Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue
Abstract
1. Introduction
2. Results
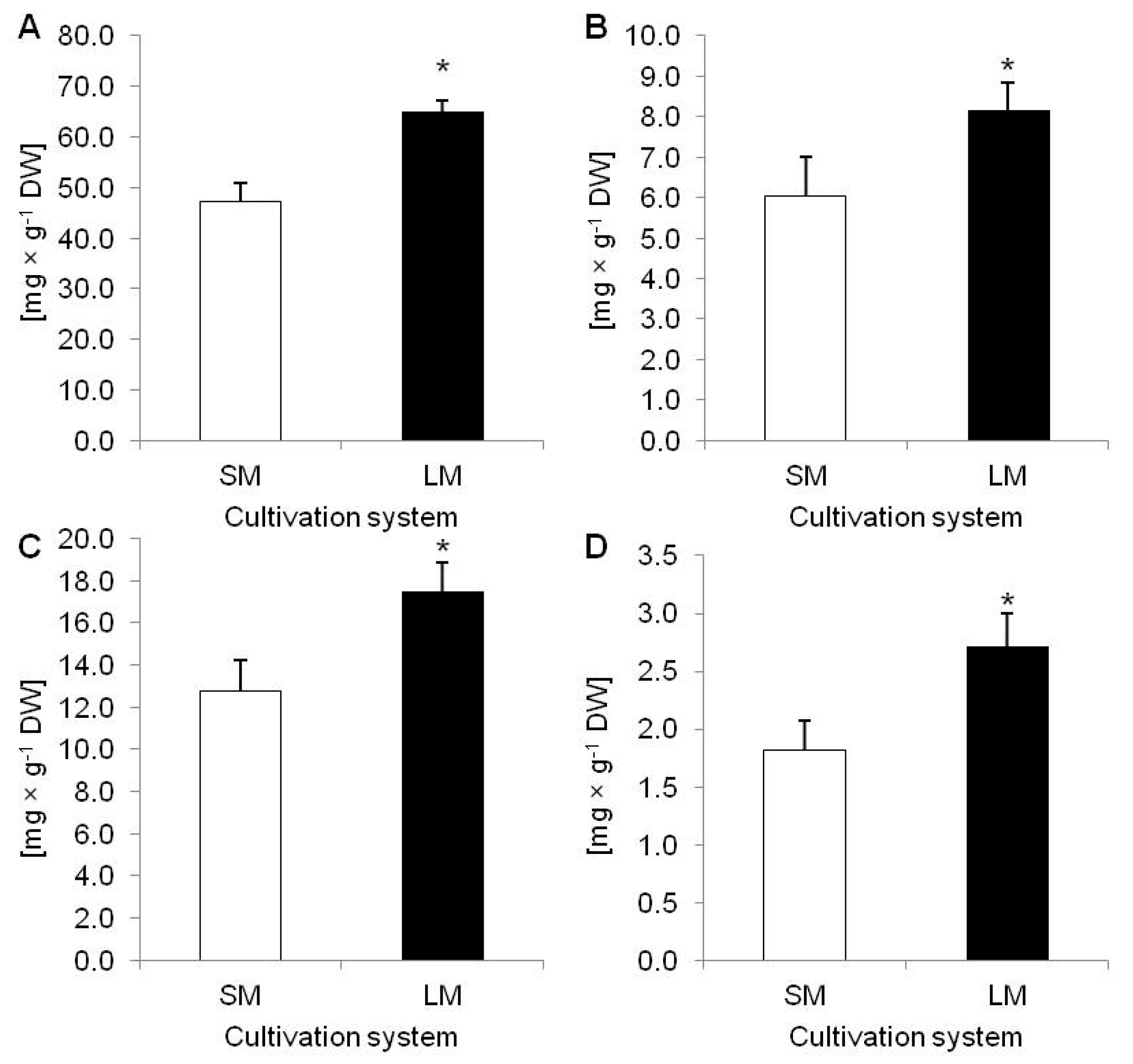
2.1. Biometric and Biochemical Parameters of Plants Growing in LM (Experiment 1)
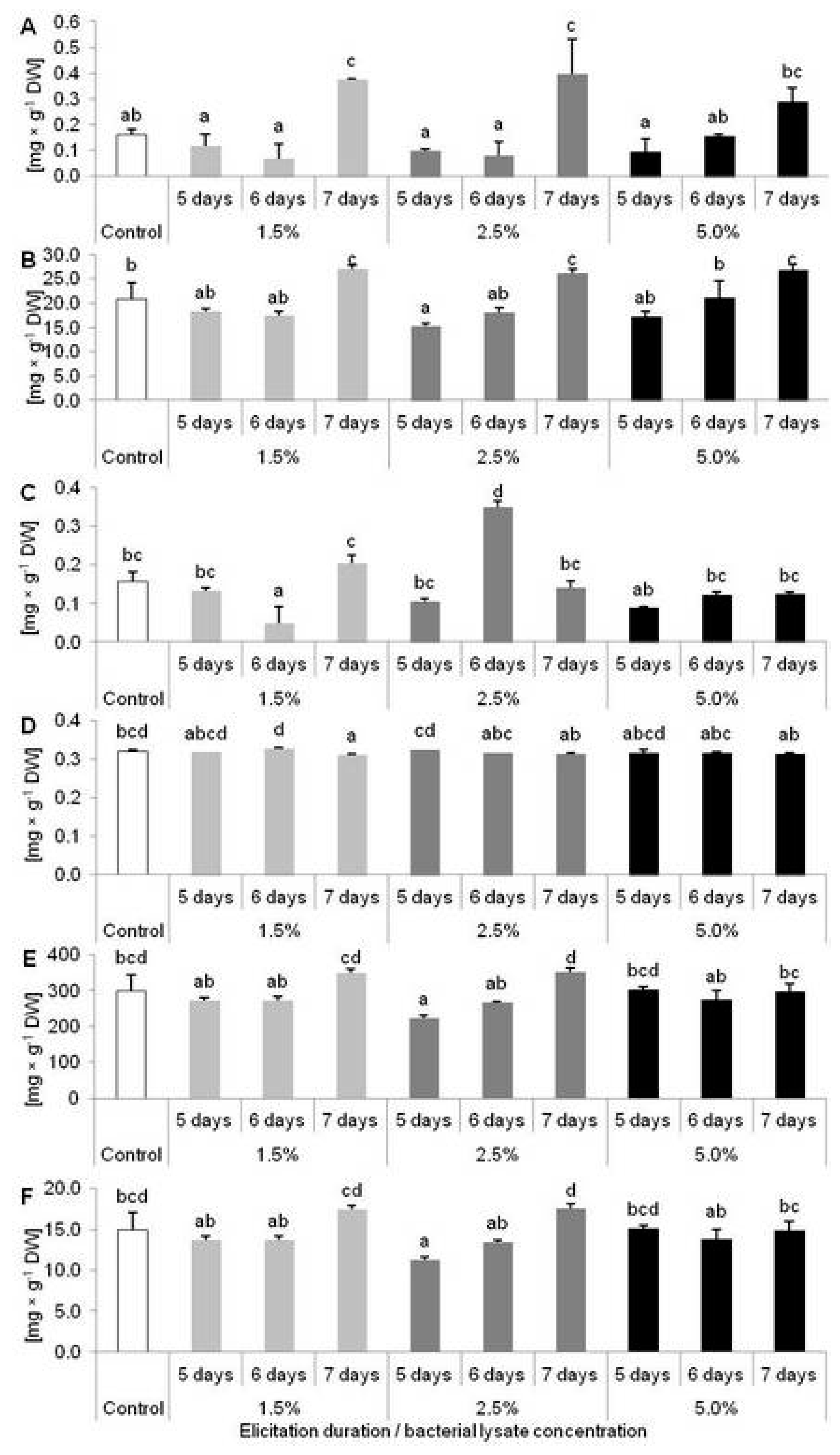
2.2. Results of D. muscipula Elicitation with C. sakazakii Lysate (Experiment 2)
2.3. Biological Activity of Extracts from D. muscipula Plants Elicited with C. sakazakii Lysate
2.3.1. Reactive Oxygen Species Scavenging Activity
2.3.2. Antibacterial Activity
3. Discussion
3.1. The Effect of Shaking on Plant Growth and Secondary Metabolite Levels
3.2. Impact of Biotic Elicitation on Plant Growth and Secondary Metabolite Levels
3.3. Impact of Biotic Elicitation on Biological Properties of D. muscipula Plants
4. Materials and Methods
4.1. Plant Material and Experiments Design
4.1.1. Plant Material
4.1.2. Experiment 1: Cultivation of Plants in Liquid Media with Rotary Shaking
4.1.3. Experiment 2: Elicitation of Plants Growing in Liquid Media with Rotary Shaking
4.2. Growth Parameters Estimation
Growth Index (GI) and Dry Weight (DW) Content
4.3. Biochemical Analysis
4.3.1. Spectrophotometric Estimation of Total Phenolic Content (TPC)
4.3.2. Spectrophotometric Estimation of Phenylpropanoids (PHE), Flavonoids (FLA) and Anthocyanins (ANT) Content
4.3.3. High Pressure Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
4.4. Analysis of Biological Activity of Examined Plants
4.4.1. Spectrophotometric Estimation of Antioxidative Properties of Plant Extract Using DPPH Method
4.4.2. Antibacterial Activity
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANT | Anthocyanins |
| CA | Caffeic acid |
| CFU | Colony-forming unit |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl |
| DW | Dry weight |
| EA | Ellagic acid |
| FLA | Flavonoids |
| GI | Growth index |
| HYP | Hyperoside |
| LM | Liquid medium |
| MBC | Minimal Bactericidal Concentration |
| MYR | Myricetin |
| PHE | Phenylpropanoids |
| PLU | Plumbagin |
| PPFD | Photosynthetic photon flux density |
| QUE | Quercetin |
| SA | Salicylic acid |
| SM | Solid medium |
| THF | Tetrahydrofuran |
| TPC | Total phenolic content |
References
- Krolicka, A.; Szpitter, A.; Gilgenast, E.; Romanik, G.; Kaminski, M.; Lojkowska, E. Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants by addition of elicitors. Enzyme Microb. Technol. 2008, 42, 216–221. [Google Scholar] [CrossRef]
- Gaascht, F.; Dicato, M.; Diederich, M. Venus Flytrap (Dionaea muscipula Solander ex Ellis) contains powerful compounds that prevent and cure cancer. Front. Oncol. 2013, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.P.; Kumaria, S.; Rao, S.R.; Tandon, P. Carnivorous plants as a source of potent bioactive compound: Naphthoquinones. Trop. Plant Biol. 2016, 9, 267–279. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Amato, M.; De Feo, V.; Camele, I. Chemical composition and antimicrobial activity of Chia (Salvia hispanica l.) Essential oil. Europ. Food Res. Technol. 2018, 244, 1675–1682. [Google Scholar] [CrossRef]
- Krolicka, A.; Szpitter, A.; Maciag, A.; Biskup, E.; Gilgenast, E.; Romanik, G.; Kaminski, M.; Wegrzyn, G.; Lojkowska, E. Antibacterial and antioxidant activity of the secondary metabolites from in vitro cultures of the Alice sundew (Drosera aliciae). Biotechnol. Appl. Biochem. 2009, 53, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Krychowiak, M.; Grinholc, M.; Banasiuk, R.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Królicka, A. Combination of silver nanoparticles and Drosera binata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS ONE 2014, 9, e115727. [Google Scholar] [CrossRef]
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev 2010, 32, 1131–1158. [Google Scholar] [CrossRef]
- Kawiak, A.; Domachowska, A.; Królicka, A.; Smolarska, M.; Łojkowska, E. 3-Chloroplumbagin Induces Cell Death in Breast Cancer Cells Through MAPK-Mediated Mcl-1 Inhibition. Front. Pharmacol. 2019, 10, 784. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Establishment of root suspension culture of Plumbago zeylanica and enhanced production of plumbagin. Ind. Crops Prod. 2019, 137, 419–427. [Google Scholar] [CrossRef]
- Okatch, H.; Ngwenya, B.; Raletamo, K.M.; Andrae-Marobela, K. Determination of potentially toxic heavy metals in traditionally used medicinal plants for HIV/AIDS opportunistic infections in Ngamiland District in Northern Botswana. Anal. Chim. Acta 2012, 730, 42–48. [Google Scholar] [CrossRef]
- Jaisi, A.; Panichayupakaranant, P. Enhanced plumbagin production in Plumbago indica root cultures by l-alanine feeding and in situ adsorption. Plant Cell Tissue Organ Cult. 2017, 129, 53–60. [Google Scholar] [CrossRef]
- Jaisi, A.; Panichayupakaranant, P. Chitosan elicitation and sequential Diaion® HP-20 addition a powerful approach for enhanced plumbagin production in Plumbago indica root cultures. Process Biochem. 2017, 53, 210–215. [Google Scholar] [CrossRef]
- Tokarz, K.; Makowski, W.; Banasiuk, R.; Krolicka, A.; Piwowarczyk, B. Response of Dionaea muscipula J. Ellis to light stress in in vitro: Physiological study. Plant Cell, Tissue Organ Cult. 2018, 134, 65–77. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, B.; Banasiuk, R.; Krolicka, A.; Dziurka, M.; Wojciechowska, R.; Tokarz, K.M. Is a blue–red light a good elicitor of phenolic compounds in the family Droseraceae? A comparative study. J. Photochem. Photobiol. B, Biol. 2019, 201, 111679. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hort. Res. 2016, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Banasiuk, R.; Kawiak, A.; Krolicka, A. In vitro cultures of carnivorous plants from the Drosera and Dionaea genus for the production of biologically active secondary metabolites. Biotechnologia 2012, 93, 87–96. [Google Scholar] [CrossRef]
- Boonsnongcheepa, P.; Sae-fooa, W.; Banpakoata, K.; Channaronga, S.; Chitsaithana, S.; Uafuaa, P.; Puthaa, W.; Kerdsiria, K.; Putaluna, W. Artificial color light sources and precursor feeding enhance plumbagin production of the carnivorous plants Drosera burmannii and Drosera indica. J. Photochem. Photobiol. B, Biol. 2019, 199, 111628. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Krol, A.; Luczkiewicz, M.; Ekiert, H. Improved production of dibenzocyclooctadiene lignans in the elicited microshoot cultures of Schisandra chinensis (Chinese magnolia vine). Appl. Microbiol. Biotechnol. 2018, 102, 945–959. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Krolicka, A.; Zabiegala, B.; Luczkiewicz, M. Elicitation strategies for the improvement of essential oil content in Rhododendron tomentosum (Ledum palustre) bioreactor-grown microshoots. Ind. Crops Prod. 2018, 123, 461–469. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Ziaratnia, S.M.; Kunert, K.J.; Lall, N. Elicitation of 7-methyljuglone in Drosera capensis. S. Afr. J. Bot. 2009, 75, 97–103. [Google Scholar] [CrossRef][Green Version]
- Putalun, W.; Udomsin, O.; Yusakul, G.; Juengwatanatrakul, T.; Sakamoto, S.; Tanaka, H. Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnol. Lett. 2010, 32, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Krolicka, A.; Szpitter, A.; Stawujak, K.; Baranski, R.; Gwizdek-Wisniewska, A.; Skrzypczak, A.; Kaminski, M.; Lojkowska, E. Teratomas of Drosera capensis var. alba as a source of naphthoquinone: Ramentaceone. Plant Cell Tissue Organ Cult. 2010, 103, 285–292. [Google Scholar] [CrossRef]
- Krolicka, A.; Staniszewska, I.; Maliński, E.; Szafranek, J.; Łojkowska, E. Stimulation of furanochromone accumulation in callus cultures of Ammi visnaga L. by addition of elicitors. Pharmazie 2003, 58, 590–592. [Google Scholar]
- Farmer, J.J., III; Asbury, M.A.; Hickmann, F.W.; Brenner, D.J. Enterobacter sakazakii: A new species of ‘‘Enterobacteriaceae’’ isolated from clinical specimens. Int. J. Syst. Bacteriol. 1980, 30, 569–584. [Google Scholar] [CrossRef]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant, J. 1999, 18, 265–276. [Google Scholar] [CrossRef]
- Szafranek, J.; Czerwicka, M.; Kumirska, J.; Paszkiewicz, M.; Łojkowska, E. Repeating unit structure of Enterobacter sakazakii ZORB A 741 o-polysaccharide. Polish, J. Chem. 2005, 79, 287–295. [Google Scholar]
- Klockner, W.; Buchs, J. Advances in shaking technologies. Trends Biotechnol. 2012, 30, 307–314. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Kannan, N.; Manokari, M.; Ramanujam, M.P. An efficient micropropagation protocol for high-frequency plantlet regeneration from liquid culture of nodal tissues in a medicinal plant Turnera ulmifolia L. J. Sustain. For. 2014, 33, 327–336. [Google Scholar] [CrossRef]
- Liu, C.Z.; Murch, S.J.; EL-Demerdash, M.; Saxena, P.K. Artemisia judaica L.: Micropropagation and antioxidant activity. J. Biotechnol. 2004, 110, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J.; Towler, M.J.; Xu, J. Bench to batch: Advances in plant cell culture for producing useful products. Appl. Microbiol. Biotechnol. 2010, 85, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Morone Fortunato, I.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Lattanzio, V.; Caretto, S.; Linsalata, V.; Colella, G.; Mita, G. Signal transduction in artichoke [Cynara cardunculus L. subsp. scolymus (L.) Hayek] callus and cell suspension cultures under nutritional stress. Plant Physiol. Biochem. 2018, 127, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; del Pilar Nicasio-Torres, M.; Sarmiento-Lopez, L.G.; Rodriguez-Monroy, M. Production of anti-inflammatory compounds in Sphaeralcea angustifolia cell suspension cultivated in stirred tank bioreactor. Eng. Life Sci. 2019, 19, 196–205. [Google Scholar] [CrossRef]
- Niazian, M. Application of genetics and biotechnology for improving medicinal plants. Planta 2019, 249, 953–973. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Joseph, C.; Hagege, D. Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tissue Organ Cult. 2007, 89, 1–13. [Google Scholar] [CrossRef]
- Orlita, A.; Sidwa-Gorycka, M.; Malinski, E.; Czerwicka, M.; Kumirska, J.; Golebiowski, M.; Lojkowska, E.; Stepnowski, P. Effective biotic elicitation of Ruta graveolens L. shoot cultures by lysates from Pectobacterium atrosepticum and Bacillus sp. Biotechnol. Lett. 2008, 30, 541–545. [Google Scholar] [CrossRef]
- Staniszewska, I.; Krolicka, A.; Malinski, E.; Lojkowska, E.; Szafranek, J. Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enzyme Microb. Technol. 2003, 33, 565–568. [Google Scholar] [CrossRef]
- Finlay, B.B.; Falkow, S. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 1997, 61, 123–169. [Google Scholar] [CrossRef]
- Orlita, A.; Sidwa-Gorycka, M.; Paszkiewicz, M.; Malinski, E.; Kumirska, J.; Siedlecka, E.M.; Łojkowska, E.; Stepnowski, P. Application of chitin and chitosan as elicitors of coumarins and furoquinolone alkaloids in Ruta graveolens L. (common rue). Biotechnol. Appl. Biochem. 2008, 51, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.L.; Yam, M.F.; Asmawi, M.Z.; Chan, L.K. Elicitation of Orthosiphon stamineus cell suspension culture for enhancement of phenolic compounds biosynthesis and antioxidant activity. Ind. Crops Prod. 2013, 50, 436–442. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Dewanjee, S.; Bhattacharya, S. Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J. Biosci. Bioeng. 2011, 111, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Silja, P.K.; Satheeshkumar, K. Establishment of adventitious root cultures from leaf explants of Plumbago rosea and enhanced plumbagin production through elicitation. Ind. Crops Prod. 2015, 76, 479–486. [Google Scholar] [CrossRef]
- Banasiuk, R.; Krychowiak, M.; Swigon, D.; Tomaszewicz, W.; Michalak, A.; Chylewska, A.; Ziabka, M.; Lapinski, M.; Koscielska, B.; Narajczyk, M.; et al. Carnivorous plants used for green synthesis of silver nanoparticles with broad-spectrum antimicrobial activity. Arab. J. Chem. 2020, 13, 1415–1428. [Google Scholar] [CrossRef]
- Ansari, M.A.; Chung, I.M.; Rajakumar, G.; Alzohairy, M.A.; Almatroudi, A.; Khanna, V.G.; Thiruvengadam, M. Evaluation of Polyphenolic Compounds and Pharmacological Activities in Hairy Root Cultures of Ligularia fischeri Turcz. f. spiciformis (Nakai). Molecules 2019, 24, 1586. [Google Scholar] [CrossRef]
- Gabotti, D.; Locatelli, F.; Cusano, E.; Baldoni, E.; Genga, A.; Pucci, L.; Consonni, R.; Mattana, M. Cell Suspensions of Cannabis sativa (var. Futura): Effect of Elicitation on Metabolite Content and Antioxidant Activity. Molecules 2019, 24, 4056. [Google Scholar] [CrossRef]
- Ogihara, H.; Endou, F.; Furukawa, S.; Matsufuji, H.; Suzuki, K.; Anzai, H. Antimicrobial Activity of the Carnivorous Plant Dionaea muscipula Against Food-Related Pathogenic and Putrefactive Bacteria. Biocontrol Sci. 2013, 3, 151–155. [Google Scholar] [CrossRef][Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. Phenolic constituents of Prunus domestica. I. Quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.M.; Makowski, W.; Tokarz, B.; Hanula, M.; Sitek, E.; Muszyńska, E.; Jędrzejczyk, R.; Banasiuk, R.; Chajec, Ł.; Mazur, S. Can Ceylon Leadwort (Plumbago zeylanica L.) Acclimate to Lead Toxicity?—Studies of Photosynthetic Apparatus Efficiency. Int. J. Mol. Sci. 2020, 21, 1866. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.H.; Sadeek, S.A.; Camele, I. Biological investigations and spectroscopic studies of new Moxifloxacin/Glycine-Metal complexes. Chem. Biodivers. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |






| Concentration of C. sakazaki Lysate (%) | Days of Elicitation | Staphylococcus aureus ATCC 25923 | Escherichia coli ATCC 25922 |
|---|---|---|---|
| MBC (µg DW × mL−1) | |||
| 0.0 (Control) | 501 | 2087.5 | |
| 1.5 | 5 | 501 | 1670 |
| 6 | 417.5 | 1670 | |
| 7 | 334 | 1670 | |
| 2.5 | 5 | 417.5 | 1670 |
| 6 | 417.5 | 1670 | |
| 7 | 334 | 1670 | |
| 5.0 | 5 | 417.5 | 1670 |
| 6 | 417.5 | 1670 | |
| 7 | 334 | 1670 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue. Molecules 2020, 25, 1794. https://doi.org/10.3390/molecules25081794
Makowski W, Tokarz KM, Tokarz B, Banasiuk R, Witek K, Królicka A. Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue. Molecules. 2020; 25(8):1794. https://doi.org/10.3390/molecules25081794
Chicago/Turabian StyleMakowski, Wojciech, Krzysztof Michał Tokarz, Barbara Tokarz, Rafał Banasiuk, Karolina Witek, and Aleksandra Królicka. 2020. "Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue" Molecules 25, no. 8: 1794. https://doi.org/10.3390/molecules25081794
APA StyleMakowski, W., Tokarz, K. M., Tokarz, B., Banasiuk, R., Witek, K., & Królicka, A. (2020). Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue. Molecules, 25(8), 1794. https://doi.org/10.3390/molecules25081794







