Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas
Abstract
1. Introduction
2. Materials and Methods
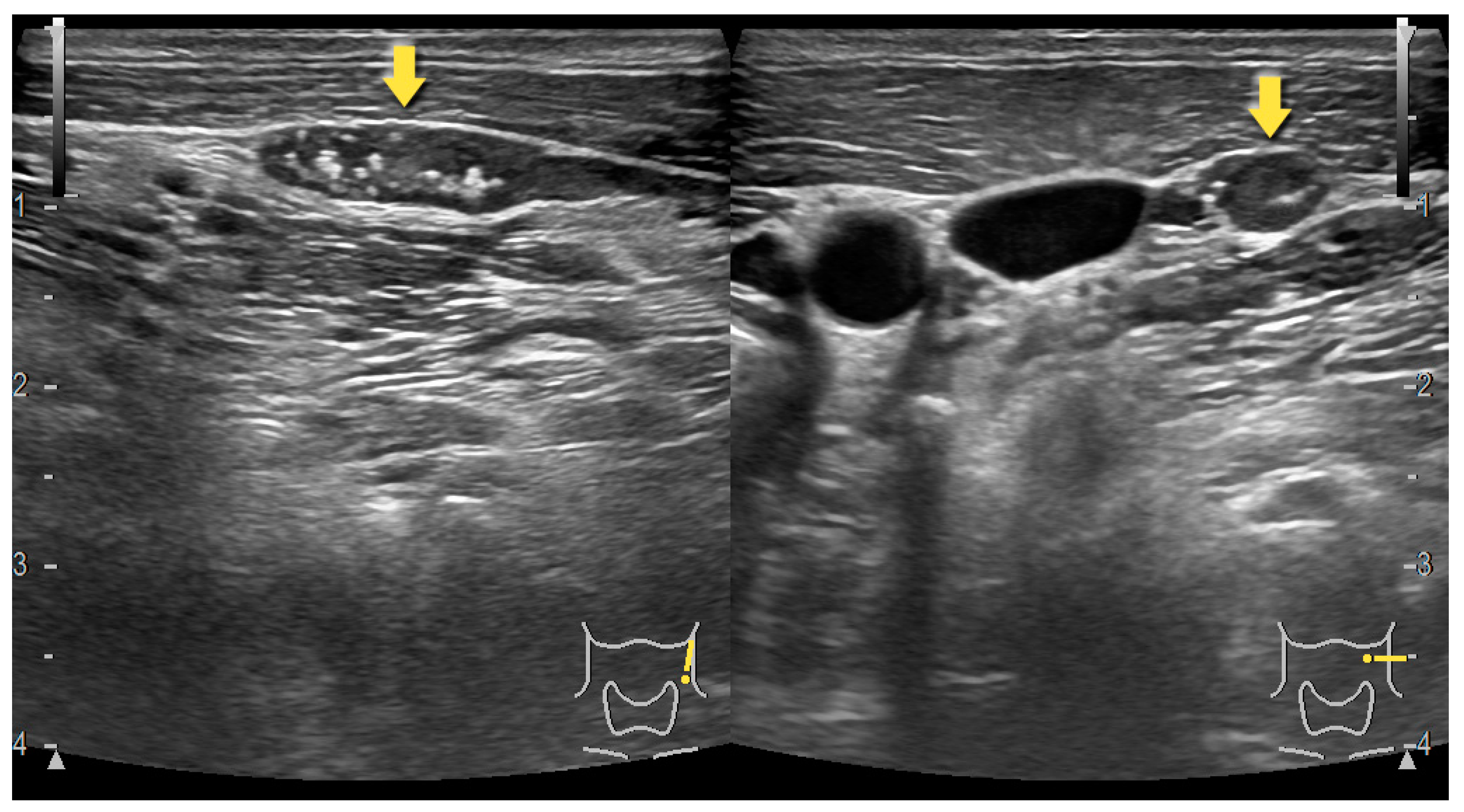
3. Preoperative Imaging Techniques–Ultrasonography, Computed Tomography and Sestamibi Scintigraphy
4. Carbon Nanoparticles
4.1. Carbon Nanoparticles Characterization
4.2. Carbon Nanoparticles in Parathyroid Glands Localization
5. Carbon Nanoparticles Suspension and Technetium Sestamibi (99mTc-MIB)
6. Raman Spectroscopy
Raman Spectroscopy–Differentiation Between Parathyroid Adenomas and Hyperplasia
7. Near-Infrared Autofluorescence
8. Dynamic Optical Contrast Imaging
9. Laser Speckle Contrast Imaging
10. Shear Wave Elastography
11. Indocyanine Green
12. Indocyanine Green Fluorescence Vs Parathyroid Autofluorescence
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4D-CT | Four-dimensional computed tomography |
| ARFI | Acoustic radiation force impulse |
| CT | Computed tomography CT |
| DOCI | Dynamic optical contrast imaging |
| FCH | 18F-fluorocholine |
| ICG | Indocyanine green |
| ICGA | Indocyanine green fluorescence angiography |
| LSCI | Laser speckle contrast imaging |
| NIR | Near-infrared autofluorescence |
| PGs | Parathyroid glands |
| PHPT | Primary hyperparathyroidism |
| SPECT-CT | Single-photon emission CT |
| SWE | Shear-wave elastography |
| USG | Ultrasonography |
| VTIQ | Virtual Touch tissue imaging quantification |
References
- Goltzman, D.; Mannstadt, M.; Marcocci, C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front. Horm. Res. 2018, 50, 1–13. [Google Scholar] [PubMed]
- Giudice, M.L.; Mihalik, B.; Dinnyes, A.; Kobolak, J. The nervous system relevance of the calcium sensing receptor in health and disease. Molecules 2019, 24, 2546. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Silverberg, S.J. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2017, 14, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, C.E.; Jackson, C.E. Serum Calcium Survey for Hyperparathyroidism. Am. J. Clin. Pathol. 1971, 55, 523–526. [Google Scholar] [CrossRef]
- Wermers, R.A.; Khosla, S.; Atkinson, E.J.; Achenbach, S.J.; Oberg, A.L.; Grant, C.S. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: An update on the changing epidemiology of the disease. J. Bone Miner. Res. 2006, 21, 171–177. [Google Scholar] [CrossRef]
- Machado, W. Parathyroid cancer: A review. Cancers 2019, 11, 1676. [Google Scholar] [CrossRef]
- Chai, Y.; Chae, H.; Kim, K.; Lee, H.; Choi, S.; Lee, K. Comparative gene expression profiles in parathyroid adenoma and normal parathyroid tissue. J. Clin. Med. 2019, 8, 297. [Google Scholar] [CrossRef]
- Bringhurst, R.F. Hormones and Disorders of Mineral Mtabolism. Williams Textb Endocrinol. 1998. Available online: https://ci.nii.ac.jp/naid/10007000361/ (accessed on 16 July 2019).
- Novodvorsky, P.; Hussein, Z.; Arshad, M.F.; Iqbal, A.; Fernando, M.; Munir, A. Two cases of spontaneous remission of primary hyperparathyroidism due to auto-infarction: Different management and their outcomes. Endocrinol. Diabetes Metab. Case Rep. 2019. [Google Scholar] [CrossRef]
- Mihai, R.; Wass, J.A.H.; Sadler, G.P. Asymptomatic hyperparathyroidism–Need for multicentre studies. Clin. Endocrinol. 2008, 68, 155–164. [Google Scholar] [CrossRef]
- Van der Walt, J. Pathology of the parathyroid glands. Diagn. Histopathol. 2012, 18, 221–233. [Google Scholar] [CrossRef]
- Verdelli, C.; Corbetta, S. Epigenetic alterations in parathyroid cancers. Int. J. Mol. Sci. 2017, 18, 310. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Weber, F. Complications in thyroid and parathyroid surgery. In Surgery of the Thyroid and Parathyroid Glands; Springer: Berlin/Heidelberg, Germany, 2007; pp. 217–224. [Google Scholar]
- Edafe, O.; Antakia, R.; Laskar, N.; Uttley, L.; Balasubramanian, S.P. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br. J. Surg. 2014, 101, 307–320. [Google Scholar] [CrossRef] [PubMed]
- McWade, M.A.; Thomas, G.; Nguyen, J.Q.; Sanders, M.E.; Solorzano, C.C.; Mahadevan-Jansen, A. Enhancing parathyroid gland visualization using a near infrared fluorescence-based overlay imaging system. J. Am. Coll. Surg. 2019, 228, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.M.; Brant, J.A.; Chai, R.L. Predictors of readmission and reoperation in patients undergoing parathyroidectomy for primary hyperparathyroidism. Otolaryngol. Head Neck Surg. 2018, 158, 828–834. [Google Scholar] [CrossRef]
- Nawrot, I.; Chudzinski, W.; Ciacka, T.; Barczynski, M.; Szmidt, J. Reoperations for persistent or recurrent primary hyperparathyroidism: Results of a retrospective cohort study at a tertiary referral center. Med. Sci. Monit. 2014, 20, 1604–1612. [Google Scholar]
- Zajac, J.D.; Danks, J.A. The development of the parathyroid gland: From fish to human. Curr. Opin. Nephrol. Hypertens. 2008, 17, 353–356. [Google Scholar] [CrossRef]
- Nakai, K.; Fujii, H.; Maeno, K.; Nishida, K.; Kobayashi, A.; Shin, J. A case of parathyroid adenoma adjacent to the thoracic spine in a hemodialysis patient. Clin. Nephrol. 2014, 81, 52–57. [Google Scholar] [CrossRef]
- Policeni, B.A.; Smoker, W.R.K.; Reede, D.L. Anatomy and embryology of the thyroid and parathyroid glands. Semin. Ultrasound CT MRI 2012, 33, 104–114. [Google Scholar] [CrossRef]
- Peissig, K.; Condie, B.G.; Manley, N.R. Embryology of the parathyroid glands. Endocrinol. Metab. Clin. N. Am. 2018, 47, 733–742. [Google Scholar] [CrossRef]
- Lopinto, M.; Rubio, G.A.; Khan, Z.F.; Vaghaiwalla, T.M.; Farra, J.C.; Lew, J.I. Science direct location of abnormal parathyroid glands: Lessons from 810 parathyroidectomies. J. Surg. Res. 2016, 6, 3–7. [Google Scholar]
- Mansberger, A.R.; Wei, J.P. Surgical embryology and anatomy of the thyroid and parathyroid glands. Surg. Clin. N. Am. 1993, 73, 727–746. [Google Scholar] [CrossRef]
- Caron, N.R.; Sturgeon, C.; Clark, O.H. Persistent and recurrent hyperparathyroidism. Curr. Treat. Options Oncol. 2004, 5, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Taterra, D.; Wong, L.M.; Vikse, J.; Sanna, B.P.; Kala, P.; Walocha, J. The prevalence and anatomy of parathyroid glands: A meta-analysis with implications for parathyroid surgery. Langenbeck’s Arch. Surg. 2019, 404, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Randone, B.; Costi, R.; Scatton, O.; Fulla, Y.; Bertagna, X.; Soubrane, O. Thoracoscopic removal of mediastinal parathyroid glands: A critical appraisal of an emerging technique. Ann. Surg. 2010, 251, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Mohebati, A.; Shaha, A.R. Imaging techniques in parathyroid surgery for primary hyperparathyroidism. Am. J. Otolaryngol. 2012, 33, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Listewnik, M.H.; Piwowarska-Bilska, H.; Safranow, K.; Iwanowski, J.; Laszczynska, M.; Chossia, M. Estimation of parameters of parathyroid glands using particle swarm optimatizaion and multivariate generalized gaussian function mixture. Appl. Sci. 2019, 9, 4511. [Google Scholar] [CrossRef]
- Schneider, M.; Dahm, V.; Passler, C.; Sterrer, E.; Mancusi, G.; Repasi, R. Complete and incomplete recurrent laryngeal nerve injury after thyroid and parathyroid surgery: Characterizing paralysis and paresis. Surgery 2019, 166. [Google Scholar] [CrossRef]
- Sapalidis, K.A.; Papanastasiou, A.; Fyntanidou, V.; Aidoni, Z.; Michalopoulos, N.; Katsaounis, A. Comparison between magnification techniques and direct vision in thyroid surgery: A systematic review and meta-analysis. Medicina 2019, 55, 725. [Google Scholar] [CrossRef]
- Johnson, N.A.; Tublin, M.E.; Ogilvie, J.B. Parathyroid imaging: Technique and role in the preoperative evaluation of primary hyperparathyroidism. Am. J. Roentgenol. 2007, 188, 1706–1715. [Google Scholar] [CrossRef]
- Lai, E.C.H.; Ching, A.S.C.; Leong, H.T. Secondary and tertiary hyperparathyroidism: Role of preoperative localization. ANZ J. Surg. 2007, 77, 880–882. [Google Scholar] [CrossRef]
- Teksoz, S.; Bukey, Y.; Ozcan, M.; Arikan, A.E.; Erbabacan, S.E.; Ozyegin, A. Minimal invasive parathyroidectomy with local anesthesia for well-localized primary hyperparathyroidism: Cerrahpasa experience. Updates Surg. 2013, 65, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.R.; Beggs, A.D.; Han, T.S. Utility of surgeon-performed pre-operative ultrasound in the localisation of parathyroid adenomas. JRSM Cardiovasc. Dis. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, F.; Chowdry, N.; Gojwari, T.; Wani, A.; Khan, S. Role of cervical ultrasonography in primary hyperparathyroidism. Indian J. Radiol. Imaging 2008, 18, 302. [Google Scholar] [CrossRef] [PubMed]
- Cakal, E.; Cakir, E.; Dilli, A.; Colak, N.; Unsal, I.; Aslan, M.S. Parathyroid adenoma screening efficacies of different imaging tools and factors affecting the success rates. Clin. Imaging 2012, 36, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-Y.; Lang, B.H.; Chan, W.; Kung, A.W.; Lam, K.S. A prospective evaluation of preoperative localization by technetium-99m sestamibi scintigraphy and ultrasonography in primary hyperparathyroidism. Am. J. Surg. 2007, 193, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Thanseer, N.; Bhadada, S.K.; Sood, A.; Mittal, B.R.; Behera, A.; Gorla, A.K.R. Comparative effectiveness of ultrasonography, 99mTc-sestamibi, and 18F-fluorocholine PET/CT in detecting parathyroid adenomas in patients with primary hyperparathyroidism. Clin. Nucl. Med. 2017, 42. [Google Scholar] [CrossRef]
- Ozkaya, M.; Elboga, U.; Sahin, E.; Kalender, E.; Korkmaz, H.; Demir, H.D. Evaluation of Conventional Imaging Techniques on Preoperative Localization in Primary Hyperparathyroidism. Bosn. J. Basic Med. Sci. 2015, 15. [Google Scholar] [CrossRef]
- Kaur, P.; Gattani, R.; Singhal, A.; Sarin, D.; Arora, S.; Mithal, A. Impact of preoperative imaging on surgical approach for primary hyperparathyroidism: Data from single institution in India. Indian J. Endocrinol. Metab. 2016, 20, 625. [Google Scholar] [CrossRef]
- Sreevathsa, M.R.; Melanta, K. Unilateral exploration for parathyroid adenoma. Indian J. Surg. Oncol. 2016, 8, 142–145. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, W.Y.; Lee, Y.-M. The role of preoperative ultrasonography, computed tomography, and sestamibi scintigraphy localization in secondary hyperparathyroidism. Ann. Surg. Treat. Res. 2015, 89, 300. [Google Scholar] [CrossRef][Green Version]
- Shi, C.; Tian, B.; Li, S.; Shi, T.; Qin, H.; Liu, S. Enhanced identification and functional protective role of carbon nanoparticles on parathyroid in thyroid cancer surgery. Medicine 2016, 95, e5148. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhao, W.; Wang, B.; Zhang, L. A novel technology for localization of parathyroid adenoma: Ultrasound-guided fine needle aspiration combined with rapid parathyroid hormone detection and nano-carbon technology. Surg. Innov. 2018, 25, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Q.; Feng, J.; Wang, J. Combined use of a nanocarbon suspension and 99mTc-MIBI for the intra-operative localization of the parathyroid glands. Am. J. Otolaryngol. 2018, 39, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Fosca, M.; Tabacco, G.; Marini, F.; Graziani, V.; Santarsia, M.C. Raman spectroscopy applied to parathyroid tissues: A new diagnostic tool to discriminate normal tissue from adenoma. Anal. Chem. 2018, 90, 847–854. [Google Scholar] [CrossRef]
- Das, K.; Stone, N.; Kendall, C.; Fowler, C.; Christie-Brown, J. Raman spectroscopy of parathyroid tissue pathology. Lasers Med. Sci. 2006, 21, 192–197. [Google Scholar] [CrossRef]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Solorzano, C.C.; Broome, J.T. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J. Clin. Endocrinol. Metab. 2014, 99, 4574–4580. [Google Scholar] [CrossRef]
- McWade, M.A.; Sanders, M.E.; Broome, J.T.; Solorzano, C.C.; Mahadevan-Jansen, A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016, 159, 193–203. [Google Scholar] [CrossRef]
- Kim, S.W.; Song, S.H.; Lee, H.S.; Noh, W.J.; Oak, C.; Ahn, Y.-C. Intraoperative real-time localization of normal parathyroid glands with autofluorescence imaging. J. Clin. Endocrinol. Metab. 2016, 101, 4646–4652. [Google Scholar] [CrossRef]
- Serra, C.; Silveira, L.; Canudo, A.; Lemos, M.C. Parathyroid identification by autofluorescence–Preliminary report on five cases of surgery for primary hyperparathyroidism. BMC Surg. 2019, 19. [Google Scholar] [CrossRef]
- Benmiloud, F.; Rebaudet, S.; Varoquaux, A.; Penaranda, G.; Bannier, M.; Denizot, A. Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: A before and after controlled study. Surgery 2018, 163, 23–30. [Google Scholar] [CrossRef]
- Paras, C.; Keller, M.; White, L.; Phay, J.; Mahadevan-Jansen, A. Near-infrared autofluorescence for the detection of parathyroid glands. J. Biomed. Opt. 2011, 16. [Google Scholar] [CrossRef] [PubMed]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Mahadevan-Jansen, A.; Broome, J.T. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013, 154, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, H.S.; Ahn, Y.-C.; Park, C.W.; Jeon, S.W.; Kim, C.H. Near-infrared autofluorescence image-guided parathyroid gland mapping in thyroidectomy. J. Am. Coll. Surg. 2018, 226, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Dip, F.; Quadri, P.; de la Fuente, M.; Rosenthal, R. Cutting edge in thyroid surgery: Autofluorescence of parathyroid glands. J. Am. Coll. Surg. 2016, 223, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, R.; Sommerey, S.; Arabi NAl Hallfeldt, K.K.J.; Stepp, H.; Gallwas, J.K.S. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg. Endosc. 2017, 31, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, F.; Breuskin, I.; Abbaci, M.; Casiraghi, O.; Mirghani, H.; Ben Lakhdar, A. Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: A feasibility study. World J. Surg. 2016, 40, 2131–2138. [Google Scholar] [CrossRef]
- Squires, M.H.; Jarvis, R.; Shirley, L.A.; Phay, J.E. Intraoperative parathyroid autofluorescence detection in patients with primary hyperparathyroidism. Ann. Surg. Oncol. 2019, 26, 1142–1148. [Google Scholar] [CrossRef]
- Kose, E.; Kahramangil, B.; Aydin, H.; Donmez, M.; Berber, E. Heterogeneous and low-intensity parathyroid autofluorescence: Patterns suggesting hyperfunction at parathyroid exploration. Surgery 2019, 165, 431–437. [Google Scholar] [CrossRef]
- Kose, E.; Rudin, A.V.; Kahramangil, B.; Moore, E.; Aydin, H.; Donmez, M. Autofluorescence imaging of parathyroid glands: An assessment of potential indications. Surgery 2020, 167, 173–179. [Google Scholar] [CrossRef]
- Henegan, J.; Mcgrath, S.; Shah, K.; Bendinelli, C. On the use of autofluorescence for detection of intrathyroidal parathyroid adenoma. ANZ J. Surg. 2019. [Google Scholar] [CrossRef]
- Alesina, P.F.; Meier, B.; Hinrichs, J.; Mohmand, W.; Walz, M.K. Enhanced visualization of parathyroid glands during video-assisted neck surgery. Langenbeck’s Arch. Surg. 2018, 403, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kahramangil, B.; Dip, F.; Benmiloud, F.; Falco, J.; de La Fuente, M.; Verna, S. Detection of parathyroid autofluorescence using near-infrared imaging: A multicenter analysis of concordance between different surgeons. Ann. Surg. Oncol. 2018, 25, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; McWade, M.A.; Paras, C.; Mannoh, E.A.; Sanders, M.E.; White, L.M. Developing a clinical prototype to guide surgeons for intraoperative label-free identification of parathyroid glands in real time. Thyroid 2018, 28, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.A.; Taylor, Z.D.; Cheng, H.; Sebastian, C.; Maccabi, A.; Garritano, J. Dynamic optical contrast imaging: A technique to differentiate parathyroid tissue from surrounding tissues. Otolaryngol. Neck Surg. 2017, 156, 480–483. [Google Scholar] [CrossRef]
- Mannoh, E.A.; Thomas, G.; Solorzano, C.C.; Mahadevan-Jansen, A. Intraoperative assessment of parathyroid viability using laser speckle contrast imaging. Sci. Rep. 2017, 7, 14798. [Google Scholar] [CrossRef]
- Hattapoğlu, S.; Goya, C.; Hamidi, C.; Tasdemir, B.; Alan, B.; Durmaz, M.S. Evaluation of parathyroid lesions with point shear wave elastography. J. Ultrasound Med. 2016, 35, 2179–2182. [Google Scholar] [CrossRef]
- Azizi, G.; Piper, K.; Keller, J.M.; Mayo, M.L.; Puett, D.; Earp, K.M. Shear wave elastography and parathyroid adenoma: A new tool for diagnosing parathyroid adenomas. Eur. J. Radiol. 2016, 85, 1586–1593. [Google Scholar] [CrossRef]
- Golu, I.; Sporea, I.; Moleriu, L.; Tudor, A.; Cornianu, M.; Vlad, A. 2D-shear wave elastography in the evaluation of parathyroid lesions in patients with hyperparathyroidism. Int. J. Endocrinol. 2017, 1–6. [Google Scholar] [CrossRef]
- Stangierski, A.; Wolinski, K.; Ruchala, M. Shear wave elastography in the diagnostics of parathyroid adenomas-new application of the method. Endocrine 2018, 60, 240–245. [Google Scholar] [CrossRef]
- Chandramohan, A.; Therese, M.; Abhraham, D.; Paul, T.V.; Mazhuvanchary, P.J. Can ARFI elastography be used to differentiate parathyroid from thyroid lesions? J. Endocrinol. Investig. 2017, 41, 111–119. [Google Scholar] [CrossRef]
- Batur, A.; Atmaca, M.; Yavuz, A.; Ozgokce, M.; Bora, A.; Bulut, M.D. Ultrasound elastography for distinction between parathyroid adenomas and thyroid nodules. J. Ultrasound Med. 2016, 35, 1277–1282. [Google Scholar] [CrossRef]
- Vidal Fortuny, J.; Karenovics, W.; Triponez, F.; Sadowski, S.M. Intra-operative indocyanine green angiography of the parathyroid gland. World J. Surg. 2016, 40, 2378–2381. [Google Scholar] [CrossRef]
- Van den Bos, J.; van Kooten, L.; Engelen, S.M.E.; Lubbers, T.; Stassen, L.P.S.; Bouvy, N.D. Feasibility of indocyanine green fluorescence imaging for intraoperative identification of parathyroid glands during thyroid surgery. Head Neck 2019, 41, 340–348. [Google Scholar] [CrossRef]
- Sound, S.; Okoh, A.; Yigitbas, H.; Yazici, P.; Berber, E. Utility of indocyanine green fluorescence imaging for intraoperative localization in reoperative parathyroid surgery. Surg. Innov. 2015, 26, 774–779. [Google Scholar] [CrossRef]
- Kahramangil, B.; Berber, E. The use of near-infrared fluorescence imaging in endocrine surgical procedures. J. Surg. Oncol. 2017, 115, 848–855. [Google Scholar] [CrossRef]
- Lang, B.H.-H.; Wong, C.K.H.; Hung, H.T.; Wong, K.P.; Mak, K.L.; Au, K.B. Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 2017, 161, 87–95. [Google Scholar] [CrossRef]
- DeLong, J.C.; Ward, E.P.; Lwin, T.M.; Brumund, K.T.; Kelly, K.J.; Horgan, S. Indocyanine green fluorescence-guided parathyroidectomy for primary hyperparathyroidism. Surgery 2018, 163, 388–392. [Google Scholar] [CrossRef]
- Chakedis, J.M.; Maser, C.; Brumund, K.T.; Bouvet, M. Indocyanine green fluorescence-guided redo parathyroidectomy. BMJ Case Rep. 2015, 2015, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.G.; O’Doherty, M.J. Parathyroid imaging: How good is it and how should it be done? Semin. Nucl. Med. 2006, 36, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Bezerra, P.; Vieira, R.; Amaral, F.; Cartaxo, H.; Lima, T.; Montarroyos, U. Better performance of four-dimension computed tomography as a localization procedure in normocalcemic primary hyperparathyroidism. J. Med. Imaging Radiat. Oncol. 2018, 62, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Tokmak, H.; Demirkol, M.O.; Alagol, F.; Tezelman, S.; Terzioglu, T. Clinical impact of SPECT-CT in the diagnosis and surgical management of hyper-parathyroidism. Int. J. Clin. Exp. Med. 2014, 7, 1028–1034. [Google Scholar] [PubMed]
- Rodgers, S.E.; Hunter, G.J.; Hamberg, L.M.; Schellingerhout, D.; Doherty, D.B.; Ayers, G.D. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 2006, 140, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.S.C.D.; Mangussi-Gomes, J.P.; Rocha, L.A.D.; Ohe, M.N.; Rosano, M.; Neves, M.C.D. Localization of ectopic and supernumerary parathyroid glands in patients with secondary and tertiary hyperparathyroidism: Surgical description and correlation with preoperative ultrasonography and Tc99m-Sestamibi scintigraphy. Braz. J. Otorhinolaryngol. 2014, 80, 29–34. [Google Scholar] [PubMed]
- Ma, J.-J.; Zhang, D.-B.; Zhang, W.-F.; Wang, X. Application of nanocarbon in breast approach endoscopic thyroidectomy thyroid cancer surgery. J. Laparoendosc. Adv. Surg. Tech. 2020. [Google Scholar] [CrossRef]
- Persky, M.S.; Lagmay, V.M. Treatment of the clinically negative neck in oral squamous cell carcinoma. Laryngoscope 1999, 109, 1160–1164. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.; Lu, C.; Zhou, J. The role of nanocarbon lymphatic tracer in thoraco-laparoscopic esophagectomy. Minerva Chir. 2017, 72, 475–482. [Google Scholar]
- Zhang, D.; Wang, T.; Dionigi, G.; Fu, Y.; Zhang, J.; Zhao, Y. Application of carbon nanoparticles in endoscopic thyroidectomy via bilateral areola approach: Total thyroidectomy plus central lymph node dissection. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 1038–1041. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, Z. Negative developing of parathyroid using carbon nanoparticles during thyroid surgery. Gland Surg. 2013, 2, 100–101. [Google Scholar]
- Huang, K.; Luo, D.; Huang, M.; Long, M.; Peng, X.; Li, H. Protection of parathyroid function using carbon nanoparticles during thyroid surgery. Otolaryngol. Head Neck Surg. 2013, 149, 845–850. [Google Scholar] [CrossRef]
- Li, Y.; Jian, W.-H.; Guo, Z.-M.; Li, Q.-L.; Lin, S.-J.; Huang, H.-Y. A meta-analysis of carbon nanoparticles for identifying lymph nodes and protecting parathyroid glands during surgery. Otolaryngol. Head Neck Surg. 2015, 152, 1007–1016. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, N.-C.; Zhang, W.; Shan, C.-X.; Jiang, Z.-G.; Liu, S. The role of carbon nanoparticles in identifying lymph nodes and preserving parathyroid in total endoscopic surgery of thyroid carcinoma. Surg. Endosc. 2015, 29, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Chaojie, Z.; Shanshan, L.; Zhigong, Z.; Jie, H.; Shuwen, X.; Peizhi, F. Evaluation of the clinical value of carbon nanoparticles as lymph node tracer in differentiated thyroid carcinoma requiring reoperation. Int. J. Clin. Oncol. 2015, 21, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tian, W.; Jiang, Y.; Zhang, S.; Guo, L.; Zhao, J. Application of carbon nanoparticles for parathyroid protection in reoperation of thyroid diseases. Int. J. Clin. Exp. Med. 2015, 8, 22254–22261. [Google Scholar] [PubMed]
- Shang, J.; Gu, J.; Wang, W.; Nie, X.; Han, Q.; Wang, K. Potential role of carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Xu, X.F.; Gu, J. The application of carbon nanoparticles in the lymph node biopsy of cN0 papillary thyroid carcinoma: A randomized controlled clinical trial. Asian J. Surg. 2017, 40, 345–349. [Google Scholar] [CrossRef]
- Yu, W.; Cao, X.; Xu, G.; Song, Y.; Li, G.; Zheng, H. Potential role for carbon nanoparticles to guide central neck dissection in patients with papillary thyroid cancer. Surgery 2016, 160, 755–761. [Google Scholar] [CrossRef]
- Wang, B.; Du, Z.-P.; Qiu, N.-C.; Liu, M.-E.; Liu, S.; Jiang, D.-Z. Application of carbon nanoparticles accelerates the rapid recovery of parathyriod function during thyroid carcinoma surgery with central lymph node dissection: A retrospective cohort study. Int. J. Surg. 2016, 36, 164–169. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Lv, J.-Y.; Yu, D.; Xin, S.-J. Application of carbon nanoparticles in lymph node dissection and parathyroid protection during thyroid cancer surgeries: A systematic review and meta-analysis. OnctoTargets Ther. 2017, 10, 1247–1260. [Google Scholar] [CrossRef]
- Gencoglu, E.A.; Aktas, A. The efficacy of low and high dose 99mTc-MIBI protocols for intraoperative identification of hyperplastic parathyroid glands in secondary hyperparathyroidism. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 210–214. [Google Scholar]
- Gencoglu, E.A.; Aras, M.; Moray, G.; Aktas, A. The effectiveness of low-dose versus high-dose 99mTc MIBI protocols for radioguided surgery in patients with primary hyperparathyroidism. Nucl. Med. Commun. 2014, 35, 398–404. [Google Scholar] [CrossRef]
- Keidar, Z.; Solomonov, E.; Karry, R.; Frenkel, A.; Israel, O.; Mekel, M. Preoperative [ 99m Tc ] MIBI SPECT / CT interpretation criteria for localization of parathyroid adenomas–Correlation with surgical findings. Mol. Imaging Biol. 2016. [Google Scholar] [CrossRef]
- Guhne, F.; Mothes, H.; Freesmeyer, M. Allocation of parathyroid adenoma and suspicious thyroid nodule by real-time 99m Tc-MIBI SPECT / US fusion imaging. Endocrine 2016, 54, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Agha, A. Recurrence of secondary hyperparathyroidism in patients after total parathyroidectomy with autotransplantation: Technical and therapeutic aspects. Eur. Arch. Otorhinolaryngol. 2012, 269, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Q.Y.; Wang, J.D. Comparison between subtotal parathyroidectomy and total parathyroidectomy with autotransplantation for secondary hyperparathyroidism in patients with chronic renal failure: A meta-analysis. Horm. Metab. Res. 2015, 47, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.; Kendall, C.; Shepherd, N.; Crow, P.; Barr, H. Near-infrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. J. Raman Spectrosc. 2002, 33, 564–573. [Google Scholar] [CrossRef]
- Reyes-Goddard, J.M.; Barr, H.; Stone, N. Photodiagnosis using Raman and surface enhanced Raman scattering of bodily fluids. Photodiagn. Photodyn. Ther. 2005, 2, 223–233. [Google Scholar] [CrossRef]
- Feng, X.; Fox, M.C.; Reichenberg, J.S.; Lopes, F.C.; Sebastian, K.R.; Dunn, A.K. Superpixel raman spectroscopy for rapid skin cancer margin assessment. J. Biophotonics 2019. [Google Scholar] [CrossRef]
- Parlatan, U.; Inanc, M.T.; Ozgor, B.Y.; Oral, E.; Bastu, E.; Unlu, M.B. Raman spectroscopy as a non-invasive diagnostic technique for endometriosis. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; He, M.; Ma, X.; Song, Y.; Liu, M. Accuracy of raman spectroscopy in differentiating brain tumor from normal brain tissue. Oncotarget 2017, 8, 36824–36831. [Google Scholar] [CrossRef]
- Wong, K.K.; Fig, L.M.; Gross, M.D.; Dwamena, B.A. Parathyroid adenoma localization with 99mTc-sestamibi SPECT/CT. Nucelar Med. Commun. 2015, 36, 363–375. [Google Scholar] [CrossRef]
- Bondeson, A.G.; Bondeson, L.; Ljungberg, O.; Tibblin, S. Fat staining in parathyroid disease–Diagnostic value and impact on surgical strategy: Clinicopathologic analysis of 191 cases. Hum. Pathol. 1985, 16, 1255–1263. [Google Scholar] [CrossRef]
- Castleman, B.; Mallory, T.B. The pathology of the parathyroid gland in hyperparathyroidism: A study of 25 cases. Am. J. Pathol. 1935, 11, 1–72. [Google Scholar]
- Demarchi, M.S.; Karenovics, W.; Bedat, B.; Triponez, F. Intraoperative autofluorescence and indocyanine green angiography for the detection and preservation of parathyroid glands. J. Clin. Med. 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell. Endocrinol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Abbaci, M.; Leeuw FDe Breuskin, I.; Casiraghi, O.; Ben, A.; Ghanem, W. Parathyroid gland management using optical technologies during thyroidectomy or parathyroidectomy: A systematic review. Oral Oncol. 2018, 87, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.M.S.; Faraji, E.; Davis, M.A.; Huang, Y.; Zhang, X.J.; Dunn, A.K. Flux or speed? Examining speckle contrast imaging of vascular flows. Biomed. Opt. Express 2015, 6, 326–330. [Google Scholar] [CrossRef]
- Kirkpatrick, S.J.; Duncan, D.D.; Wells-gray, E.M. Detrimental effects of speckle-pixel size matching in laser speckle contrast imaging. Opt. Lett. 2008, 33, 2886–2888. [Google Scholar] [CrossRef]
- Andrioli, M.; Valcavi, R. Sonography of normal and abnormal thyroid and parathyroid glands. Imaging Endocr. Disord. Front. Horm. Res. 2016, 45, 1–15. [Google Scholar]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in parathyroid imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 151–166. [Google Scholar] [CrossRef]
- Kluijfhout, W.P.; Venkatesh, S.; Beninato, T.; Vriens, M.R.; Duh, Q.-Y.; Wilson, D.M. Performance of magnetic resonance imaging in the evaluation of first-time and reoperative primary hyperparathyroidism. Surgery 2016, 160, 747–754. [Google Scholar] [CrossRef]
- Cotoi, L.; Borcan, F.; Sporea, I.; Amzar, D.; Schiller, O.; Schiller, A. Shear wave elastography in diagnosing secondary hyperparathyroidism. Diagnostics 2019, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Polat, A.V.; Ozturk, M.; Akyuz, B.; Celenk, C.; Kefeli, M.; Polat, C. The diagnostic value of shear wave elastography for parathyroid lesions and comparison with cervical lymph nodes. Med. Ultrason. 2017, 19, 386. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Patila, T.; Spillmann, T.; Tuchin, V.V. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Rudin, A.V.; Mckenzie, T.J.; Thompson, G.B.; Farley, D.R.; Lyden, M.L. Evaluation of parathyroid glands with indocyanine green fluorescence angiography after thyroidectomy. World J. Surg. 2019. [Google Scholar] [CrossRef]
- Ladurner, R.; Lerchenberger, M.; Arabi, N.A.; Gallwas, J.K.S.; Stepp, H.; Hallfeldt, K.K.J. Parathyroid autofluorescence–How does it affect parathyroid and thyroid surgery? A 5 year experience. Molecules 2019, 24, 2560. [Google Scholar] [CrossRef] [PubMed]
- Mph, A.C.R.; Ibraheem, K.; Haddad, A. Efficacy of indocyanine green fluorescence in predicting parathyroid vascularization during thyroid surgery. Head Neck 2019, 41, 1–6. [Google Scholar]
- Suh, Y.J.; Choi, J.Y.; Chai, Y.J.; Kwon, H.; Woo, J.-W.; Kim, S. Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg. Endosc. 2015, 29, 2811–2817. [Google Scholar] [CrossRef]
- Zaidi, N.; Bucak, E.; Okoh, A.; Yazici, P.; Yigitbas, H.; Berber, E. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J. Surg. Oncol. 2016, 113, 771–774. [Google Scholar] [CrossRef]
- Kuriloff, D.B. Rapid intraoperative localization of parathyroid glands utilizing methylene blue infusion. Otolaryngol Head Neck Surg. 2004, 131, 616–622. [Google Scholar] [CrossRef]
- Liu, W.W.; Li, C.Q.; Guo, Z.M.; Li, H.; Zhang, Q.; Yang, A.K. Fluorescence identification of parathyroid glands by aminolevulinic acid hydrochloride in rats. Photomed. Laser Surg. 2011, 29, 635–638. [Google Scholar] [CrossRef]
- Bewick, J.; Pfleiderer, A. The value and role of low dose methylene blue in the surgical management of hyperparathyroidism. Ann. R. Coll. Surg. Eng. 2014, 21, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, E.D.; Thambi, R.; Pytynia, K.B. Methylene blue and parathyroid adenoma localization: Three new cases of a rare cutaneous complication. Ear. Nose Throat J. 2016, 95, 70–72. [Google Scholar] [PubMed]
- Prosst, R.L.; Weiss, J.; Hupp, L.; Willeke, F.; Post, S. Fluorescence-guided minimally invasive parathyroidectomy: Clinical experience with a novel intraoperative detection technique for parathyroid glands. World J. Surg. 2010, 34, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, T.; Sato, T.; Hanazaki, K. Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green. Surg. Today 2015, 45, 1467–1474. [Google Scholar] [CrossRef]
- Aoki, T.; Murakami, M.; Yasuda, D.; Shimizu, Y.; Kusano, T.; Matsuda, K. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J. Hepatobiliary Pancreat Sci. 2010, 17, 590–594. [Google Scholar] [CrossRef]
- Golijanin, D.J.; Marshall, J.; Cardin, A.; Singer, E.A.; Wood, R.W.; Reeder, J.E. Bilitranslocase (btl) is immunolocalised in proximal and distal renal tubules and absent in renal cortical tumors accurately corresponding to intraoperative near infrared fluorescence (nirf) expression of renal cortical tumors using intravenous indocyanin. J. Urol. 2008, 179, 137. [Google Scholar] [CrossRef]
- Karampinis, I.; Di Meo, G.; Gerken, A.; Stasiunaitis, V.; Lammert, A.; Nowak, K. Intraoperative Indocyanine Green Fluorescence to Assure Vital Parathyroids in Thyroid Resections. Zentralbl Chir. 2018, 143, 380–384. [Google Scholar]

| Ref. | Authors | Year | Origin | Method | Usage | No. Patients (Studied Group) | No. Controls (Control Group) | No./% PGs Detected (Studied Group) | No./% PGs Detected (Control Group) | No. Parathyroid Adenomas Detected (Studied Group) | Accuracy | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [43] | Shi et al. | 2016 | China | Carbon nanoparticles | Intraoperative | 52 | 45 | 52/52 (100%) | 45/45 (100%) | - | - | - |
| [44] | Yan et al. | 2018 | China | Carbon nanoparticles with rapid parathyroid hormone detection and ultrasound-guided fine needle aspiration | Preoperative | 12 | - | - | - | 12 | Up to 100% | 12/12 (100%) |
| [45] | Chen et al. | 2017 | China | Carbon nanoparticles & technetium sestamibi (99mTc-MIB) | Preoperative (99mTc-MIB) Intraoperative (carbon nanoparticles) | 20 | 20 | 160* | - | - | - | - |
| [46] | Palermo et al. | 2017 | Italy | Raman spectroscopy | Intraoperative | 18 | - | - | - | 13 | 100% | - |
| [47] | Das et al. | 2006 | UK | Raman spectroscopy | Intraoperative | 15** | - | - | - | 9 | - | 95% |
| [48] | McWade et al. | 2014 | USA | Near-infrared autofluorescence spectroscopy | Intraoperative | 110 | 6 | 100% | - | - | - | 100% |
| [49] | McWade et al. | 2016 | USA | Near-infrared autofluorescence spectroscopy | Intraoperative | 137 | - | 100%*** 98%**** | - | - | 100% | - |
| [50] | Kim et al. | 2016 | Korea | Near-infrared autofluoresccence imaging | Intraoperative | 8 | - | 16/16 (100%) | - | - | 100% | 100% |
| [51] | Serra et al. | 2019 | Portugal | Near-infrared autofluoresccence imaging | Intraoperative | 5 | - | 10/10 (100%) | - | - | - | - |
| [52] | Benmiloud et al. | 2019 | France | Near-infrared autofluoresccence imaging | Intraoperative | 121 | 120 | 390 | 299 | - | - | - |
| [53] | Paras et al. | 2011 | USA | Near-infrared autofluoresccence imaging | Intraoperative | 21 | - | - | - | - | - | - |
| [54] | McWade et al. | 2013 | USA | Near-infrared autofluorescence spectroscopy | Intraoperative | 45 | - | 100% | - | - | - | - |
| [55] | Kim et al. | 2017 | Korea | Near-infrared autofluoresccence imaging | Intraoperative | 38 | - | 64 92.8% | - | 1 | 92.85% | 92.75% |
| [56] | Falco et al. | 2016 | Argentina | Near-infrared autofluorescence | Intraoperative | 28 | - | - | - | 9 | - | - |
| [57] | Ladurner et al. | 2016 | Germnay | Near-infrared autofluoresccence imaging | Intraoperative | 25 | - | 27/35 | - | - | - | - |
| [58] | De Leeuw et al. | 2016 | France | Near-infrared autofluoresccence imaging | Intraoperative | 35 | - | 81 | - | - | - | 94.1% |
| [59] | Squires et al. | 2019 | USA | Near-infrared autofluoresccence imaging | Intraoperative | 59 | - | 12 | - | - | - | 87% |
| [60] | Kose et al. | 2019 | USA | Near-infrared autofluoresccence imaging | Intraoperative | 50 | - | 192/199 (96%) | - | - | - | - |
| [61] | Kose et al. | 2020 | USA | Near-infrared autofluorescence imaging | Intraoperative | 310 | - | 496/503 (98.6%) | - | - | 97.6% | 98.5% |
| [62] | Henegan et al. | 2019 | Australia | Near-infrared autofluorescence imaging | Intraoperative | 1 | - | - | - | 1 | - | - |
| [63] | Alesina et al. | 2018 | Germany | Near-infrared autofluorescence imaging | Intraoperative | 5 | - | 11 | - | 1 | - | - |
| [64] | Kahramangil et al. | 2018 | Argentina | Near-infrared autofluorescence imaging | Intraoperative | 210 | - | (584/594) 98% | - | - | 97-99%***** | |
| [65] | Thomas et al. | 2018 | USA | Near-infrared autofluorescence imaging + PTeye | Intraoperative | 162 (near-IR auto-fluorescence imaging) 35 (PTeye) | - | 881 (near-IR auto-fluorescence imaging) 383 (PTeye) | - | 92.5% (near-infrared autoluorescene imaging) 96.1% (PTeye) | 89.1% (near-infrared autoluorescene imaging) 95.5% (PTeye) | |
| [66] | Kim et al. | 2017 | USA | Dynamic optical contrast imaging | Ex vivo study Eventually intraoperative | 81 | - | - | - | - | - | - |
| [67] | Mannoh et al. | 2017 | USA | Laser speckle contrast imaging | Intraoperative | 20 | - | 32 (well vascularized PGs) 27 (compromised PGs) | - | - | 91.5% | 92.6% |
| [68] | Hattapo ğlu et al. | 2015 | Turkey | Shear-wave elastography | Preoperative | 36 | - | - | - | - | - | 90% (for parathyroid adenomas) |
| [69] | Azizi et al. | 2016 | USA | Shear-wave elastography | Preoparative | 57 | - | - | - | - | - | - |
| [70] | Golu et al. | 2017 | Romania | Shear-wave elastography | Preoparative | 22 | 43 | - | - | 21 | - | 93% |
| [71] | Stangierski et al. | 2018 | Poland | Shear-wave elastography | Preoperative | 65 | 35 | - | - | - | - | - |
| [72] | Chandramohan et al. | 2017 | India | Shear-wave elastography | Preoperative | 44 | - | - | - | 39 | 90.5% | 91.1% |
| [73] | Batur et al. | 2015 | Turkey | Shear-wave elastography | Preoperative | 92 | - | - | 21 | - | 85.7% | |
| [74] | Vidal Fortuny et al. | 2017 | Stwitzerland | Parathyroid angiography with indocyanine green | Intraoperative | 73 | 73 | - | - | - | - | - |
| [75] | Van den Bos | 2018 | Netherlands | Indocyanine green | Intraoperative | 26 | - | - | - | - | - | - |
| [76] | Sound et al. | 2015 | USA | Indocyanine green | Intraoperative | 3 | - | - | - | 2 | - | - |
| [77] | Kahramangil and Berber | 2017 2018 | USA China | Parathydoid autofluorescence | Intraoperative | 22 | - | 61/62 (98%) | - | - | - | |
| Indocyanine green | Intraoperative | 3 | - | all | - | - | - | - | ||||
| [78] | Lang et al. | 2016 | China | Indocyanine green | Intraoperative | 70 | - | - | - | - | - | - |
| [79] | DeLong et al. | 2017 | USA | Indocyanine green | Intraoperative | 60 | - | 60/60 (100%) | - | 18/18 (100%) | - | - |
| [80] | Chakedis et al. | 2015 | USA | Indocyanine green | Intraoperative | 1 | - | 1 | - | 1 | - | - |
| Method | Advantages | Disadvantages |
|---|---|---|
| Dynamic Optical Contrast Imaging | Non-invasive No admission of exogenous substances Instant feedback | Not enough evidence |
| Laser Speckle Contrast Imaging | No admission of exogenous substances Instant feedback Assess of viability of PGs | High susceptibility to movement of the operation field |
| Autofluorescence Spectroscopy | Non-invasive No admission of exogenous substances; Instant feedback | No information about the viability of PGs Requires the blackout of the operating room light Limited ability to localize PGs covered deeply by other tissues |
| Autofluorescence Imaging | Non-invasive No admission of exogenous substance Instant feedback Contactless Possibility to differentiate adenomas Possibility to display on the operation field | No information about viability of PGs Requires the blackout of the operating room Limited ability to localize PGs covered deeply by other tissues |
| Raman Spectroscopy | Non-invasive No admission of exogenous substance; Possibility to differentiate adenomas | Requires additional time |
| Carbon Nanoparticles | Do not penetrate to tissues Visible in the operation field | Admission of exogenous substance Requires precise injection Do not differentiate adenomas |
| Shear Wave Elastography | Non-invasive No admission of exogenous substance; Possibility to differentiate adenomas. | Dimensions of parathyroid adenomas cannot be estimated |
| Indocyanine Green | Inexpensive Safe Possibility to differentiate adenomas Visible in the operation field | Admission of exogenous substance Contrast nonspecific for PGs Contains iodine |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baj, J.; Sitarz, R.; Łokaj, M.; Forma, A.; Czeczelewski, M.; Maani, A.; Garruti, G. Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas. Molecules 2020, 25, 1724. https://doi.org/10.3390/molecules25071724
Baj J, Sitarz R, Łokaj M, Forma A, Czeczelewski M, Maani A, Garruti G. Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas. Molecules. 2020; 25(7):1724. https://doi.org/10.3390/molecules25071724
Chicago/Turabian StyleBaj, Jacek, Robert Sitarz, Marek Łokaj, Alicja Forma, Marcin Czeczelewski, Amr Maani, and Gabriella Garruti. 2020. "Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas" Molecules 25, no. 7: 1724. https://doi.org/10.3390/molecules25071724
APA StyleBaj, J., Sitarz, R., Łokaj, M., Forma, A., Czeczelewski, M., Maani, A., & Garruti, G. (2020). Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas. Molecules, 25(7), 1724. https://doi.org/10.3390/molecules25071724







