Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices
Abstract
:1. Introduction
2. Results and Discussion
2.1. Co-Microencapsulation Efficiency
2.2. The Phytochemical Characterization of Co-Microencapsulated Powder
2.3. Stability over the Time of the Co-Microencapsulated Powder
2.4. Confocal Laser Microscopy
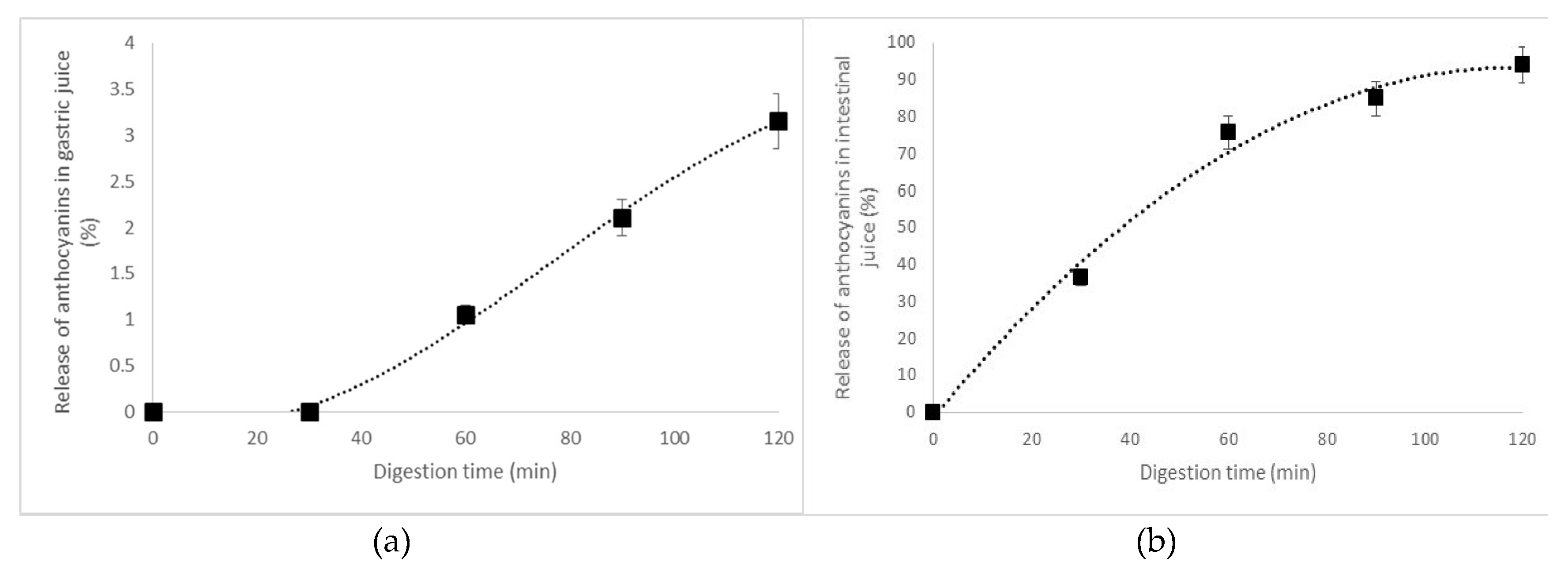
2.5. In Vitro Digestibility of the Black Currant Anthocyanins
2.6. Colorimetric Analysis
2.7. Biological Activity
2.8. The Added-Value Yogurt with Powder Addition
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals and Reagents
3.1.2. Sample Processing
3.2. Encapsulation Efficiency
3.3. The Phytochemicals Content
3.4. Storage Stability
3.5. Structure and Morphology of the Microparticles
3.6. In Vitro Digestibility of the Anthocyanins
3.7. Colorimetric Analysis of the Microencapsulated Powder Using CIEL*a*b* System
3.8. Viability of Lactic Acid Bacteria
3.9. Inhibitory Activity
3.10. Added-Value Food Products with Co-Microencapsulated Powder
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buchweitz, M.; Speth, M.; Kammerer, D.R.; Carle, R. Impact of pectin type on the storage stability of black currant (Ribes nigrum L.) anthocyanins in pectic model solutions. Food Chem. 2013, 139, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- McCann, D.; Barett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, R.; Anttonen, M.; Saviranta, N.; Stewart, D.; McDougall, G.J.; Hilz, H.; Mattila, P.; Törrönen, R. A review on bioactive compounds in black currants (Ribes Nigrum L.) and their potential health-promoting properties. ISHS BIOTECHFRUIT. 2008, 839, 301–307. [Google Scholar] [CrossRef]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.-O.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (Ribes nigrum L.): Variation due to genotype, location, and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef]
- Bishayee, A.; Mbimba, T.; Thoppil, R.J.; Háznagy-Radnai, E.; Sipos, P.; Darvesh, A.S.; Folkesson, H.G.; Hohmann, J. Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Bioch. 2011, 22, 1035–1045. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of black currants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from Black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 9, 107–116. [Google Scholar] [CrossRef]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT-Food Sci. Technol. 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of Fingered citron extract with gum Arabic, modified starch, whey protein, and maltodextrin using spray drying. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nualkaekul, S.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res. Int. 2013, 53, 304–311. [Google Scholar] [CrossRef]
- Ilha, E.C.; da Silva, T.; Lorenz, J.G.; de Oliveira Rocha, G.; Sant’Anna, E.S. Lactobacillus paracasei isolated from grape sourdough: Acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. Eur. Food Res. Technol. 2015, 240, 977–984. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Nunes, G.L.; Etchepare, M.A.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; Barin, J.S.; de Moraes Flores, E.M.; de Bonada Silva, C.; Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT-Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Vanden Braber, N.L.; Díaz Vergara, L.I.; Rossi, Y.E.; Aminahuel, C.A.; Mauri, A.N.; Cavaglieri, L.R.; Montenegro, M.A. Effect of microencapsulation in whey protein and water-soluble chitosan derivative on the viability of the probiotic Kluyveromyces marxianus VM004 during storage and in simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2020, 108844. [Google Scholar] [CrossRef]
- Rosolen, M.D.; Weber Bordini, F.; Diazde Oliveira, P.; Rochedo Conceição, F.; Scherer Pohndorf, R.; Fiorentini, A.M.; Padilha da Silva, W.; Pieniz, S. Symbiotic microencapsulation of Lactococcus lactis subsp. lactis R7 using whey and inulin by spray drying. LWT-Food Sci. Technol. 2019, 115, 108411. [Google Scholar] [CrossRef]
- Loyeau, P.A.; Spotti, M.J.; Vanden Braber, N.L.; Montenegro, M.A.; Vinderola, G.; Carraraa, C.R. Microencapsulation of Bifidobacterium animalis subsp. lactis INL1 using whey proteins and dextrans conjugates as wall materials. Food Hydrocol. 2018, 85, 129–135. [Google Scholar] [CrossRef]
- Eckert, C.; Garcia Serpa, V.; Felipe dos Santos, A.C.; da Costa Viviane Dalpubel, S.M.; Neutzling Lehn, D.; Volken de Souza, C.F. Microencapsulation of Lactobacillus plantarum ATCC 8014 through spray drying and using dairy whey as wall materials. LWT-Food Sci. Technol. 2017, 82, 176–183. [Google Scholar] [CrossRef]
- Mansour, M.; Salah, M.; Xu, X. Effect of microencapsulation using soy protein isolate and gum arabic as wall material on red raspberry anthocyanin stability, characterization, and simulated gastrointestinal conditions. Ultrason. Sonoch. 2019. [Google Scholar] [CrossRef] [PubMed]
- Aprodu, I.; Milea, Ș.A.; Anghel, R.-M.; Enachi, E.; Barbu, V.; Crăciunescu, O.; Râpeanu, G.; Bahrim, G.E.; Oancea, A.; Stănciuc, N. New functional ingredients based on microencapsulation of aqueous anthocyanin-Rich extracts derived from black rice (Oryza sativa L.). Molecules 2019, 24, 3389. [Google Scholar] [CrossRef] [Green Version]
- Yee, W.L.; Yee, C.L.; Lin, N.K.; Phing, P.L. Microencapsulation of Lactobacillus acidophilus NCFM incorporated with mannitol and its storage stability in mulberry tea. Ciência e Agrotecn. 2019, 43, 005819. [Google Scholar] [CrossRef] [Green Version]
- Shinde, T.; Sun-Waterhouse, D.; Brooks, J. Co-extrusion Encapsulation of Probiotic Lactobacillus acidophilus alone or together with apple skin polyphenols: An aqueous and value-added delivery system using alginate. Food Bioprocess Technol. 2013, 7, 1581–1596. [Google Scholar] [CrossRef]
- Boulet, J.C.; Ducasse, M.A.; Cheynier, V. Ultraviolet spectroscopy study of phenolic substances and other major compounds in red wines: Relationship between astringency and the concentration of phenolic substances. Aust. J. Grape Wine Res. 2017, 23, 193–199. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, A. White Light Emission from Vegetable Extracts. Sci. Rep.- Nat. 2015, 5, 9. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; del Hurtado-Sánchez, M.C.; Galeano-Díaz, T.; Durán-Merás, I. Front-face fluorescence spectroscopy combined with second-order multivariate algorithms for the quantification of polyphenols in red wine samples. Food Chem. 2017, 220, 168–176. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Walton, M.C.; Hendriks, W.H.; Broomfield, A.M.; McGhie, T.K. Viscous food matrix influences absorption and excretion but not Metabolism of blackcurrant anthocyanins in rats. J. Food Sci. 2009, 74, 22–29. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, O.; Ruiz-Espinosa, H.; Luna-Guevara, J.J.; Ochoa-Velasco, C.E.; Luna Vital, D.; Luna-Guevara, M.L. A potential natural coloring agent with antioxidant properties: Microencapsulates of Renealmia alpinia (Rottb.) Maas fruit pericarp. NFS J. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT-Food Sci. Technol. 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Li, X.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.A.R.; Son, Y. Microencapsulation and the characterization of polyherbal formulation (PHF) rich in natural polyphenolic compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sant’Anna, V.; Gurak, P.D.; Ferreira Marczak, L.D.; Tessaro, I.C. Tracking bioactive compounds with colour changes in foods- A review. Dyes Pigm. 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Schanda, J. Colorimetry: Understanding the CIE System; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-0-470-04904-4. [Google Scholar]
- Browning, W.D.; Contreras-Bulnes, R.; Brackett, M.G.; Brackett, W.W. Color differences: Polymerized composite and corresponding Vitapan Classical shade Table. J. Dent. 2009, 37, 34–39. [Google Scholar] [CrossRef]
- International Organization for Standardization Milk and Milk Products - General Guidance for the Preparation of Test Samples, Initial Suspensions and Decimal Dilutions for Microbiological Examination; ISO: Geneva, Switzerland, 2001.
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |

| Phytochemicals | 0 | 90 Days |
|---|---|---|
| Anthocyanins (mg CGE/g DW) | 71.85 ± 2.33 a | 52.99 ± 5.18 b |
| Polyphenols (mg GAE/g DW) | 12.36 ± 0.08 a | 6.16 ± 0.18 b |
| Flavonoids (mg CE/g DW) | 13.96 ± 0.21 a | 7.76 ± 0.88 b |
| Antioxidant activity (mMol Trolox/g DW) | 63.64 ± 0.75 a | 62.36 ± 0.03 b |
| Phytochemicals | Storage Time (d) | Control | S1 | S2 |
|---|---|---|---|---|
| Antioxidant activity (mMol Trolox/g DW) | 0 | 2.3 ± 0.26 B,c | 11.95 ± 3.45 A,b | 27.33 ± 3.17 A,a |
| 21 | 9.44 ± 2.84 A,b | 14.22 ± 2.00 A,b | 26.24 ± 0.68 A,a | |
| Polyphenols (mg GAE/g DW) | 0 | 3.91 ± 0.02 B,a | 5.32 ± 0.09 B,b | 6.48 ± 0.49 B,c |
| 21 | 5.76 ± 0.53 A,b | 7.1 ± 0.46 A,a | 8.15 ± 0.23 A,a | |
| Flavonoids (mg CE/g DW) | 0 | 95.63 ± 1.38 A,a | 99.59 ± 5.56 A,a | 101.50 ± 6.50 A,a |
| 21 | 71.26 ± 6.23 B,b | 97.50 ± 2.26 A,a | 90.09 ± 0.72 B,a | |
| Anthocyanins (mg CGE/g DW) | 0 | 0.80 ± 0.11 A,c | 11.84 ± 4.43 A,b | 27.75 ± 0.24 B,a |
| 21 | 0.98 ± 0.52 A,c | 15.52 ± 0.55 A,b | 32.64 ± 0.42 A,a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices. Molecules 2020, 25, 1700. https://doi.org/10.3390/molecules25071700
Enache IM, Vasile AM, Enachi E, Barbu V, Stănciuc N, Vizireanu C. Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices. Molecules. 2020; 25(7):1700. https://doi.org/10.3390/molecules25071700
Chicago/Turabian StyleEnache, Iuliana Maria, Aida Mihaela Vasile, Elena Enachi, Vasilica Barbu, Nicoleta Stănciuc, and Camelia Vizireanu. 2020. "Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices" Molecules 25, no. 7: 1700. https://doi.org/10.3390/molecules25071700
APA StyleEnache, I. M., Vasile, A. M., Enachi, E., Barbu, V., Stănciuc, N., & Vizireanu, C. (2020). Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices. Molecules, 25(7), 1700. https://doi.org/10.3390/molecules25071700









