The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste
Abstract
:1. Introduction
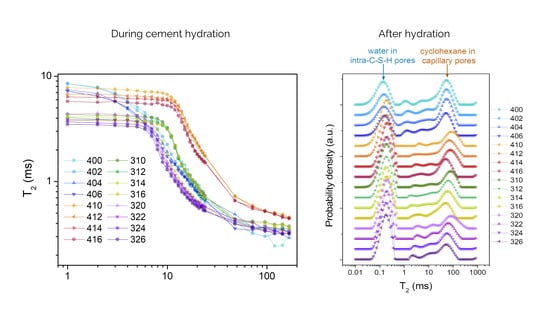
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. NMR Investigations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heede, P.V.D.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Lee, J.; Mahendra, S.; Alvarez, P.J. Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Bribian, I.Z.; Capilla, A.V.; Uson, J.A.A. Life cycle assessment of building materials: Comparative analysis of energy and environmental impacts and evaluation of the eco-efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Salas, D.A.; Ramirez, A.D.; Rodriguez, C.; Petroche, D.M.; Boero, A.; Duque-Rivera, J. Environmental impacts, life cycle assessment and potential improvement measures for cement production: A literature review. J. Clean. Prod. 2016, 113, 114–122. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Birgisson, B.; Taylor, P.; Attoh-Okine, N.O. (Eds.) Nano-technology in civil infrastructure; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-16656-3. [Google Scholar]
- Oltulu, M.; Şahin, R. Pore structure analysis of hardened cement mortars containing silica fume and different nano-powders. Constr. Build. Mater. 2014, 53, 658–664. [Google Scholar] [CrossRef]
- Badea, C.; Bede, A.; Ardelean, I. The effect of silica fume on early hydration of white Portland cement via fast field cycling-NMR relaxometry. AIP Conf. Proc. 2017, 1917, 040001. [Google Scholar]
- Muller, A.; Scrivener, K.; Skibsted, J.; Gajewicz, A.; McDonald, P.J. Influence of silica fume on the microstructure of cement pastes: New insights from 1H NMR relaxometry. Cem. Concr. Res. 2015, 74, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Hou, P.; Qian, J.; Cheng, X.; Shah, S.P. Effects of the pozzolanic reactivity of nanoSiO2 on cement-based materials. Cem. Concr. Compos. 2015, 55, 250–258. [Google Scholar] [CrossRef]
- Lilkov, V.; Petrov, O.; Tzvetanova, Y.; Savov, P. Mössbauer, DTA and XRD study of Portland cement blended with fly ash and silica fume. Constr. Build. Mater. 2012, 29, 33–41. [Google Scholar] [CrossRef]
- Yajun, J.; Cahyadi, J.H. Simulation of silica fume blended cement hydration. Mater. Struct. 2004, 37, 397–404. [Google Scholar] [CrossRef]
- Esteves, L.P. On the hydration of water-entrained cement–silica systems: Combined SEM, XRD and thermal analysis in cement pastes. Thermochim. Acta 2011, 518, 27–35. [Google Scholar] [CrossRef]
- Rossen, J.; Lothenbach, B.; Scrivener, K. Composition of C–S–H in pastes with increasing levels of silica fume addition. Cem. Concr. Res. 2015, 75, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Torii, K.; Kawamura, M. Pore structure and chloride ion permeability of mortars containing silica fume. Cem. Concr. Compos. 1994, 16, 279–286. [Google Scholar] [CrossRef]
- Cheng-Yi, H.; Feldman, R. Hydration reactions in portland cement-silica fume blends. Cem. Concr. Res. 1985, 15, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Cheng-Yi, H.; Feldman, R. Influence of silica fume on the microstructural development in cement mortars. Cem. Concr. Res. 1985, 15, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Siddique, R. Utilization of silica fume in concrete: Review of durability properties. Resour. Conserv. Recycl. 2011, 57, 30–35. [Google Scholar] [CrossRef]
- Kong, X.-M.; Liu, H.; Lu, Z.-B.; Wang, D.-M. The influence of silanes on hydration and strength development of cementitious systems. Cem. Concr. Res. 2015, 67, 168–178. [Google Scholar] [CrossRef]
- Bede, A.; Pop, A.; Moldovan, M.; Ardelean, I. The influence of silanized nano-SiO2 on the hydration of cement paste: NMR investigations. AIP Conf. Proc. 2015, 1700, 60009. [Google Scholar]
- Švegl, F.; Šuput-Strupi, J.; Skrlep, L.; Kalcher, K. The influence of aminosilanes on macroscopic properties of cement paste. Cem. Concr. Res. 2008, 38, 945–954. [Google Scholar] [CrossRef]
- Pop, A.; Bede, A.; Dudescu, M.C.; Popa, F.; Ardelean, I. Monitoring the Influence of Aminosilane on Cement Hydration Via Low-field NMR Relaxometry. Appl. Magn. Reson. 2015, 47, 191–199. [Google Scholar] [CrossRef]
- Plassais, A.; Pomies, M.-P.; Lequeux, N.; Korb, J.-P.; Petit, D.; Barberon, F.; Bresson, B. Microstructure evolution of hydrated cement pastes. Phys. Rev. E 2005, 72, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Stark, J. Recent advances in the field of cement hydration and microstructure analysis. Cem. Concr. Res. 2011, 41, 666–678. [Google Scholar] [CrossRef]
- Korb, J.-P. Multiscale nuclear magnetic relaxation dispersion of complex liquids in bulk and confinement. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 104, 12–55. [Google Scholar] [CrossRef]
- Pop, A.; Ardelean, I. Monitoring the size evolution of capillary pores in cement paste during the early hydration via diffusion in internal gradients. Cem. Concr. Res. 2015, 77, 76–81. [Google Scholar] [CrossRef]
- Bede, A.; Scurtu, A.-D.; Ardelean, I. NMR relaxation of molecules confined inside the cement paste pores under partially saturated conditions. Cem. Concr. Res. 2016, 89, 56–62. [Google Scholar] [CrossRef]
- Cretu, A.; Mattea, C.; Stapf, S.; Ardelean, I. The effect of silica nanoparticles on the pore structure of hydrating cement paste: A spatially resolved low-field NMR study. Mol. Phys. 2018, 117, 1006–1014. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Venkataramanan, L.; Hurlimann, M.; Flaum, M.; Frulla, P.; Straley, C. T1–T2 Correlation Spectra Obtained Using a Fast Two-Dimensional Laplace Inversion. J. Magn. Reson. 2002, 154, 261–268. [Google Scholar] [CrossRef]
- Pop, A.; Badea, C.; Ardelean, I. Monitoring the ettringite formation in cement paste using low field T2-NMR. AIP Conf. Proc. 2013, 144, 141–144. [Google Scholar]
- Korb, J.-P. Nuclear magnetic relaxation of liquids in porous media. New J. Phys. 2011, 13, 35016. [Google Scholar] [CrossRef] [Green Version]
- Scrivener, K.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.-M.; Lu, Z.-B.; Lu, Z.-C.; Hou, S.-S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Pop, A.; Badea, C.; Ardelean, I. The Effects of Different Superplasticizers and Water-to-Cement Ratios on the Hydration of Gray Cement Using T2-NMR. Appl. Magn. Reson. 2013, 44, 1223–1234. [Google Scholar] [CrossRef]
- Bede, A.; Ardelean, I. Revealing the influence of water-cement ratio on the pore size distribution in hydrated cement paste by using cyclohexane. AIP Conf. Proc. 2017, 1917, 040002. [Google Scholar]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |




| Sample | Water/Cement | Organosilane (%) | Silica Fume (%) |
|---|---|---|---|
| 400 | 0.4 | 0 | 0 |
| 402 | 0.4 | 0 | 2 |
| 404 | 0.4 | 0 | 4 |
| 406 | 0.4 | 0 | 6 |
| 410 | 0.4 | 1 | 0 |
| 412 | 0.4 | 1 | 2 |
| 414 | 0.4 | 1 | 4 |
| 416 | 0.4 | 1 | 6 |
| 310 | 0.3 | 1 | 0 |
| 312 | 0.3 | 1 | 2 |
| 314 | 0.3 | 1 | 4 |
| 316 | 0.3 | 1 | 6 |
| 320 | 0.3 | 2 | 0 |
| 322 | 0.3 | 2 | 2 |
| 324 | 0.3 | 2 | 4 |
| 326 | 0.3 | 2 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crețu, A.; Mattea, C.; Stapf, S.; Ardelean, I. The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste. Molecules 2020, 25, 1762. https://doi.org/10.3390/molecules25081762
Crețu A, Mattea C, Stapf S, Ardelean I. The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste. Molecules. 2020; 25(8):1762. https://doi.org/10.3390/molecules25081762
Chicago/Turabian StyleCrețu, Andrea, Carlos Mattea, Siegfried Stapf, and Ioan Ardelean. 2020. "The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste" Molecules 25, no. 8: 1762. https://doi.org/10.3390/molecules25081762
APA StyleCrețu, A., Mattea, C., Stapf, S., & Ardelean, I. (2020). The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste. Molecules, 25(8), 1762. https://doi.org/10.3390/molecules25081762