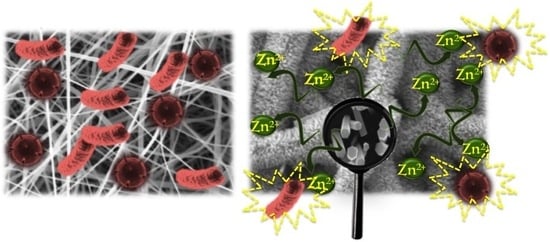
Optimization of ZnO Nanorods Growth on Polyetheresulfone Electrospun Mats to Promote Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results
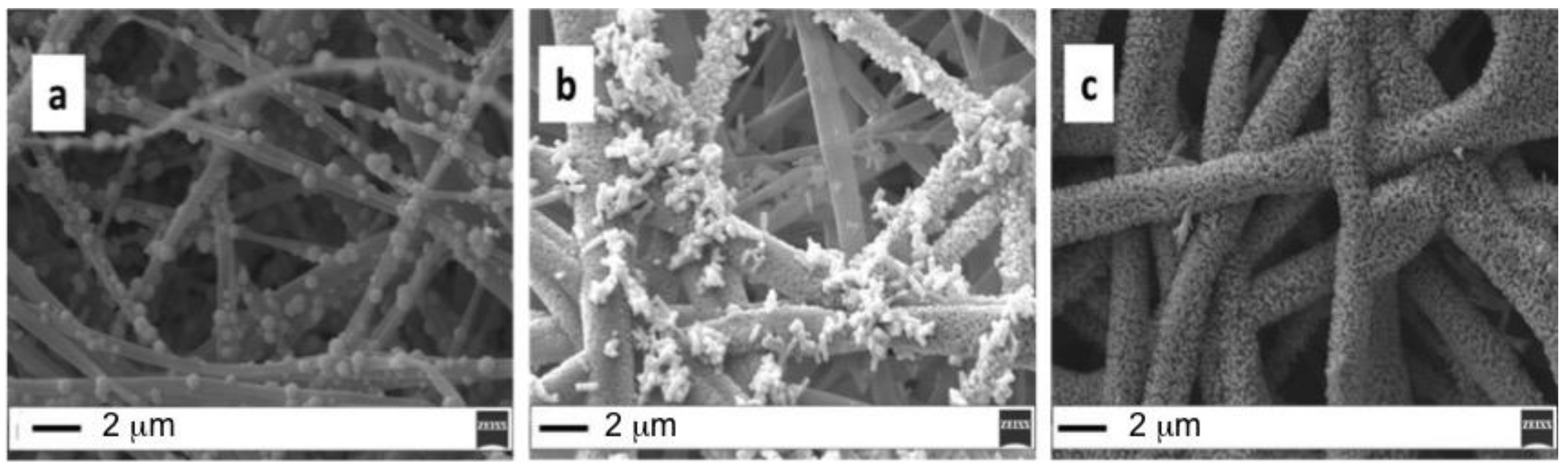
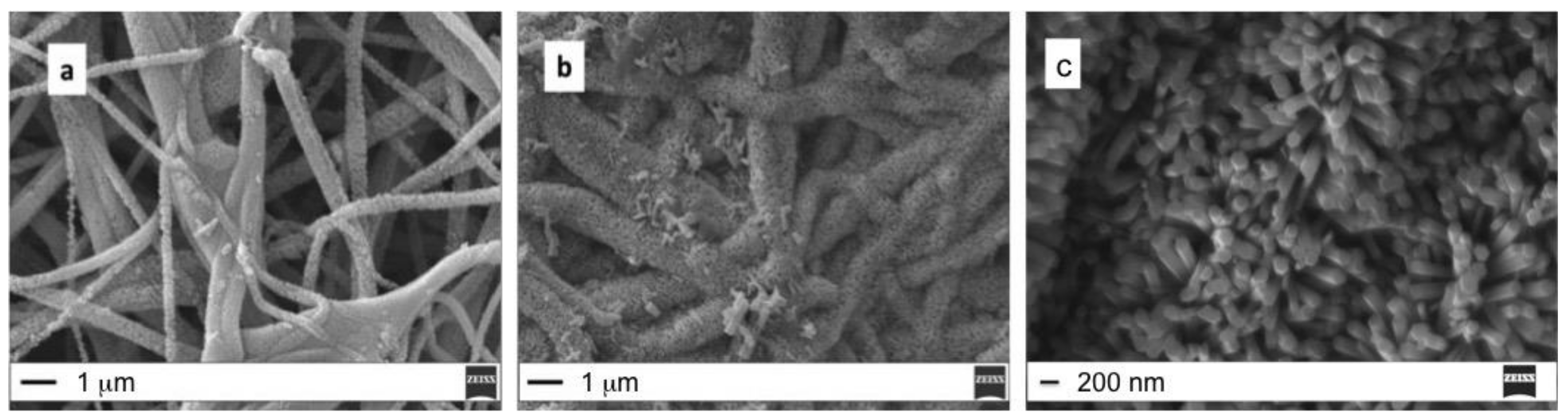
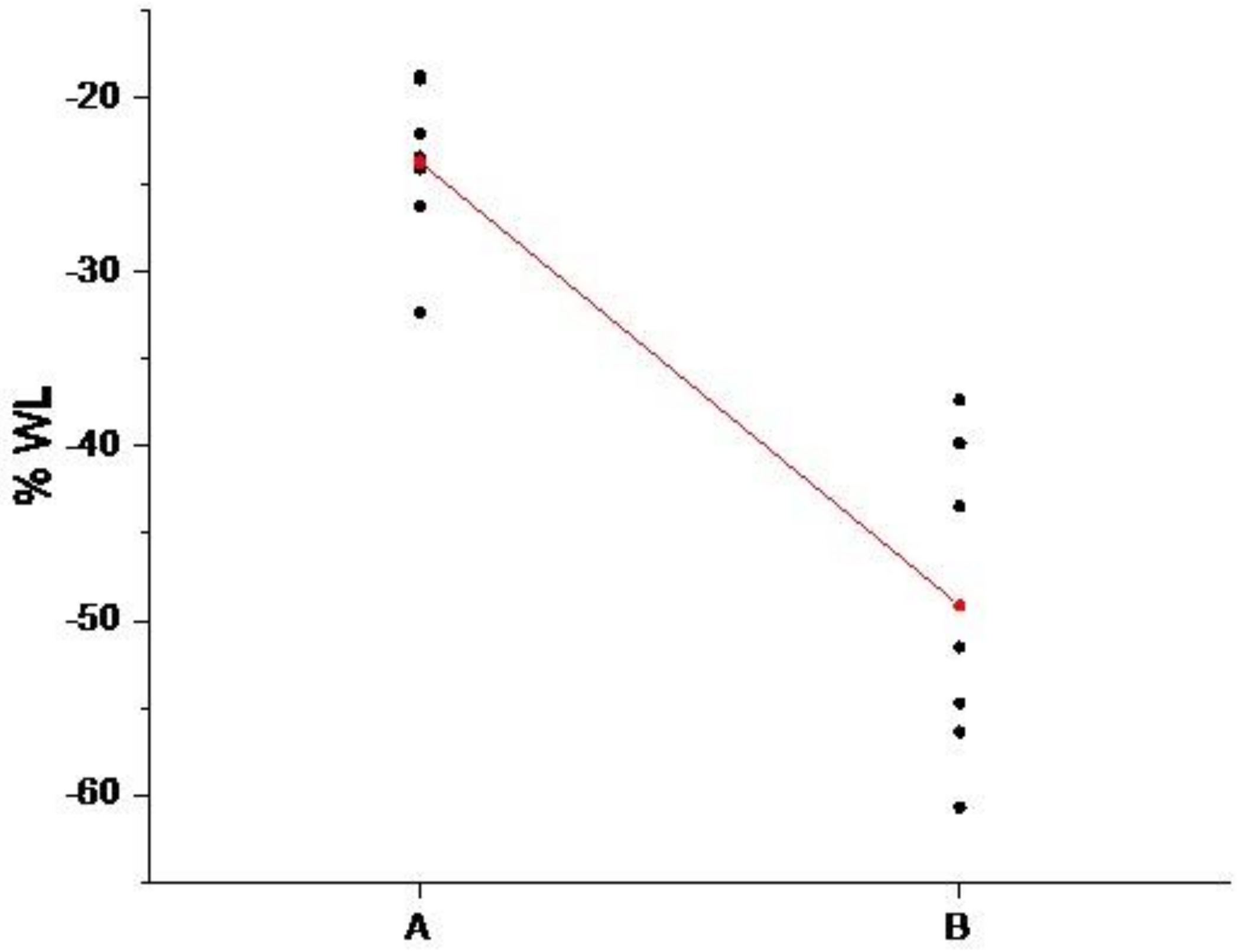
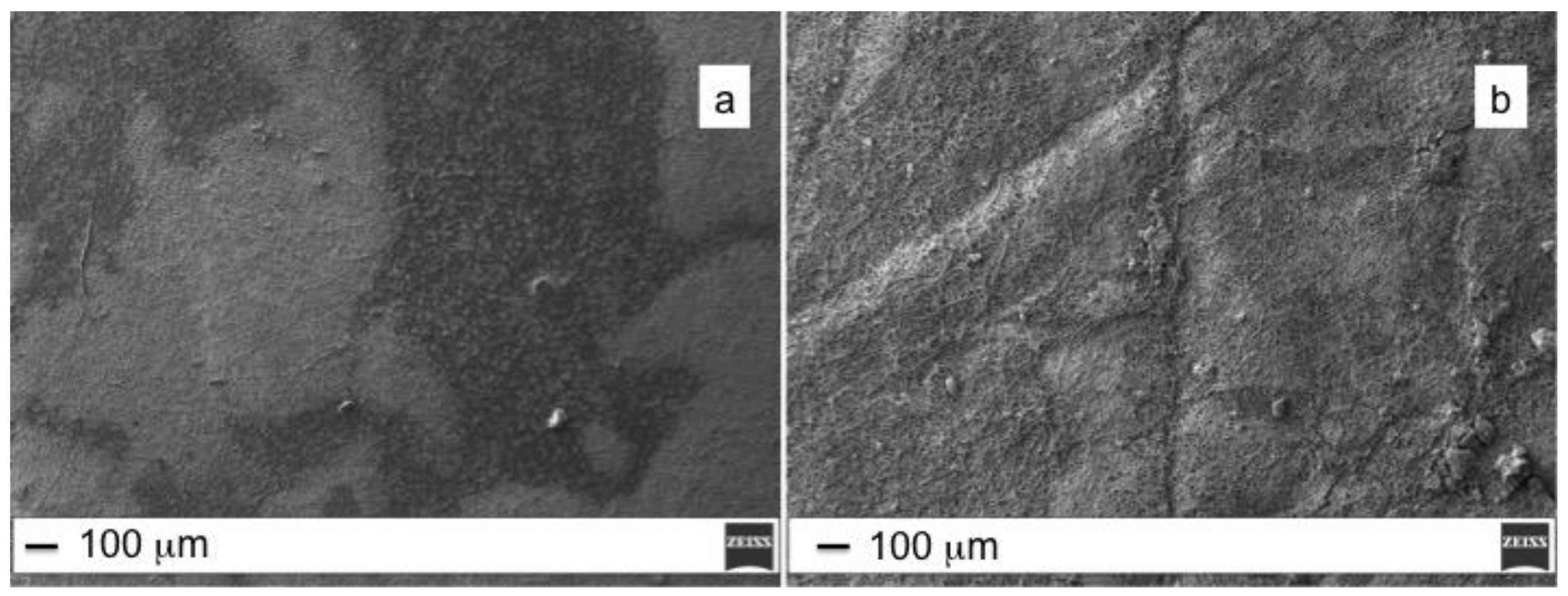
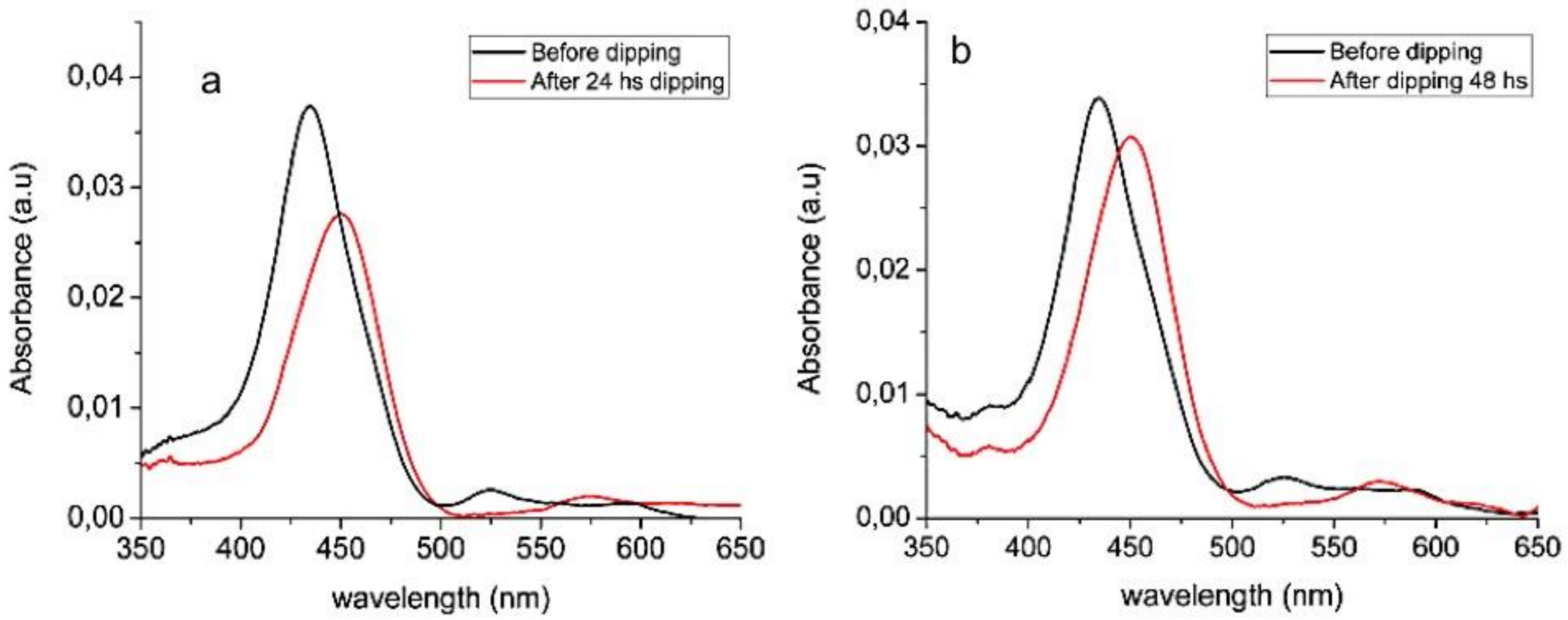
3.1. ZnO Growth Optimization
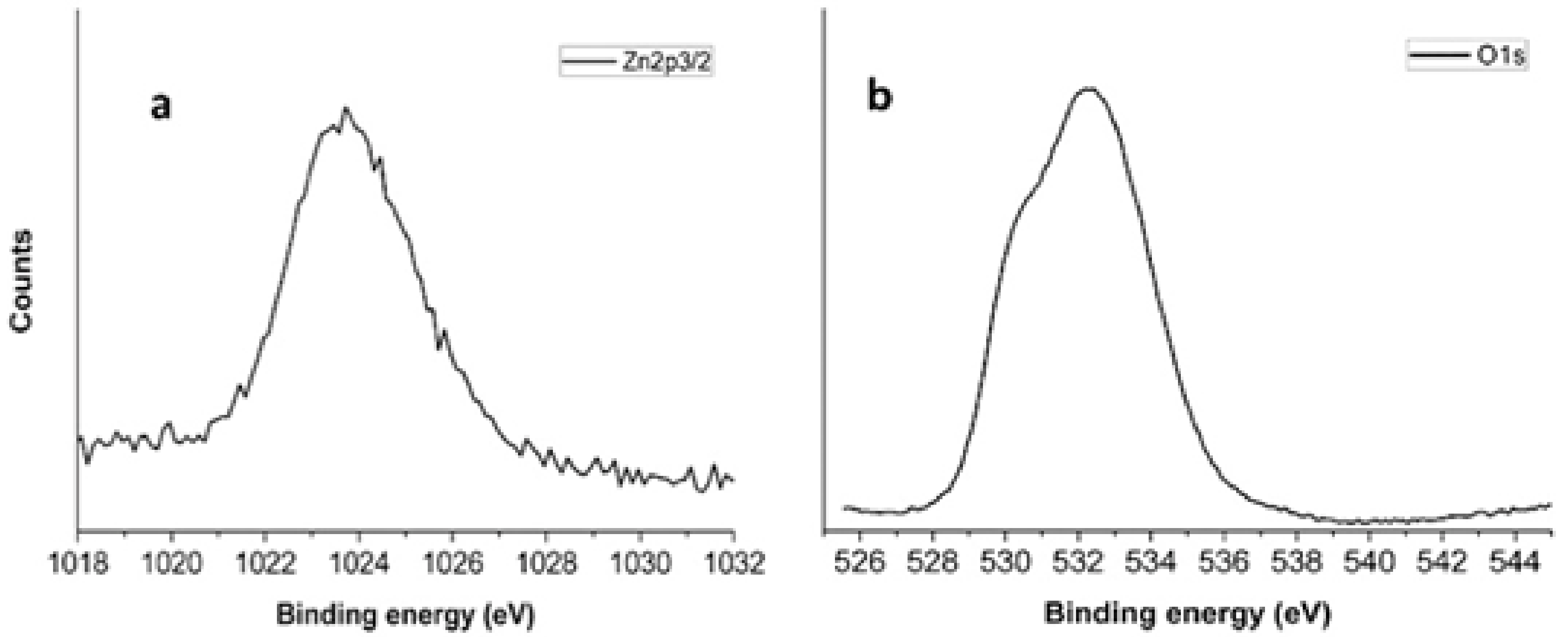
XPS Characterization
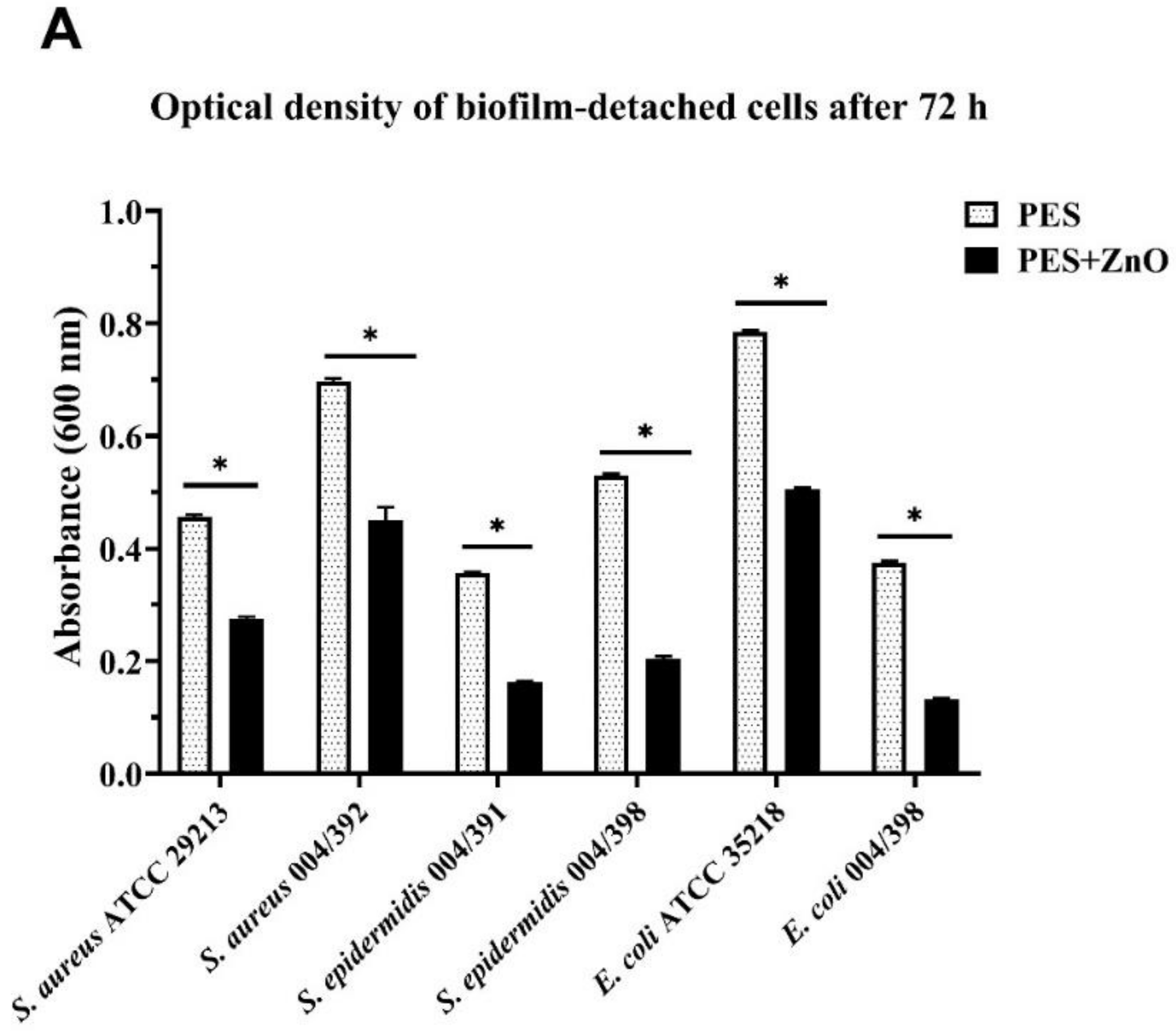
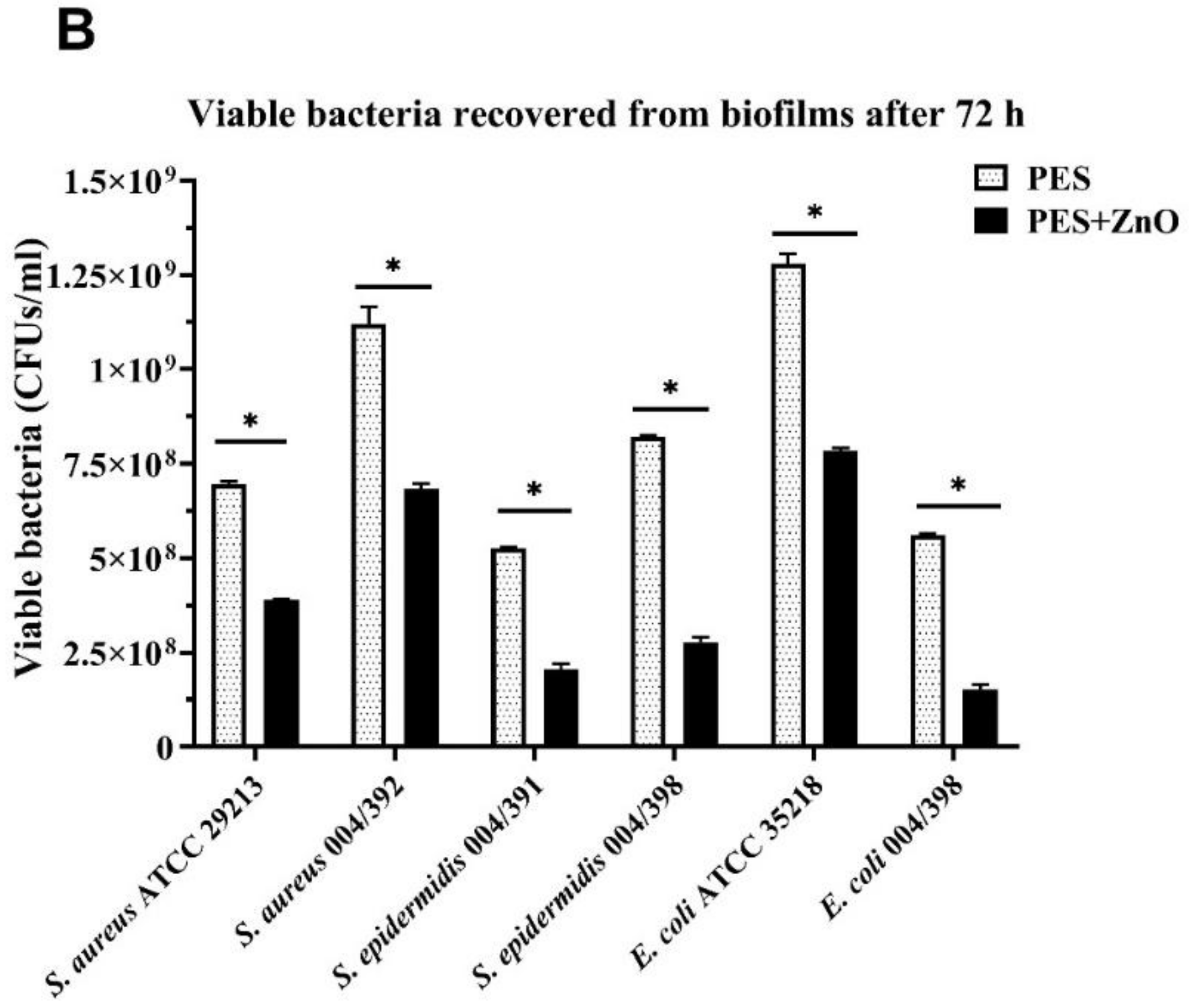
3.2. Antimicrobial Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Polyetheresulphone (PES) |
| Chemical bath deposition (CBD) |
| Zinc oxide (ZnO) |
| Titanium oxide (TiO2) |
| Zinc acetate dihydrate (Zn(Ac)2 ·2H2O) |
| Thermogravimetric analysis (TGA) |
| X-ray photoelectron spectroscopy (XPS) |
| Scanning electron microscopy (SEM) |
| Tetracationic tetrakis (N-methylpyridinium-4-yl)porphyrin (H2T4) |
| Phosphate buffered saline (PBS) |
| Colony-forming unit/mL (CFU/mL) |
References
- Xue Tong Wu, J.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev 2019, 119, 5298–5415. [Google Scholar]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Jordan, A.M.; Viswanath, V.; Kim, S.-E.; Pokorski, J.K.; Korley, L.T.J. Processing and surface modification of polymer nanofibers for biological scaffolds: A review. J. Mater. Chem. B 2016, 4, 5958–5974. [Google Scholar] [CrossRef]
- Joly, D.; Jung, J.-W.; Kim, I.-D.; Demadrille, R. Electrospun materials for solar energy conversion: Innovations and trends. J. Mater. Chem. C 2016, 4, 10173–10197. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyena, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. Rsc Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Applications of polymer nanofibers in biomedicine and biotechnology. Appl. Biochem. Biotechnol. 2005, 125, 147–157. [Google Scholar] [CrossRef]
- Ognibene, G.; Cristaldi, D.A.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scirè, S.; Fragalà, M.E. Photoactivity of hierarchically nanostructured ZnO–PES fibre mats for water treatments. Rsc Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Tudisco, C.; Fragalà, M.E.; Giuffrida, A.E.; Dertani, F.; Pinalli, R.; Dalcanale, E.; Compagnini, G.; Condorelli, G.G. Hierarchical Route for the Fabrication of Cavitand-Modified Nanostructured ZnO Fibers for Volatile Organic Compound Detection. J. Phys. Chem. C 2016, 120, 12611–12617. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Zhang, J.; Aslan, H.; Dong, M. Photoactive antimicrobial nanomaterials. J. Mater. Chem. B 2017, 5, 8631–8652. [Google Scholar] [CrossRef]
- Kurtz, I.S.; Schiffman, J.D. Review Current and Emerging Approaches to Engineer Antibacterial and Antifouling Electrospun. Nanofibers Mater. 2018, 11, 1059. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Superhydrophobic PVDF and PVDF-HFP nanofibrous mats with antibacterial and anti-biofouling properties. Appl. Surf. Sci. 2016, 363, 363–371. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, X.; Bonsu, E.O.; Sintim, H. Biofilm formation mechanisms and target for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chinese Medical Association 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Kim, C.J.; Lee, J.C.; Cho, M.H.; Lee, J. Indole-3-acetaldehyde from Rhodococcus sp. BFI 332 inhibits Escherichia coli O157: H7 biofilm formation. Appl. Microb. Biotechnol. 2012, 96, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Galdiero, E.; Lombardi, L.; Falanga, A.; Libralato, G.; Guida, M.; Carotenuto, R. Biofilms: Novel Strategies Based on Antimicrobial Peptides. Pharmaceutics 2019, 11, 322. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections: A review. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Romero-Lastra, P.; Ribeiro-Vidal, H.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Comparative gene expression analysis of planktonic Porphyromonas gingivalis ATCC 33277 in the presence of a growing biofilm versus planktonic cells. BMC Microbiology 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Besharova, O.; Suchanek, V.M.; Hartmann, R.; Drescher, K.; Sourjik, V. Diversification of gene expression during formation of static submerged biofilms by Escherichia coli. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dadi, R.; Azouani, R.; Traore, M.; Mielcarek, C.; Kanaev, A. Antibacterial activity of ZnO and CuO nanoparticles against gram positive and gram negative strains. Mater. Sci. Eng.: C 2019, 104, 109968. [Google Scholar] [CrossRef] [PubMed]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on Gram-positive and Gram-negative bacteria. Afr. J. Microbiol. Res. 2011, 5, 1368–1373. [Google Scholar]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H. Superior Antibacterial Activity of Zinc Oxide/Graphene Oxide Composites Originating from High Zinc Concentration Localized around Bacteria. Acs Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, H.; Samal, M.; Yun, K. Synthesis of ZnO nanoparticles-decorated spindle-shaped graphene oxide for application in synergistic antibacterial activity. J. Photochem. Photobiol. B: Biol. 2018, 183, 293–301. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J Polym. Res. 2014, 21, 1–17. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Ledezma, A.; Romero, J.; Grande, D. Novel antibacterial electrospun mats based on poly(d,l-lactide) nanofibers and zinc oxide nanoparticles. J. Mater. Sci. 2014, 49, 8373–8385. [Google Scholar] [CrossRef]
- Koao, L.; Dejene, F.B.; Swart, H.C. Properties of flower-like ZnO nanostructures synthesized using the chemical bath deposition. Mater. Sci. Semiconduct. Proc. 2014, 27, 33–40. [Google Scholar] [CrossRef]
- Babaeijandaghi, F.; Shabani, I.; Seyedjafari, E.; Safaei Naraghi, Z.; Vasei, M.; Haddadi-Asl, V.; Kamyab Hesari, K.; Soleimani, M. Accelerated epidermial regeneration and improved dermal reconstruction achieved by polyethersulfone nanofibers. Tissue Eng. A 2010, 16, 3527–3536. [Google Scholar] [CrossRef]
- Fragalà, M.E.; Aleeva, Y.; Malandrino, G. ZnO nanorod arrays fabrication via chemical bath deposition: Ligand concentration effect study. Superlattices Microstruct. 2010, 48, 408–415. [Google Scholar] [CrossRef]
- Fragalà, M.E.; Aleeva, Y.; Malandrino, G. Effects of Metal-Organic Chemical Vapour Deposition grown seed layer on the fabrication of well aligned ZnO nanorods by Chemical Bath Deposition. Thin Solid Film. 2011, 519, 7694–7701. [Google Scholar] [CrossRef][Green Version]
- Briggs, D.; Grant, J.T. Surface Analysis by Auger and X-Ray Photoelectron Spectroscopy; Briggs, D., Grant, T., Eds.; IM Publications: Chichester, UK; Surface Spectra Ltd.: Manchester, UK, 2003; p. 900. [Google Scholar]
- Gulino, A. Structural and electronic characterization of self-assembled molecular nanoarchitectures by X-ray photoelectron spectroscopy. Anal. Bioanal. Chem. 2013, 405, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Cicala, G.; Giordano, D.; Tosto, C.; Filippone, G.; Recca, A.; Blanco, I. Polylactide (PLA) Filaments a Biobased Solution for Additive Manufacturing: Correlating Rheology and Thermomechanical Properties with Printing Quality. Materials 2018, 11, 1191. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-seventh Informational Supplement M100-S28; CLSI: 950 West Valley Road, Wayne, PA, USA, 2018. [Google Scholar]
- Gangemi, C.M.A.; Ognibene, G.; Randazzo, R.; D’Urso, A.; Purrello, R.; Fragalà, M.E. Easy sensing of lead and zinc in water using smart glass based on cationic porphyrin layers. New. J. Chem 2018, 42, 8717–8723. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; D’Urso, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Combined approach to remove and fast detect heavy metals in water based on PES–TiO2 electrospun mats and porphyrin chemosensors. Acs Omega 2018, 3, 7182–7190. [Google Scholar] [CrossRef]
- Gulino, A.; Lupo, F.; Fragalà, M.E. Substrate-free, self-standing ZnO thin films. J. Phys. Chem. C 2008, 112, 13869–13872. [Google Scholar] [CrossRef]
- Gulino, A.; Dapporto, P.; Rossi, P.; Fragalà, I. Synthesis and characterization of liquid MOCVD precursors for thin films of cadmium oxide. Chem. Mater. 2002, 14, 4955–4962. [Google Scholar] [CrossRef]
- Contino, A.; Maccarrone, G.; Spitaleri, L.; Torrisi, L.; Nicotra, G.; Gulino, A. One Pot Synthesis of Au_ZnO Core–shell Nanoparticles Using a Zn Complex Acting as ZnO Precursor, Capping and Reducing Agent During the Au NPs Formation. Eur. J. Inorg. Chem. 2018, 43, 4678–4683. [Google Scholar] [CrossRef]
- Spitaleri, L.; Nicotra, G.; Zimbone, M.; Contino, A.; Maccarrone, G.; Alberti, A.; Gulino, A. Fast and Efficient Sun Light Photocatalytic Activity of Au_ZnO Core−Shell Nanoparticles Prepared by a One-Pot Synthesis. Acs Omega 2019, 4, 15061–15066. [Google Scholar] [CrossRef]
- Gulino, A.; Fragala, I. Deposition and characterization of transparent thin films of zinc oxide doped with Bi and Sb. Chem. Mater. 2002, 14, 116–121. [Google Scholar] [CrossRef]
- Gulino, A.; Castelli, F.; Dapporto, P.; Rossi, P.; Fragalà, I. Synthesis and Characterization of Novel Self-Generating Liquid MOCVD Precursors for Thin Films of Zinc Oxide. Chem. Mater. 2000, 12, 548–554. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [PubMed]
- Stanković, A.; Dimitrijević, S.; Uskokov, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothermally synthesized using different surface stabilizing agents. Colloids Surf. B: Bioint. 2013, 102, 21–28. [Google Scholar]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Xia, S.; Yao, L.; Zhao, Y.; Li, N.; Zheng, Y. Preparation of graphene oxide modified polyamide thin film composite membranes with improved hydrophilicity for natural organic matter removal. Chem. Eng. J. 2015, 280, 720–727. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B: Biology 2013, 120, 66–73. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110319. [Google Scholar] [CrossRef]
- Malis, D.; Jeršek, B.; Tomšič, B.; Štular, D.; Golja, B.; Kapun, G.; Simončič, B. Antibacterial activity and biodegradation of cellulose fiber blends with incorporated ZnO. Materials 2019, 12, 3399. [Google Scholar] [CrossRef]
- Dincă, V.; Mocanu, A.; Isopencu, G.; Busuioc, C.; Brajnicov, S.; Vlad, A.; Icriverzi, M.; Roseanu, A.; Dinescu, M.; Stroescu, M.; et al. Biocompatible pure ZnO nanoparticles-3D bacterial cellulose biointerfaces with antibacterial properties. Arab. J. Chem. 2020, 13, 3521–3533. [Google Scholar]
- Gottenbos, B.; Grijpma, D.W.; van der Mei, H.C.; Feijen, J.; Busscher, H.J. Antimicrobial effects of positively charged surface on adhering Gram-positive and Gram-negative bacteria. J. Antimicr. Chemother. 2001, 48, 7–13. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Bacterial Strains | Source |
|---|---|
| Gram-positive | |
| Staphylococcus aureus ATCC 29213 | Standard |
| Staphylococcus aureus 004/392 | Septicemia |
| Staphylococcus epidermidis 004/391 | Endovascular catheter-associated infection |
| Staphylococcus epidermidis 004/398 | Endovascular catheter-associated infection |
| Gram-negative | |
| Escherichia coli ATCC 35218 | Standard |
| Escherichia coli 004/398 | Peritonitis |
| Sample | Seed Annealing Time (min) | Annealing (°C) | CBD Growth Time (min) |
|---|---|---|---|
| A | 60 | 180 | 60 |
| B | 60 | 180 | 30 |
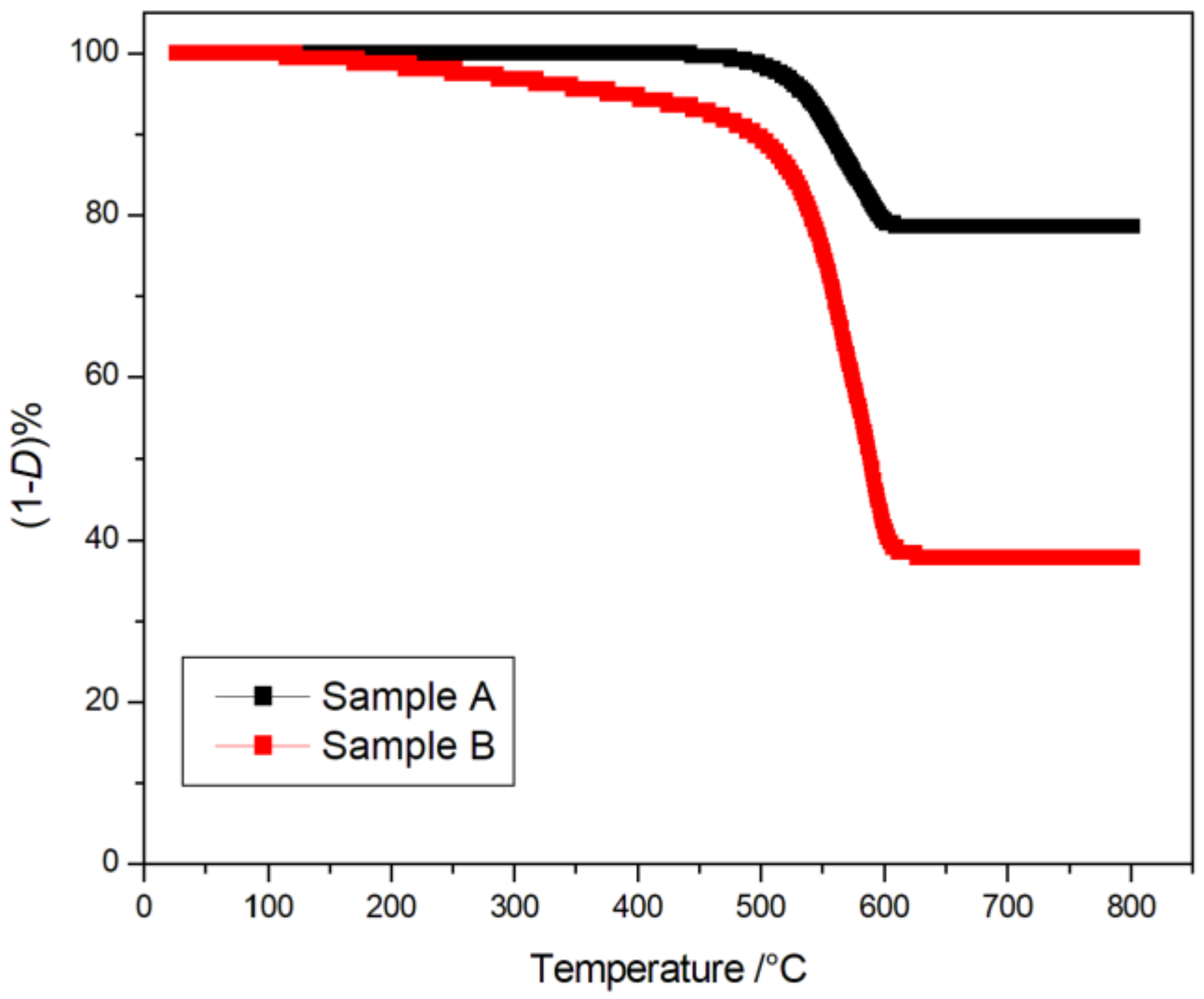
| Sample | Mean D% | StDev | SE Mean |
|---|---|---|---|
| A | −23.66 | 4.68 | 3.4 |
| B | −49.14 | 8.97 | 1.8 |
| Bacterial Strains | PES | PES/ZnO | ||
|---|---|---|---|---|
| Gram-positive | OD600 | CFUs/mL | OD600 | CFUs/mL |
| S. aureus ATCC 29213 | 0.460 ± 0.003 | 6.96 × 108 ± 7.02 × 106 | 0.280 ± 0.004 | 3.89 ×108 ± 3.51 ×106 |
| S. aureus 004/392 | 0.700 ± 0.005 | 1.12 × 109 ± 4.58 ×107 | 0.450 ± 0.024 | 6.84 ×108 ± 1.45 ×107 |
| S. epidermidis 004/391 | 0.360 ± 0.003 | 5.26 × 108 ± 3.61 ×106 | 0.160 ± 0.003 | 2.04 ×108 ± 1.64 ×107 |
| S. epidermidis 004/398 | 0.530 ± 0.003 | 8.22 × 108 ± 3.00 ×106 | 0.200 ± 0.005 | 2.78 ×108 ± 1.35 ×107 |
| Gram-negative | OD600 | CFUs/mL | OD600 | CFUs/mL |
| E. coli ATCC 35218 | 0.790 ± 0.002 | 1.28 ×109 ± 2.65 ×107 | 0.510 ± 0.003 | 7.84 ×108 ± 7.64 ×106 |
| E. coli 004/398 | 0.380 ± 0.003 | 5.61 ×108 ± 4.00 ×106 | 0.130 ± 0.003 | 1.53 ×108 ± 1.23 ×107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmeri, M.; Ognibene, G.; Saitta, L.; Lombardo, C.; Genovese, C.; Barcellona, M.; D’Urso, A.; Spitaleri, L.; Blanco, I.; Cicala, G.; et al. Optimization of ZnO Nanorods Growth on Polyetheresulfone Electrospun Mats to Promote Antibacterial Properties. Molecules 2020, 25, 1696. https://doi.org/10.3390/molecules25071696
Salmeri M, Ognibene G, Saitta L, Lombardo C, Genovese C, Barcellona M, D’Urso A, Spitaleri L, Blanco I, Cicala G, et al. Optimization of ZnO Nanorods Growth on Polyetheresulfone Electrospun Mats to Promote Antibacterial Properties. Molecules. 2020; 25(7):1696. https://doi.org/10.3390/molecules25071696
Chicago/Turabian StyleSalmeri, Mario, Giulia Ognibene, Lorena Saitta, Cinzia Lombardo, Carlo Genovese, Matteo Barcellona, Alessandro D’Urso, Luca Spitaleri, Ignazio Blanco, Gianluca Cicala, and et al. 2020. "Optimization of ZnO Nanorods Growth on Polyetheresulfone Electrospun Mats to Promote Antibacterial Properties" Molecules 25, no. 7: 1696. https://doi.org/10.3390/molecules25071696
APA StyleSalmeri, M., Ognibene, G., Saitta, L., Lombardo, C., Genovese, C., Barcellona, M., D’Urso, A., Spitaleri, L., Blanco, I., Cicala, G., Gulino, A., & Fragalà, M. E. (2020). Optimization of ZnO Nanorods Growth on Polyetheresulfone Electrospun Mats to Promote Antibacterial Properties. Molecules, 25(7), 1696. https://doi.org/10.3390/molecules25071696