scholar] [CrossRef]
- Maglione, M.; Spano, S.; Ruaro, M.E.; Salvador, E.; Zanconati, F.; Tromba, G.; Turco, G. In vivo evaluation of chitosan-glycerol gel scaffolds seeded with stem cells for full-thickness mandibular bone regeneration. J. Oral Sci. 2017, 59, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qian, J.; Ding, F. Recent advances in engineered chitosan-based nanogels for biomedical applications. J. Mater. Chem. B 2017, 5, 6986–7007. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Haddouchi, F.; Belkaid, A.B.; Sek, F.; Chaouche, T.M.; Zaouali, Y.; Ksouri, R.; Attou, A.; Benmansour, A. Chemical composition and antimicrobial activity of the essential oils from four Ruta species growing in Algeria. Food Chem. 2013, 14, 253–258. [Google Scholar] [CrossRef]
- Reddy, D.N.; Al-Rajab, A.J. Chemical composition, antibacterial and antifungal activities of Ruta graveolens L. volatile oils. Cogent Chem. 2016, 2, 1220055. [Google Scholar] [CrossRef]
- Nogueira, J.C.R.; Diniz, M.d.F.M.; Lima, E.O. In vitro antimicrobial activity of plants in Acute Otitis Externa. Braz. J. Otorhinolaryngol. 2008, 74, 118–124. [Google Scholar] [CrossRef] [Green Version]
- da Silva, F.G.E.; Mendes, F.R.d.S.; Assunção, J.C.d.C.; Maria Pinheiro Santiago, G.; Aislania Xavier Bezerra, M.; Barbosa, F.G.; Mafezoli, J.; Rodrigues Rocha, R. Seasonal variation, larvicidal and nematicidal activities of the lef essential oil of Ruta graveolens L. J. Essent. Oil Res. 2014, 26, 204–209. [Google Scholar] [CrossRef]
- Orlanda, J.F.F.; Nascimento, A.R. Chemical composition and antibacterial activity of Ruta graveolens L.(Rutaceae) volatile oils, from São Luís, Maranhão, Brazil. S. Afr. J. Bot. 2015, 99, 103–106. [Google Scholar] [CrossRef]
- De Feo, V.; De Simone, F.; Senatore, F. Potential allelochemicals from the essential oil of Ruta graveolens. Phytochemistry 2002, 61, 573–578. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Schrader, K.K.; Wedge, D.E.; Duke, S.O. Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry 2005, 66, 2689–2695. [Google Scholar] [CrossRef]
- Al-Shuneigat, J.M.; Al-Tarawneh, I.N.; Al-Qudah, M.A.; Al-Sarayreh, S.A.; Al-Saraireh, Y.M.; Alsharafa, K.Y. The chemical composition and the antibacterial properties of Ruta graveolens L. essential oil grown in Northern Jordan. Jordan J. Biol. Sci. 2015, 147, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chaftar, N.; Girardot, M.; Labanowski, J.; Ghrairi, T.; Hani, K.; Frère, J.; Imbert, C. Comparative evaluation of the antimicrobial activity of 19 essential oils. In Advances in Microbiology, Infectious Diseases and Public Health; Springer: Basel, Switzerland, 2015; pp. 1–15. [Google Scholar]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Oliva, A.; Lahoz, E.; Contillo, R.; Aliotta, G. Fungistatic activity of Ruta graveolens extract and its allelochemicals. J. Chem. Ecol. 1999, 25, 519–526. [Google Scholar] [CrossRef]
- Wolters, B.; Eilert, U. Antimicrobial substances in callus cultures of Ruta graveolens. Planta Med. 1981, 43, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Grande Tovar, C.D.; Delgado-Ospina, J.; Navia Porras, D.P.; Peralta-Ruiz, Y.; Cordero, A.P.; Castro, J.I.; Valencia, C.; Noé, M.; Mina, J.H.; Chaves López, C. Colletotrichum Gloesporioides Inhibition In Situ by Chitosan-Ruta graveolens Essential Oil Coatings: Effect on Microbiological, Physicochemical, and Organoleptic Properties of Guava (Psidium guajava L.) during Room Temperature Storage. Biomolecules 2019, 9, 399. [Google Scholar] [CrossRef] [Green Version]
- Thangavel, P.; Ramachandran, B.; Muthuvijayan, V. Fabrication of chitosan/gallic acid 3D microporous scaffold for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 750–760. [Google Scholar] [CrossRef]
- São Pedro, A.; Cabral-Albuquerque, E.; Ferreira, D.; Sarmento, B. Chitosan: An option for development of essential oil delivery systems for oral cavity care? Carbohydr. Polym. 2009, 76, 501–508. [Google Scholar] [CrossRef]
- Silva, S.S.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaacob, K.B.; Abdullah, C.M.; Joulain, D. Essential oil of Ruta graveolens L. J. Essent. Oil Res. 1989, 1, 203–207. [Google Scholar] [CrossRef]
- Kunicka-Styczyńska, A.; Gibka, J. Antimicrobial Activity of Undecan-x-ones (x = 2–4). Pol. J. Microbiol. 2010, 59, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute and synergistic effects of some monoterpenoid essential oil compounds on the house fly (Musca domestica L.). J. Essent. Oil Bear. Pl. 2008, 11, 451–459. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [Green Version]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, antiviral and antifungal activity of essential oils: Mechanisms and applications. In Antimicrobial Compounds; Springer: Basel, Switzerland, 2014; pp. 51–81. [Google Scholar]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Argillier, J.F.; Zeilinger, S.; Roche, P. Enhancement of aqueous emulsion and foam stability with oppositely charged surfactant/polyelectrolyte mixed systems. Oil Gas Sci. Technol. 2009, 64, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Bonilla Lagos, M.J.; Atarés Huerta, L.M.; Vargas, M.; Chiralt, A. Physicochemical properties of chitosan-essential oils film-forming dispersions. Effect of homogenization treatments. Procedia Food Sci. 2011, 1, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-González, L.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Physical and antimicrobial properties of chitosan – tea tree essential oil composite films. J. Food Eng. 2010, 98, 443–452. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Characterization of chitosan–oleic acid composite films. Food Hydrocoll. 2009, 23, 536–547. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma Gutiérrez Castañeda, C.; Valencia, E.M.; Grande Tovar, D.C. The Effect of Edible Chitosan Coatings Incorporated with Thymus capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria x ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaivani, T.; Rajasekaran, C.; Suthindhiran, K.; Mathew, L. Free radical scavenging, cytotoxic and hemolytic activities from leaves of Acacia nilotica (L.) wild. ex. delile subsp. indica (benth.) brenan. Evidence-Based Complement. Altern. Med. 2011, 2011, 274741. [Google Scholar] [CrossRef] [Green Version]
- Atrooz, O.M. The effects of Cuminum cyminum L. and Carum carvi L. seed extracts on human erythrocyte hemolysis. Int. J. Biol. 2013, 5, 57. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Khan, M.T.H.; Ather, A.; Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; de Moraes, M.E.A.; de Moraes, M.O. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J. Ethnopharmacol. 2005, 99, 21–30. [Google Scholar] [CrossRef]
- Quihui-Cota, L.; Morales-Figueroa, G.G.; Valbuena-Gregorio, E.; Campos-García, J.C.; Silva-Beltrán, N.P.; López-Mata, M.A. Membrana de Quitosano con Aceites Esenciales de Romero y Árbol de Té: Potencial como Biomaterial. Rev. Mex. Ing. biomédica 2017, 38, 255–264. [Google Scholar]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crop. Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant and antimicrobial properties of chitosan–cinnamon leaf oil films as affected by oleic acid. Food Hydrocolloid. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Park, S.; Zhao, Y. Incorporation of a high concentration of mineral or vitamin into chitosan-based films. J. Agric. Food Chem. 2004, 52, 1933–1939. [Google Scholar] [CrossRef]
- García, M.A.; Pinotti, A.; Martino, M.N.; Zaritzky, N.E. Characterization of composite hydrocolloid films. Carbohydr. Polym. 2004, 56, 339–345. [Google Scholar] [CrossRef]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/clay films’ properties as affected by biopolymer and clay micro/nanoparticles’ concentrations. Food Hydrocolloid. 2009, 23, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- de Moura, M.R.; Aouada, F.A.; Avena-Bustillos, R.J.; McHugh, T.H.; Krochta, J.M.; Mattoso, L.H.C. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J. Food Eng. 2009, 92, 448–453. [Google Scholar] [CrossRef]
- Grande Tovar, C.D.; Castro, J.I.; Valencia, C.H.; Navia Porras, D.P.; Hernandez, M.; Herminsul, J.; Valencia, M.E.; Velásquez, J.D.; Chaur, M.N. Preparation of Chitosan/Poly (Vinyl Alcohol) Nanocomposite Films Incorporated with Oxidized Carbon Nano-Onions (Multi-Layer Fullerenes) for Tissue-Engineering Applications. Biomolecules 2019, 9, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutiérrez, G.; Chiralt, A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocolloid. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- Ruprai, H.; Romanazzo, S.; Ireland, J.; Kilian, K.; Mawad, D.; George, L.; Wuhrer, R.; Houang, J.; Ta, D.; Myers, S. Porous chitosan films support stem cells and facilitate sutureless tissue repair. ACS Appl. Mater. Interfaces 2019, 11, 32613–32622. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocolloid. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Prateepchanachai, S.; Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Mechanical properties improvement of chitosan films via the use of plasticizer, charge modifying agent and film solution homogenization. Carbohydr. Polym. 2017, 174, 253–261. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Rheological and structural characterisation of film-forming solutions and biodegradable edible film made from kefiran as affected by various plasticizer types. Int. J. Biol. Macromol. 2011, 49, 814–821. [Google Scholar] [CrossRef]
- Cerqueira, M.A.P.R.; Pereira, R.N.C.; da Silva Ramos, O.L.; Teixeira, J.A.C.; Vicente, A.A. Edible food packaging: Materials and processing technologies; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1315373173. [Google Scholar]
- Baklagina, Y.G.; Klechkovskaya, V.V.; Kononova, S.V.; Petrova, V.A.; Poshina, D.N.; Orekhov, A.S.; Skorik, Y.A. Polymorphic Modifications of Chitosan. Crystallogr. Reports 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa protein–chitosan–sunflower oil edible film: Mechanical, barrier and structural properties. LWT Food Sci. Technol. 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef]
- Noor, I.S.; Majid, S.R.; Arof, A.K. Poly(vinyl alcohol)-LiBOB complexes for lithium-air cells. Electrochim. Acta 2013, 102, 149–160. [Google Scholar] [CrossRef]
- Sangeetha, K.; Angelin, V.P.; Sudha, P.N.; Alsharani, F.A.; Sukumaran, A. Novel chitosan based thin sheet nanofiltration membrane for rejection of heavy metal chromium. Int. J. Biol. Macromol. 2019, 132, 939–953. [Google Scholar]
- Salama, H.E.; Abdel Aziz, M.S.; Sabaa, M.W. Development of antibacterial carboxymethyl cellulose/chitosan biguanidine hydrochloride edible films activated with frankincense essential oil. Int. J. Biol. Macromol. 2019, 139, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Jahed, E.; Khaledabad, M.A.; Almasi, H.; Hasanzadeh, R. Physicochemical properties of Carum copticum essential oil loaded chitosan films containing organic nanoreinforcements. Carbohydr. Polym. 2017, 164, 325–338. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Crica, L.; Dinescu, S.; Costache, M.; Iovu, H. Synthesis, characterization, and in vitro studies of graphene oxide/chitosan-polyvinyl alcohol films. Carbohyd. Polym. 2014, 102, 813–820. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Phase transitions in starch based films containing fatty acids. Effect on water sorption and mechanical behaviour. Food Hydrocoll. 2013, 30, 408–418. [Google Scholar]
- Malafaya, P.B.; Santos, T.C.; van Griensven, M.; Reis, R.L. Morphology, mechanical characterization and in vivo neo-vascularization of chitosan particle aggregated scaffolds architectures. Biomaterials 2008, 29, 3914–3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tığlı, R.S.; Karakeçili, A.; Gümüşderelioğlu, M. In vitro characterization of chitosan scaffolds: Influence of composition and deacetylation degree. J. Mater. Sci. Mater. Med. 2007, 18, 1665–1674. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Pella, M.C.G.; Lima-Tenório, M.K.; Tenorio-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Fujita, M.; Ishihara, M.; Simizu, M.; Obara, K.; Ishizuka, T.; Saito, Y.; Yura, H.; Morimoto, Y.; Takase, B.; Matsui, T. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials 2004, 25, 699–706. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic Antimicrobial Activities of Natural Essential Oils with Chitosan Films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Milas, M.; Le Dung, P. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Jones, R.M. Particle size analysis by laser diffraction: ISO 13320, standard operating procedures, and Mie theory. Am. Lab. 2003, 35, 44–47. [Google Scholar]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: East Lansing, MI, USA, 1996; ISBN 0963203614. [Google Scholar]
- Porras, D.P.N.; Suárez, M.G.; Umaña, J.H.; Perdomo, L.G.P. Optimization of Physical, Optical and Barrier Properties of Films Made from Cassava Starch and Rosemary Oil. J. Polym. Environ. 2019, 27, 127–140. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the Relationship Between Water-satured State and Crystallinity by the Diffraction Method for Moistened Potato Starch. Starch Stärke 1983, 35, 407–410. [Google Scholar] [CrossRef]
- Ruiz, S.; Tamayo, A.J.; Delgado Ospina, J.; Navia Porras, P.D.; Valencia Zapata, E.M.; Mina Hernandez, H.J.; Valencia, H.C.; Zuluaga, F.; Grande Tovar, D.C. Antimicrobial Films Based on Nanocomposites of Chitosan/Poly(vinyl alcohol)/Graphene Oxide for Biomedical Applications. Biomolecules. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Valencia, C.; Valencia, C.; Zuluaga, F.; Valencia, M.; Mina, J.; Grande-Tovar, C. Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [Green Version]
- Tamayo Marín, A.J.; Londoño, R.S.; Delgado, J.; Navia Porras, P.D.; Valencia Zapata, E.M.; Mina Hernandez, H.J.; Valencia, H.C.; Grande Tovar, D.C. Biocompatible and Antimicrobial Electrospun Membranes Based on Nanocomposites of Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide. Int. J. Mol. Sci. 2019, 20, 2987. [Google Scholar] [CrossRef] [Green Version]



| Formulation | Thickness (µm) | Moisture Content (%) | Solubility in Water (%) | Water vapor Permeability Coefficient (gs−1m−1Pa × 10−10) |
|---|---|---|---|---|
| F1 | 48.32 ± 0.02 a | 42 ± 5 a | 28 ± 5 a | 5.81 ± 0.07 a |
| F2 | 52.11 ± 0.05 b | 42 ± 2 a | 26 ± 1 a,b | 1.60 ± 0.02 c |
| F3 | 59.34 ± 0.01 c | 43 ± 1 a | 24 ± 11a | 2.29 ± 0.04 b |
| F4 | 66.75 ± 0.02 d | 41 ± 1 a | 15 ± 7 b | 2.04 ± 0.07 b,c |
| Sample | L* | a* | b* | a*/b* | ΔE |
|---|---|---|---|---|---|
| F1 | 58 ± 1 a | −1.2 ± 0.1 a | 9 ± 2 a | −0.14 a | - |
| F2 | 54 ± 3 b | −0.9 ± 0.3 a,b | 12 ± 4 b | −0.085 b | 6 ± 4 a |
| F3 | 50 ± 2 c | −0.4 ± 0.6 c | 16 ± 3 c | −0.030 c | 10 ± 3 b |
| F4 | 52 ± 2 b,c | −0.6 ± 0.7 b,c | 18 ± 5 c | −0.043 b,c | 11± 5 b |
| Formulation | Xc XRD (%) | Xc DSC (%) | Tm (°C) |
|---|---|---|---|
| F1 | 10.9 | 100 | 247.3 |
| F2 | 10.7 | 78.6 | 254.4 |
| F3 | 5.0 | 78.2 | 250.1 |
| F4 | 6.4 | 87.6 | 252.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande Tovar, C.D.; Castro, J.I.; Valencia Llano, C.H.; Navia Porras, D.P.; Delgado Ospina, J.; Valencia Zapata, M.E.; Herminsul Mina Hernandez, J.; Chaur, M.N. Synthesis, Characterization, and Histological Evaluation of Chitosan-Ruta Graveolens Essential Oil Films. Molecules 2020, 25, 1688. https://doi.org/10.3390/molecules25071688
Grande Tovar CD, Castro JI, Valencia Llano CH, Navia Porras DP, Delgado Ospina J, Valencia Zapata ME, Herminsul Mina Hernandez J, Chaur MN. Synthesis, Characterization, and Histological Evaluation of Chitosan-Ruta Graveolens Essential Oil Films. Molecules. 2020; 25(7):1688. https://doi.org/10.3390/molecules25071688
Chicago/Turabian StyleGrande Tovar, Carlos David, Jorge Iván Castro, Carlos Humberto Valencia Llano, Diana Paola Navia Porras, Johannes Delgado Ospina, Mayra Eliana Valencia Zapata, José Herminsul Mina Hernandez, and Manuel N. Chaur. 2020. "Synthesis, Characterization, and Histological Evaluation of Chitosan-Ruta Graveolens Essential Oil Films" Molecules 25, no. 7: 1688. https://doi.org/10.3390/molecules25071688





