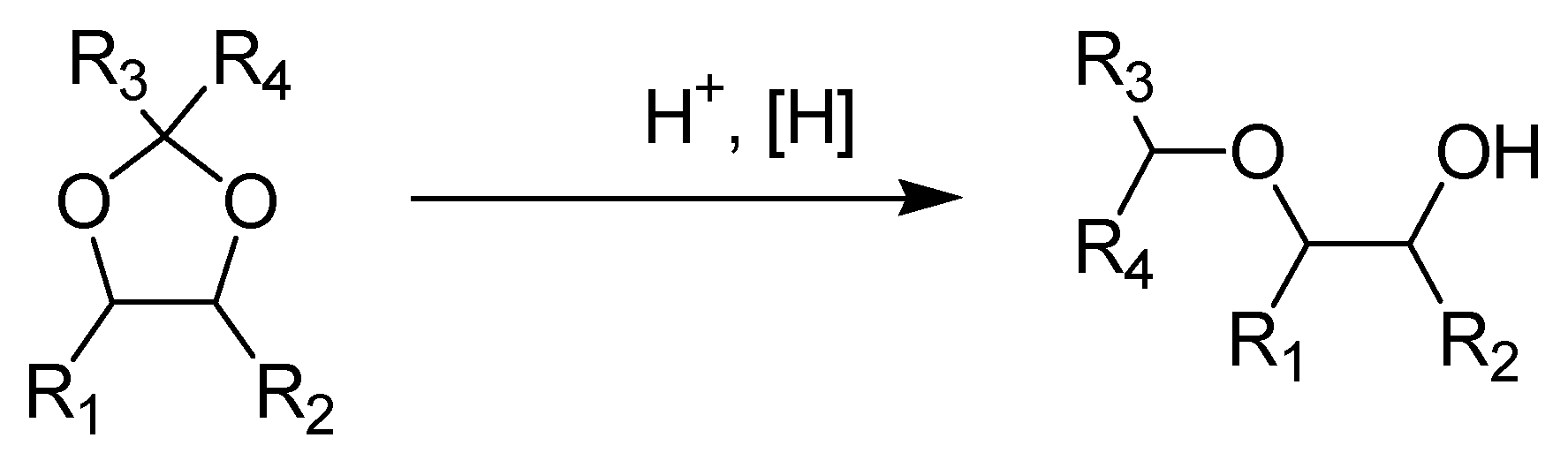2.1. The Ketalization of 1,2-PD and 2,3-BD with Acetone and MEK
The ketalization between diols and acetone (or MEK) should comply with the general rules known for the reactions of this type. As is known, the ketalization process is slightly exothermic and results in a decrease in entropy [
47]. Thus, to obtain the maximum ketal yields lower temperatures are preferred. At the same time, the thermodynamic stability of the cyclic ketal products depends on the molecular structure of the precursor diols and carbonyl compounds, what is supported by the different equilibrium yields obtained either within the reactions of the diol with the different carbonyl compounds [
48,
49,
50] or in transacetalization (transketalization) reactions [
51]. In order to determine the thermodynamic equilibrium for the reactions of interest (yielding the corresponding cyclic acetals from 1,2-PD and 2,3-BD) and to evaluate the relation between the reactants molecular structure and the reaction thermodynamic, experimental measurements of the equilibrium constant temperature dependence were conducted.
The determination of equilibrium compositions was carried out under the conditions employed earlier for the ketalization between acetone and glycerol [
52,
53]. During the analysis of the reaction mixtures, no byproducts were observed. The experimental results obtained (
Table 1) show the dependence between the equilibrium yield and the temperature typical for the homologous ketalization reactions. The data was plotted in the Arrhenius coordinates (
Figure 1) and fitted with a linear function with good precision.
Using the Van ’t Hoff equation in the form:
and the equation which expresses the relation between the changes Gibbs free energy, the enthalpy and the entropy upon the reaction:
the corresponding values of Δ
G0, Δ
H0 and Δ
S0 for the reaction were calculated (
Table 2).
A comparison between the values obtained in this work with the previously reported ones permits us to conclude that in the present case the differences in the molecular structure do not affect the thermodynamic stability of the cyclic ketals formed. The only result which shows a significant difference seems to be the result of Nanda et al. [
52] for the reaction of glycerol with acetone, probably due to the use of ethyl alcohol as solvent, while other results were obtained in solventless reactions. The values of Δ
H0r and Δ
S0r obtained for the reaction between 1,2-PD and acetone by Anteunis and Rommelaere are likely to be underestimated, what might be connected with the known precision limits of the NMR measurements. At the same time, the data from the
Table 2 proves the postulate of Anteunis and Rommelaere about the isoequilibrium relationship for the homological reactions of the cyclic ketal formation. For those typical values of Δ
H0r, Δ
S0r are about −17.2 ± 2.6 kJ mol
−1 and 57.2 ± 7.3 J mol
−1 K
−1, respectively.
The equilibrium yield values for the reactions of 2,3-BD and 1,2-PD ketalization are close to the values measured for glycerol ketalization under the similar conditions [
52,
53]. Pure TMD and ETMD samples were obtained via the direct ketalization under the same ketone molar excess (6:1) conditions. The isolated yields of TMD and ETMD (83.7 and 81.6%) turned out to be close to the equilibrium yields determined by GC (89.7 and 84.9%), hence the isolated yields for TMD and ETMD amounted to 0.933 and 0.961 of the corresponding equilibrium yields. Thus, the direct synthesis approach offers excellent yields along with a relatively simple set-up, and thus might be further potentially developed with a view to an industrial process. The thermodynamic data obtained here could be of interest for the necessary reaction engineering purposes.
2.2. The Glycol Ethers Synthesis Via the Hydrogenolysis of the Corresponding Cyclic Ketals
The properties of the catalysts used for 1,2-PD and 2,3-BD monoether synthesis are given in
Table 3. The performance of Pd/Al-HMS materials in the cyclic glycerol ketal hydrogenolysis reaction and the characterization details have been previously reported [
39], so in this paper only the brief description is given. The values of the specific surface areas for the catalysts indicate well-developed pore structures (between 680 m
2 g
−1 and 850 m
2 g
−1) and a narrow pore size distribution with mean pore sizes of about 3–5 nm. The acidity of the mesoporous aluminosilicas demonstrates a non-linear growth with the increase in the Al content. The acidity strength distribution pattern for Al-HMS aluminosilicas determined previously has shown that strong acid sites are practically absent, what might provide the high selectivity in the reaction of the cyclic ketals hydrogenolysis [
39].
The palladium particles are well dispersed, with mean sizes between 3 nm and 5 nm, what allows us to reach the conclusion that the metal particles size seems to be limited by the pore size and that the metal particles are predominately located inside the pores of the Al-HMS. The metal particles dispersion degrees in the catalysts with the supports of the different Si/Al ratio are practically identical.
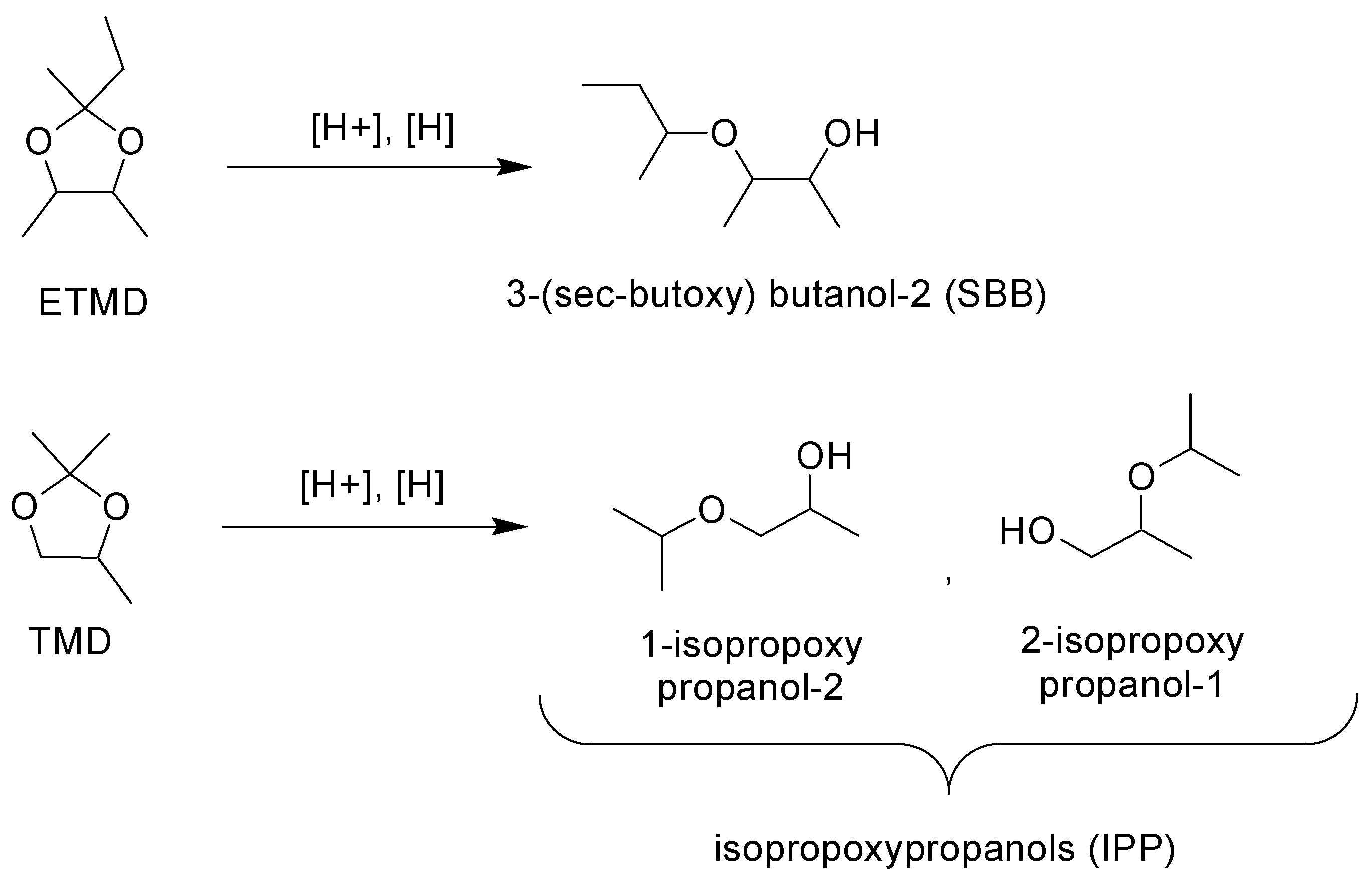
The catalysts were tested in the cyclic ketal (both TMD and ETMD) hydrogenolysis reaction yielding the corresponding glycol ethers (
Scheme 3). The reaction is known for its high selectivity: the yields are usually nearly quantitative for the reduction with hydrogen [
38,
41], LiAlH
4 [
55] or 1,1,3,3-tetramethyldisiloxane [
36]. The results of the catalytic tests (
Table 4) demonstrate that in the present case the selectivity values were also very high, normally reaching 97–98 mol %. The corresponding glycol ether (IPP for TMD, SBB for ETMD) was actually the main and only reaction product with the remaining 2–3% of selectivity accounted for by the hydrolysis products (the ketone and the diol), originated from the trace water.
The hydrogenolysis of the glycerol-acetone cyclic ketal (solketal) over these catalysts has been studied earlier. It has been revealed by the authors that the Si/Al ratio of the support influences the overall catalytic activity [
39]. The observed differences were attributed to the different activity of the supports in the acid-catalyzed reactions, e.g., ketalization, as more hydrophobic supports with greater Si/Al ratio showed to be more active. The strong adsorption of polar species (for example, water, polar organic compounds), which are typical for the relatively hydrophilic materials, might be the reason for their limited catalytic activity [
50,
52]. Thus, in the case of solketal catalytic hydrogenolysis, more hydrophilic material is likely to adsorb the primary hydrogenolysis product that is the diol.
In the present case, the authors have not observed any significant differences between the palladium catalysts supported over the Al-HMS materials with the Si/Al ratios ranging from 10 to 30 (
Table 4). The Al-HMS(10) catalyst was slightly less active at the lower temperature, while the activities of all the catalysts under
T = 160 °C were practically of the same value. This lets one suppose that in the present case there is no significant correlation between the support hydrophobicity and the catalytic activity, since the difference in the polarity of the reactant and the product (the ketal and the corresponding ether) is likely to be relatively low. As shown below, the physical properties in the “diol ketal–glycol monoether” pairs (for example, the water miscibility, boiling points and vapor pressures) are much closer compared to the “solketal–glycerol monoisopropyl ether” pair.
By conducting the reaction for more time, a nearly quantitative yield of the ether might be obtained. Under the optimized conditions (
Table 4, entry 5) both the TMD and ETMD were converted into the corresponding ethers with yields over 90 mol % (91.4 and 92.1 mol %, respectively), thus indicating at the feasibility of the approach for the catalytic synthesis of the renewable glycol ethers.
For the synthesis of ethers via the catalytic hydrogenolysis of the corresponding acetals and ketals high regioselectivity values are typical [
39,
40]. While the hydrogenolysis of ETMD yields only one structural isomer, the hydrogenolysis of TMD could yield two isomeric 1,2-PD monoethers: 2-isopropoxy-1-propanol and 1-isopropoxy-2-propanol. According to our results, the regioselectivity for 1-isopropoxy-2-propanol reached 87–91%. This value is lower than the previously observed one in solketal hydrogenolysis and it is closer to the regioselectivity of the solketal
O-isopropyl ether reduction [
39]. Close regioselectivity values have been also reported for the direct reductive alkylation of glycerol with aliphatic aldehydes [
41]. In the preparative syntheses carried out in the given work upon the reduction of TMD in the LiAlH
4-AlCl
3 system the regioselectivity for the 1-isopropoxy-2-propanol was about 82%, being here typical for this approach [
29].
Since ETMD might be obtained from 2,3-BD with the excellent yield in the one-step process, this compound seems to be the more preferable one as the starting material for the synthesis of the corresponding glycol ether by the pure ketal hydrogenolysis comparing with 2,3-BD. The renewable 1,2-PD may be obtained by the catalytic hydrogenolysis of bioglycerol. 1,2-PD cost is comparable to the price of acetone, so the feasibility of the one-step conversion of 1,2-PD to TMD in the manner that is similar to 2,3-BD conversion to ETMD seems to be controversial. Thus, the conversion of 1,2-PD into the corresponding glycol ether by reductive alkylation with acetone (the latter can originate from sugars by the ABE fermentation) seems to be of value (
Scheme 4).
The reductive alkylation reaction is generally believed to run through the formation of the intermediate ketal followed by its catalytic hydrogenation [
38]. Therefore, the influence of the reaction temperature should be crucial: its increase enhances the hydrogenolysis rate, while decreasing the equilibrium concentration of the intermediate ketal described above in the ketalization thermodynamic study. The experimental results (
Table 5) have shown that there is a temperature optimum for the reductive alkylation reaction. For example, upon the reductive alkylation of 1,2-PD with acetone under
T = 120, 140, 160 °C (
Table 5, entries 7–9), the yields of IPP amounted to 15.2, 58.7 and 7.9%, respectively. Hence, at the lower temperature the ether yield decreased along with the hydrogenation rate. At 160 °C the obtained ether yield was connected with the unfavorable influence of the temperature on the equilibrium between diol and the corresponding ketal, which is the reaction intermediate. The decrease of the diol:ketone ratio from 40:1 to 20:1 (mol/mol) (
Table 5, the entries 7 and 12) also led to an ether yield decrease (from 15.2 to 6.8%), thus supporting the determinant influence of the ketal equilibrium concentration on the target product yield. Regarding the reaction with the greater reaction time, the glycol ether yields are likely to be as high as 92.6 and 93.3 mol % for IPP and SBB, respectively (
Table 5, entries 5 and 11). The regioselectivity between the IPP structure isomers was close to the values obtained in the direct TMD hydrogenolysis (about 90–91% to 1-isopropoxy-2-propanol).
The reusability of the bifunctional catalyst was checked in both the pure ETMD hydrogenolysis and 2,3-BD + MEK reductive alkylation reactions (
Table 6). After the first ketal hydrogenolysis cycle a slight increase in the catalyst activity was observed (the SBB yield increased from 35.6 to 36.9 mol %), probably, due to the pre-reduction of the catalyst. For the subsequent cycles, a slight decrease in activity was observed: in the fifth cycle, the target product yield amounted to 92.5% of the initial value. In the case of the 2,3-BD reductive etherification with MEK, after the first cycle the target product yield decreased sharply from 62.6 to 54.7 mol %. In the next cycles, the activity change was lower, despite the fact that the SBB yield gradually decreased to 52.1 mol % (83.8% of the initial value). This pattern of the activity change observed in the latter reaction might be attributed to the adsorption of water on the catalyst active sites: the fresh catalyst sample showed the higher activity as it was dry, while the spent catalyst contacted with the water that was released in the catalytic reaction. It should be taken into the account that the presence of water might influence the reductive etherification by the affecting on the ketalization equilibrium [
39] or the palladium reduction process [
42].
Based on the results obtained, these approaches, namely the hydrogenolysis of the pure ketal obtained in the separate synthesis from the diol and the ketone, and the one-step reductive ketalization alkylation, both turned out to be feasible for the synthesis of the renewable glycol ethers. The use of bifunctional Pd/Al-HMS catalysts permits the reactions to be carried out under relatively mild conditions (T = 120–140 °C) with excellent selectivities (97–98 mol % and 94–95 mol % for the ketal hydrogenolysis and the reductive ketalization alkylation) and in nearly quantitative yields. The recovery of the target product from the reaction mixtures is likely to be easily conducted by simple distillation.
2.3. The Characterization of the Products
The search of the new bio-based organic solvents for the substitution of the petrochemical-derived ones is an important problem of sustainable chemistry. Investigations of solvent properties of compounds derived from propylene glycol, glycerol, levoglucosane and isosorbide are to be mentioned herein as the examples [
27,
43,
44,
46,
56,
57]. In the given study, the efforts have been taken to describe the properties of the synthesized compounds, namely, of two cyclic ketals—TMD and ETMD and of two corresponding glycol ethers—IPP and SBB. The estimate of the properties which might be relevant for the organic solvents was performed with the respect to the criteria for the green solvents recognizing reported earlier by Jessop [
58].
All the compounds tested appear to be the low-viscosity liquids with low melting points (<−60 °C) and the pleasant fruity smell. The value of the TMD viscosity is in good accordance with the data reported earlier by Kapkowski et al. [
25]; it might be compared to the kinematic viscosity of
n-butyl acetate (0.78 mm
2 s
−1), while the viscosity of ETMD is of the same order as that of diethyl ether (0.31 mm
2 s
−1). The viscosities of IPP and SBB are close to the value of 2-butoxyethanol (3.64 mm
2 s
−1). The boiling points values (
Table 7) being less than 250 °C under atmospheric pressure place these compounds in the VOC group according to the EU classification.
For volatile organic compounds not only the boiling points, but also the saturated vapor pressure and the evaporation rate values are important. In the given study, the evaporation rates were estimated according to the thermogravimetric method, whose applicability was demonstrated earlier by the Aubry group [
59]. The results (
Figure 2,
Table 8) demonstrate that TMD evaporates at the greater rate than the reference BuOAc (the evaporation rate is 1.30), while other compounds evaporate slightly slower (ETMD – 0.91, IPP – 0.84 and SBB – 0.73). The evaporation rates of the ketals might be expected to be higher than the values obtained for the corresponding ethers.
In order to characterize the volatility, the temperature dependences of saturated vapor pressure have been determined experimentally (
Table S1). The coefficients of the Antoine equation have been calculated (
Table 9) by means of the mathematical regression of the experimental results. It has been reported for the case of glycol and glycerol monoalkyl ethers that the TGA-derived evaporation rates correlate with the saturated vapor pressure values, but not with the boiling points [
59]. In the given study the linear correlations have been observed between both the boiling point–evaporation rate (RSD = 0.984) and the saturated vapor pressure–evaporation rate (RSD = 0.977) (
Figures S1 and S2). One should note that the results of the TGA evaporation rate measurements should be employed only for the primary qualitative estimate: for the isomer of IPP with the close boiling point (propylene glycol
n-propyl ether C
3P
1, bp = 149 °C), a RER of 0.56 was reported [
59], which is about a third lower than the value obtained by us. Moreover, the reported RER value for C
3P
1 showed to be about 23% lower than the one obtained for SBB, which has the highest boiling point among the compounds tested. At the same time, the experimental SVP values (at 50 °C) for IPP (1956 Pa) and its isomer, propylene glycol
n-propyl ether 1756 Pa) [
59], are in the proper relation.
All the compounds tested appeared to be completely miscible with methanol, ethanol, isopropyl alcohol, diethyl ether, toluene and dodecane. Except for IPP, the compounds were slightly miscible with water (
Table 10): the miscibility with water decreased with the increase in alkyl substituents molecular weight.
The miscibility with water measured for the ketals turned out to be in all the cases lower than that one of the corresponding glycol ethers. IPP being miscible with water is likely to be considered as the component of the low-melting water-based liquids, that is why for this compound the properties of aqueous solutions have been estimated (
Table 11).
One should note that if a liquid with the freezing point of −15 °C is needed, the IPP-based aqueous solution might contain less organic matter (22 vol. %) than in case of ethylene glycol and propylene glycol (both 30 vol. % for −14 °C), glycerol and isopropanol (both 40 vol. % for −15 °C). At the same time, if a lower freezing temperature is required, the only advantage of IPP over propylene glycol is a lower solution viscosity; the lower flash point of IPP compared to 1,2-PD should be considered in this case disadvantageous.
One of the major areas of organic solvents usage is the dissolution of polymers as the search for the new greener solvents for the preparation of the polymer solutions, e.g., for dyes and coatings applications is of great interest. The ketals, TMD and ETMD, turned out to be the excellent solvents for PS and polybutadiene (
Table 12): 300 mg of the polymer per 1 mL of the solvent were completely dissolved to give a clear solution, and only the high viscosities of the solutions hindered a further increase in the test polymer concentration. The same polymers appeared to be just slightly soluble in the glycol ethers, which bear OH-groups in the molecular structure. The chlorinated poly(vinylchloride) (CPVC) sample, which has the high solubility in dichloroethane, might dissolve in the ketals and the glycol ethers in quite the low concentrations thus making our hopes that these compounds might partially substitute chlorinated organic solvents for the dissolution of chlorinated polymers fade.
The solvatochromic parameters of the renewable diol derivatives are given in
Table 13. The positioning of the diol derivatives on the Kamlet-Taft β vs π* plots has been examined (
Figure 3). TMD and ETMD, as far as their polarity-basicity properties are concerned, are found to be close to such the aprotic solvents as diethyl ether,
n-butyl acetate, eucalyptol and 1,1-diethoxymethane, and thus might be related to the group of solvents with the moderate basicity and the moderate polarity (
Figure 3a). The higher boiling point and the lower volatility that may propose the lower evaporation losses and the lower flammability hazards are of potential benefit over Et
2O and DEM.
ETMD is totally comparable with BuOAc in terms of volatility; the remarkable difference between these compounds is that the former (being the cyclic ketal) is stable in basic media, while the latter undergoes rapid saponification. Thus, the ketal solvents may be employed in those cases, when an organic solvent should be used in contact with the aqueous alkali solution. One should note that it was impossible for us to measure the value for these compounds, since the Reichardt’s dye solutions gave the inadequate wavenumbers, probably due to the presence of some minor impurities, which were not obliged to detecting by neither NMR nor GC. The determination of this abnormal behavior is of the further interest for the solvatochromic characterization of the cyclic ketals.
SBB and IPP on the Kamlet-Taft plot for the protic solvents have been found among the aliphatic alcohols (
Figure 3b). The boiling points of the glycol ethers are slightly lower than those of aliphatic alcohols with the same carbon atom content: the boiling points for IPP and 1-hexanol are 144 and 157 °C; 163 and 195 °C for SBB and 1-octanol, respectively. Simultaneously, in the aforementioned pairs the glycol ethers have higher miscibility with water, compared to the corresponding alcohols. While combining these two facets one can conclude that the closest alcohol analogues of IPP and SBB are 1-pentanol (bp = 138 °C, solubility in water 22 g L
−1) and 1-hexanol (bp = 157 °C, solubility in water 6 g L
−1), respectively. The main difference in case of IPP and pentanol is that the former is miscible with water in all the ratios. Hence, if the Ziegler process or the hydration of an oil-derived olefin is considered as the main source of fatty alcohols, IPP and SBB glycol ethers possibly have an advantage, since they are obtained from renewable resources, although 1-pentanol derived from levulinic acid might be bio-based as well.
The synthesis trees for the compounds tested in the given study (
Figure 4) were made up based on the following assumptions: 2,3-butanediol is obtained by the microbial fermentation of carbohydrates with the subsequent one-step conversion to ETMD, according to the protocol described by Neish [
23] and Harvey [
22]; SBB is obtained by the one-step hydrogenolysis of ETMD. Thus, for these compounds the number of synthetic steps is two and three, respectively (
Figure 4a). The synthetic trees for 1,2-PD derivatives have been made up starting from bioglycerol-derived propylene glycol.
Although there are precedents for a one-step 1,2-PD synthesis by a retroaldol glucose conversion [
61,
62], currently only the glycerol hydrogenolysis process is employed on an industrial scale. Carbohydrate fermentation is supposed to be an appropriate source of the renewable acetone. The synthesis of TMD is implemented via the direct ketalization of 1,2-PD with acetone, since the feasibility of the one-step 1,2-PD to TMD conversion seems to be rather arguable in essence, representing here the dehydration of 1,2-PD to acetone. Thus, there are three synthetic steps in the TMD synthesis. For IPP obtained by the TMD hydrogenolysis it amounts to four (
Figure 4b), but if the reductive alkylation route were chosen, IPP also would require only three steps to be recovered (
Figure 4c).
In the final stage of our primary sustainability assessment for TMD, ETMD, IPP and SBB as organic solvents, the questions about the synthetic process formulated by Jessop [
58] have been here addressed (
Table 14). It is obvious that there are neither phosphorous- or nitrogen-containing wastes nor volatile heteroatomic compounds of nitrogen, sulfur and halogens in the production processes. The compounds under investigation might be considered to be fully (ETMD) or partially (TMD, IPP) renewable ones. Though providing the excellent yields and selectivity in rather mild conditions, the using of palladium catalysts for the cyclic ketal hydrogenolysis does not actually seem sustainable enough, thus employing a non-noble metal catalyst (e.g., nickel-based) for this reaction is of interest. Except the latter issue, the results of the primary sustainability estimate make it possible to suppose that the compounds investigated, particularly 2,3-butanediol-based, might be tentatively considered as potential green solvents.
As reported by Harvey et al., ETMD might have some potential as a gasoline component thanks to its appropriate volatility, relatively high calorific value and antiknock performance [
22]. At the same time, there is no data on the influence of ETMD additives on the gasoline volatility properties and the antiknock performance. One can suppose that the hydrogenolysis of the cyclic ketals such as TMD and ETMD might give derivatives with increased antiknock performance, since the latter compounds bear an alcohol moiety. Alcohols are likely to be mostly efficient octane boosters when they are added to gasoline. Finally, the ketals possess relatively low stability in contact with acidic water [
27,
49], and despite the fact there is no data on the possible degradation of ketal-containing gasoline blends, this issue should not be disregarded. One should note here that the ethers which derive from ketal hydrogenolysis might have far higher hydrolytic stability than their precursors. Therefore, for characterizing the synthesized compounds in terms of the gasoline-blending properties the corresponding tests have been performed (
Table 15). The extra purpose here was to estimate the relationships between the molecular structures of these oxygenates and their octane-enhancing efficiency.
As the tested oxygenates’ boiling points are inside the gasoline boiling range (35–193 °C), their volatilities can be considered acceptable. The main concern here is that the “excessively heavy” oxygenates can decrease the overall gasoline volatility, what could be particularly relevant for high-boiling solketal [
53], γ-valerolactone [
63], methyl pentanoate and alkyl levulinates [
64]. The results of the fractional composition, namely, the 5 and 10 vol. % recovery temperatures allow to make the “indirect” assertion on the gasoline cold start properties. As the differences in the
T5% and
T10% between the neat and additive-containing gasolines are within the method precision, one can conclude that none of the compounds tested affect the gasoline volatility.
The blending octane numbers and the calculated AKI for ETMD (
Table 15) appeared to be slightly higher than the intrinsic ONs, reported previously by Harvey et al. [
22]. Other tested oxygenates showed excellent antiknock performance enhancing the knock resistance more efficiently than ETMD: the AKI values for TMD, IPP and SBB amounted to 95.2, 99.2 and 99.7 points, respectively. The impacts on the RON are not very high in comparison with, e.g., ethanol and MTBE (blending RONs about 110–120 and 115–120, respectively), whereas the calculated values of bMON are comparable. Oxygenates providing a higher effect on MON are particularly preferable, when gasoline contains significant amounts of FCC gasoline, which normally has a high octane sensitivity. As expected, TMD turned out to have a higher octane number than ETMD: for the former, there is only one atom at the tertiary carbon per molecule, while for the latter there are two, being less resistant to oxidation.
It is of big concern that the molecular structure of TMD resembles the solketal molecule but without an OH-group. The methylation of the solketal OH-group was shown earlier to result in a dramatic ON decrease [
53], and it was the OH-group (not the 2,2-dimethyl-1,3-dioxolane moiety) which was responsible for the good antiknock performance. In the present case, the octane rating of the 2,2-dimethyl-1,3-dioxolane derivative is obviously quite high. The understanding of the data on the solketal and its methylated derivative should be thus reconsidered: it seems that it is not only the positive influence of the OH-group in the solketal molecule, but the methoxy substituent is sure to affect the antiknock performance as well (
Figure 5). An investigation on the relations between the molecular structure and the antiknock performance of 1,3-dioxolane homologues might be of further interest.
The mild hydrogenation of the cyclic ketals occurs with the release of the free alcohol group which might be considered as a “bearer” of the antiknock properties, as has been reported earlier [
53,
65]. There is little difference between the efficiency of the glycol ethers. SBB shows a slightly higher performance, although the higher impact from IPP having fewer C-H bonds at tertiary carbon atoms might be expected. However, “octane number–additive concentration” dependencies often demonstrate nonlinearity, that is why IPP and SBB might be considered to demonstrate a similar antiknock performance with the volume bMON/bRON about 101/98. The calculated molar bRON/bMON for the aforementioned glycol ethers are 101/98 and 100/97, respectively.
One should note that the volumetric calorific value of the renewable diols derivatives is quite high, compared to the alcohols with close antiknock performance. For example, 2-butanol with RON/MON = 105/93 has a density (at 20 °C) of 0.808 kg L
−1 and a volumetric NHOC of 26.7 MJ L
−1. The weight calorific value of SBB (32.5 MJ kg
−1) might also be compared with that of 2-butanol and 2-methyl tetrahydrofuran (32.9 MJ kg
−1). The SBB volumetric NHOC amounts to 28.48 MJ L
−1 with an antiknock performance similar to that of the aforementioned alcohol [
64]. Upon adding 10 vol. % of 2-butanol and SBB, the volumetric NHOC values for the blends might amount to 26.70 and 28.48 MJ L
−1 with AKI of the both blends of 89.2–89.3 points. Thus, the loss in the overall fuel volumetric calorific value upon the adding of the octane booster is remarkably lower for SBB. What is interesting, is that both the compounds could be obtained by bio-2,3-butanediol treatment involving the dehydration with the subsequent hydrogenation. On the Van Krevelen diagram the coordinates ([O:C; H:C]) of SBB and 2-BuOH are [2.25; 0.25] and [2.50; 0.25], respectively. Thus, although alcohol has a higher hydrogen content, the efficiency of SBB as the gasoline component is higher, so one can make the conclusion that the conversion of 2,3-BD to SBB represents a method to the renewable energy recovery which might be potentially efficient and sustainable.