Experimental Investigation of the Fluidization Reduction Characteristics of Iron Particles Coated with Carbon Powder under Pressurized Conditions
Abstract
:1. Introduction
2. Methodology
2.1. Experimental Materials
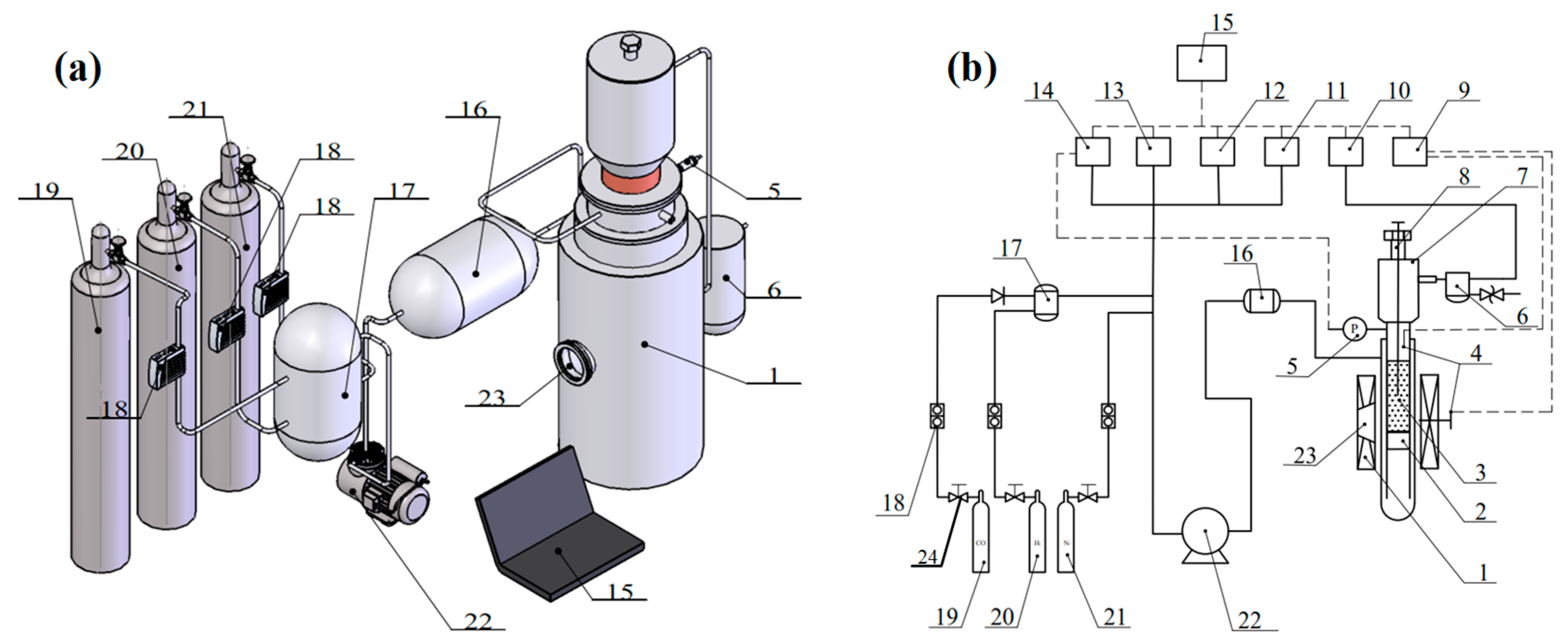
2.2. Experimental Instruments and Methods
2.3. Experimental Design
3. Experimental Results and Discussion
3.1. Optimal Operating Parameters
3.2. Bonding Mechanism of Fine Iron Ore Particles
- (1)
- The reducing gas (H2) diffuses through the gas film to the surface of the solid product layer (Fe). This process is called external diffusion;
- (2)
- The reducing gas (H2) diffuses to the reaction interface through the product layer, and iron ions diffuse into the interior through the product layer. This process is called internal diffusion
- (3)
- The adsorption of the reducing gas (H2), the interface chemical reaction, and the desorption of the oxidizing gas have the characteristics of adsorption autocatalysis, i.e., their rate has a phenomenon of small to large autocatalysis.
3.3. Mechanism of the Inhibition of Mineral Powder Bonding by Carbon Coating
4. Conclusions
- (1)
- Under carbon powder coating conditions, the metallization rate can be increased, the fluidization state can be improved, and the adhesion ratio can be reduced by properly increasing the pressure;
- (2)
- The best operating parameters of the coated toner were as follows. The temperature was selected to be 923–1023 K, the reduction gas velocity was selected to be 0.6 m/s, the reduction time was selected to be 30–50 min, the reduction pressure was selected to be 0.25 MPa, the particle size of the toner particles was selected to be 4–7 μm, and the toner content was selected to be 2–6%;
- (3)
- The agglomeration of iron whiskers is the main reason for the adhesion of iron powder particles. The carbon powder coating was found to effectively change the morphology of iron metal, and the iron metal formed spherical particles around the carbon powder to improve the fluidization state.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.X. Progress of blast furnace iron-making technology in China. Steel 2005, 40, 8–12. [Google Scholar]
- Yang, T.J. Development of blast furnace iron-making technology in China. China Metall. 2004, 1–7. [Google Scholar]
- Che, Y.M.; Guo, T.Y.; Sun, P.; Jiang, Z.; Yao, S.; Fei, J.; Liu, B.N. Application and development of big data cloud platform technology in blast furnace process. Angang Technol. 2019, 5–10. [Google Scholar]
- Ying, Z.W.; Chu, M.S.; Tang, J.; Liu, Z.G.; Zhou, Y.S. Analysis of current situation and future adaptability of non blast furnace iron-making process. Hebei Metall. 2019, 1–7. [Google Scholar]
- Gong, J.F. Comparative analysis of blast furnace iron-making and non blast furnace iron-making technology. Shanxi Metall. 2017, 40, 86–88. [Google Scholar]
- Li, S.K. Comparison of energy consumption based on blast furnace iron-making and non blast furnace iron-making. Ind. Des. 2015, 185–187. [Google Scholar]
- Xu, Q.; Liu, Z.; Li, Z.; Wang, J.; Zhou, L. The Effect of Carbon Dissection of Waste Plastics on Inhibiting the Adhesion of Fine Iron Ore Particles during Hydrogen Reduction. Metals 2018, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.Y.; Wang, J.J.; Guo, L. Analysis of the bonding mechanism in the fluidization reduction process of iron ore fines. Met. Mine 2016, V45, 108–111. [Google Scholar]
- Song, Y.F.; Zhu, Q.S. Experimental study on fluidization and reduction of ultrafine iron oxide powder in a stirred bed. J. Process Eng. 2011, 11, 361–367. [Google Scholar]
- Li, S.L. Review of reduction of iron ore by fluidizing gas. J. Process Eng. 1976, 55–79. [Google Scholar]
- Zhao, Z.L.; Tang, H.Q.; Guo, Z.C. In situ observation and Mechanism Study on the effect of CaO on the growth of iron whiskers in CO atmosphere. J. Beijing Univ. Sci. Technol. 2011, 33, 817–821. [Google Scholar]
- Xu, Q.Y.; Ren, X.; Ma, H.B.; Yang, D.; Wang, H.C.; Wang, J.Q. The effect of reduction temperature on the bond loss of reduced iron ore powder. J. Anhui Univ. Technol. (Nat. Sci. Ed.) 2017, 34, 107–110. [Google Scholar]
- Samane, M.; Mohannad, M.; Veena, S. Novel Synthesis of Silicon Carbide Nanowires from e-Waste. ACS Sustain. Chem. Eng. 2017, 5, 4171–4178. [Google Scholar]
- Maroufi, S.; Mayyas, M.; Mansuri, I.; O’Kane, P.; Skidmore, C.; Jin, Z.; Fontana, A.; Sahajwalla, V. Study of Reaction Between Slag and Carbonaceous Materials. Met. Mater. Trans. A 2017, 48, 2316–2323. [Google Scholar] [CrossRef]
- Bo, H.; Hao, S.J.; Zhang, L.Y.; Jiang, W.F.; Zhang, Y.Z.; Fang, J. Study on the inhibition of adhesion loss behavior in the process of fluidization reduction by different inerts. J. Mater. Metall. 2017, 16, 159–164. [Google Scholar]
- Wang, J.J.; Li, Z.F. Study on the mechanism of iron ore powder fluidization reduction viscosity control. J. Process Eng. 2010, 10, 31–36. [Google Scholar]
- Zhu, K.S.; Li, W.G.; Zhang, X. Thermodynamic properties of fluidization pre reduction of attached carbon (CA) Au ore. J. East China Inst. Metall. 1996, 13–18. [Google Scholar]
- Shao, J.H.; Guo, Z.C.; Tang, H.Q. Influence of reducing atmosphere on the sticking during reduction of iron ore fines in a fluidized bed. J. Univ. Sci. Technol. Beijing 2013, 35, 273–281. [Google Scholar]
- Gransden, J.F.; Sheasby, J.S. The sticking of iron ore during reduction by hydrogen in a fluidized bed. Can. Metall. Q. 2013, 11, 649–657. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; Liu, Z.; Wang, J.; Wang, H. The Effect of Pressurized Decarbonization of CO on Inhibiting the Adhesion of Fine Iron Ore Particles. Metals 2018, 8, 525. [Google Scholar] [CrossRef] [Green Version]
- Hamed, E.; Frostig, Y. Natural frequencies of bonded and unbonded prestressed beams–prestress force effects. J. Sound Vib. 2006, 295, 28–39. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Gong, X.; Guo, Z. Influence of Fluidization Conditions on Sticking Time During Reduction of Fe2O3 Particles with CO. Spec. Publ. 2011, 59, 190–197. [Google Scholar]
- Guo, L.; Gao, H.; Yu, J.-T.; Zhang, Z.-L.; Guo, Z. Influence of hydrogen concentration on Fe2O3 particle reduction in fluidized beds under constant drag force. Int. J. Miner. Met. Mater. 2015, 22, 12–20. [Google Scholar] [CrossRef]
- Guo, L.; Yu, J.-T.; Tang, J.-K.; Lin, Y.-H.; Guo, Z.; Tang, H. Influence of coating MgO on sticking and functional mechanism during fluidized bed reduction of vanadium titano-magnetite. J. Iron Steel Res. Int. 2015, 22, 464–472. [Google Scholar] [CrossRef]
- Guo, L.; Tang, J.; Tang, H.; Guo, Z. Influence of Different MgO Coating Methods on Preventing Sticking During Reduction of Fe2O3 Particles in a Fluidized Bed. J. Mater. Today: Proc. 2015, 2, S332–S341. [Google Scholar] [CrossRef]
- Sui, D.; Cui, Z. Application of orthogonal experimental design and Tikhonov regularization method for the identification of parameters in the casting solidification process. Acta Met. Sin. (Engl. Lett.) 2009, 22, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ye, W.-M.; Zhang, K.-N. Strength of copolymer grouting material based on orthogonal experiment. J. Cent. South Univ. Technol. 2009, 16, 143–148. [Google Scholar] [CrossRef]
- Zheng, X.; Ye, Y.C.; Liu, F.Y. Study of the Pure Tantalum Plate Process by Means of an Orthogonal Experimental Design. Rare Met. Mater. Eng. 1998, 27, 173–176. [Google Scholar]
- Xue, H.F. Flow properties of a fine powder fluidized bed under the action of large particles. J. Process Eng. 1993, 345–349. [Google Scholar]
- Noda, K.; Mawatari, Y.; Uchida, S. Flow patterns of fine particles in a vibrated fluidized bed under atmospheric or reduced pressure. J. Powder Technol. 1998, 99, 11–14. [Google Scholar] [CrossRef]
- Guo, P.M.; Zhao, P.; Wang, L.; Kong, L.B. Effect of process parameters on the reduction effect of iron ore powder in a continuous fluidized bed. J. Eng. Sci. 2018, 40, 1231–1236. [Google Scholar]
- Kuramoto, K.; Tsutsumi, A.; Chiba, T. High-velocity fluidization of solid particles in a liquid-solid circulating fluidized bed system. Can. J. Chem. Eng. 1999, 77, 291–298. [Google Scholar] [CrossRef]
- Cai, P.; Chen, S.P.; Jin, Y.; Yu, Z.Q.; Wang, Z.W. Effect of operating temperature and pressure on the transition from bubbling to turbulent fluidization. J. Chem. Eng. 1990, 85, 122–132. [Google Scholar]
- Patience, P.A.; Boffito, D.C.; Patience, G.S. How do you write and present research well? 11-Respect SI writing conventions. Can. J. Chem. Eng. 2016, 94, 1431–1434. [Google Scholar] [CrossRef]
- Boffito, D.C.; Patience, P.A.; Srinivasan, B.; Perrier, M.; Patience, G.S.; Srinivisan, B. How do you write and present research well? 10-State the uncertainty, but not too precisely. Can. J. Chem. Eng. 2016, 94, 1425–1430. [Google Scholar] [CrossRef]
- Patience, G.S.; Srinivisan, B.; Perrier, M.; Boffito, D.C.; Patience, P.A. How do you write and present research well? 9-show and state what error bars represent. Can. J. Chem. Eng. 2016, 94, 1221–1224. [Google Scholar] [CrossRef]
- Menéndez, M.; Herguido, J.; Bérard, A.; Patience, G.S. Experimental methods in chemical engineering: Reactors—fluidized beds. Can. J. Chem. Eng. 2019, 97, 2383–2394. [Google Scholar] [CrossRef]
- Mohannad, M.; Veena, S. Carbon nano-sponge with enhanced electrochemical properties: A new understanding of carbon activation. Chem. Eng. J. 2019, 358, 980–991. [Google Scholar]
- Mohannad, M.; Rasoul, K.N.; Veena, S. Valorization of lignin biomass as a carbon feedstock in steel industry: Iron oxide reduction, steel carburizing and slag foaming. J. Clean. Prod. 2019, 219, 971–980. [Google Scholar]
- Mohannad, M.; Farshid, P.; Martin, B.; Samane, M.; Yi, Y.; Zhao, L.; Veena, S. Thermocatalytic Conversion of Automotive Shredder Waste and Formation of Nanocarbons as a Process Byproduct. ACS Sustain. Chem. Eng. 2017, 5, 5440–5448. [Google Scholar]
- Baker, R. The relationship between particle motion on a graphite surface and Tammann temperature. J. Catal. 1982, 78, 473–476. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W. Study of the Viscosity of Mold Flux Based on the Vogel–Fulcher–Tammann (VFT) Model. Met. Mater. Trans. A 2016, 48, 220–226. [Google Scholar] [CrossRef]
Sample Availability: The reduced Brazilian iron ore powder in the experiment can be obtained from the author |








| TFe | FeO | CaO | MgO | SiO2 | P | MnO | Al2O3 |
|---|---|---|---|---|---|---|---|
| 52.31 | 0.88 | 0.16 | <0.05 | 22.15 | 0.043 | 0.195 | 0.88 |
| Fixed Carbon | Ash | Volatile Matter | Phosphorus Content | Moisture |
|---|---|---|---|---|
| >82 | <15 | <3 | <0.015 | <5 |
| Scheme | Reduction Pressure/MPa | Particle Size/µm | Toner Content/% | Metallization Rate/% | Sticking Ratio/% |
|---|---|---|---|---|---|
| 1 | 0.4 | 0 | 0 | 85.00 | 18.35 |
| 2 | 0.4 | 48–75 | 4 | 81.13 | 9.43 |
| 3 | 0.1 | 0 | 0 | 82.56 | 19.21 |
| 4 | 0.1 | 48–75 | 4 | 78.63 | 10.30 |
| A Reduction Temperature/K | B Gas Linear Velocity/m/s | C Reduction Time/min | D Reduction Pressure/MPa | E Particle Size/µm | F Toner Content/% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | D1 | D2 | D3 | E1 | E2 | E3 | F1 | F2 | F3 |
| 923 | 1023 | 1123 | 0.4 | 0.6 | 0.8 | 30 | 50 | 70 | 0.1 | 0.25 | 0.4 | 4–7 | 48–75 | 150–180 | 2 | 4 | 6 |
| Reduction Temperature/K | Gas linear Velocity/m/s | Reduction Time/min | Reduction Pressure/MPa | Particle Size /µm | Toner Content/% | |
|---|---|---|---|---|---|---|
| 1 | 923 | 0.4 | 30 | 0.1 | 4–7 | 2 |
| 2 | 923 | 0.4 | 30 | 0.1 | 48–75 | 4 |
| 3 | 923 | 0.4 | 30 | 0.1 | 150–180 | 6 |
| 4 | 923 | 0.6 | 50 | 0.25 | 4–7 | 2 |
| 5 | 923 | 0.6 | 50 | 0.25 | 48–75 | 4 |
| 6 | 923 | 0.6 | 50 | 0.25 | 150–180 | 6 |
| 7 | 923 | 0.8 | 70 | 0.4 | 4–7 | 2 |
| 8 | 923 | 0.8 | 70 | 0.4 | 48–75 | 4 |
| 9 | 923 | 0.8 | 70 | 0.4 | 150–180 | 6 |
| 10 | 1023 | 0.4 | 50 | 0.4 | 4–7 | 4 |
| 11 | 1023 | 0.4 | 50 | 0.4 | 48–75 | 6 |
| 12 | 1023 | 0.4 | 50 | 0.4 | 150–180 | 2 |
| 13 | 1023 | 0.6 | 70 | 0.1 | 4–7 | 4 |
| 14 | 1023 | 0.6 | 70 | 0.1 | 48–75 | 6 |
| 15 | 1023 | 0.6 | 70 | 0.1 | 150–180 | 2 |
| 16 | 1023 | 0.8 | 30 | 0.25 | 4–7 | 4 |
| 17 | 1023 | 0.8 | 30 | 0.25 | 48–75 | 6 |
| 18 | 1023 | 0.8 | 30 | 0.25 | 150–180 | 2 |
| 19 | 1123 | 0.4 | 70 | 0.25 | 4–7 | 6 |
| 20 | 1123 | 0.4 | 70 | 0.25 | 48–75 | 2 |
| 21 | 1123 | 0.4 | 70 | 0.25 | 150–180 | 4 |
| 22 | 1123 | 0.6 | 30 | 0.4 | 4–7 | 6 |
| 23 | 1123 | 0.6 | 30 | 0.4 | 48–75 | 2 |
| 24 | 1123 | 0.6 | 30 | 0.4 | 150–180 | 4 |
| 25 | 1123 | 0.8 | 50 | 0.1 | 4–7 | 6 |
| 26 | 1123 | 0.8 | 50 | 0.1 | 48–75 | 2 |
| 27 | 1123 | 0.8 | 50 | 0.1 | 150–180 | 4 |
| Reduction Temperature/K | Gas linear Velocity/m/s | Reduction Time/min | Reduction Pressure/MPa | Particle Size /µm | Toner Content/% | Metallization Rate/% | Sticking Ratio/% | |
|---|---|---|---|---|---|---|---|---|
| 1 | A1 | B1 | C1 | D1 | E1 | F1 | 65.13 | 13.48 |
| 2 | A1 | B1 | C1 | D1 | E2 | F2 | 59.72 | 10.70 |
| 3 | A1 | B1 | C1 | D1 | E3 | F3 | 57.96 | 6.54 |
| 4 | A1 | B2 | C2 | D2 | E1 | F1 | 70.42 | 7.86 |
| 5 | A1 | B2 | C2 | D2 | E2 | F2 | 72.88 | 8.78 |
| 6 | A1 | B2 | C2 | D2 | E3 | F3 | 76.94 | 11.78 |
| 7 | A1 | B3 | C3 | D3 | E1 | F1 | 67.69 | 15.65 |
| 8 | A1 | B3 | C3 | D3 | E2 | F2 | 65.11 | 10.90 |
| 9 | A1 | B3 | C3 | D3 | E3 | F3 | 69.47 | 11.47 |
| 10 | A2 | B1 | C2 | D3 | E1 | F2 | 83.96 | 8.03 |
| 11 | A2 | B1 | C2 | D3 | E2 | F3 | 75.78 | 6.41 |
| 12 | A2 | B1 | C2 | D3 | E3 | F1 | 81.06 | 22.32 |
| 13 | A2 | B2 | C3 | D1 | E1 | F2 | 84.35 | 12.06 |
| 14 | A2 | B2 | C3 | D1 | E2 | F3 | 88.54 | 10.70 |
| 15 | A2 | B2 | C3 | D1 | E3 | F1 | 84.19 | 11.26 |
| 16 | A2 | B3 | C1 | D2 | E1 | F2 | 79.56 | 7.03 |
| 17 | A2 | B3 | C1 | D2 | E2 | F3 | 82.09 | 11.08 |
| 18 | A2 | B3 | C1 | D2 | E3 | F1 | 87.61 | 17.57 |
| 19 | A3 | B1 | C3 | D2 | E1 | F3 | 84.72 | 13.38 |
| 20 | A3 | B1 | C3 | D2 | E2 | F1 | 83.75 | 18.83 |
| 21 | A3 | B1 | C3 | D2 | E3 | F2 | 84.46 | 16.14 |
| 22 | A3 | B2 | C1 | D3 | E1 | F3 | 82.68 | 13.45 |
| 23 | A3 | B2 | C1 | D3 | E2 | F1 | 81.38 | 15.33 |
| 24 | A3 | B2 | C1 | D3 | E3 | F2 | 79.58 | 20.49 |
| 25 | A3 | B3 | C2 | D1 | E1 | F3 | 84.08 | 5.13 |
| 26 | A3 | B3 | C2 | D1 | E2 | F1 | 85.74 | 29.72 |
| 27 | A3 | B3 | C2 | D1 | E3 | F2 | 80.41 | 18.44 |
| Factor | Indicators | A | B | C | D | E | F |
|---|---|---|---|---|---|---|---|
| Metallization rate/% | K1 | 605.32 | 676.54 | 675.71 | 690.12 | 702.59 | 706.97 |
| K2 | 747.14 | 720.96 | 711.27 | 722.43 | 694.99 | 690.03 | |
| K3 | 746.80 | 701.76 | 712.28 | 686.71 | 701.68 | 702.26 | |
| k1 | 67.26 | 75.17 | 75.08 | 76.68 | 78.07 | 78.55 | |
| k2 | 83.02 | 80.11 | 79.03 | 80.27 | 77.22 | 76.67 | |
| k3 | 82.98 | 77.97 | 79.14 | 76.30 | 77.96 | 78.03 | |
| R | 15.72 | 4.94 | 3.95 | 3.59 | 0.84 | 1.88 | |
| Primary and secondary factors ABCDFE | |||||||
| Optimization scheme A2B2C3D2F1E1 | |||||||
| Factor | Indicators | A | B | C | D | E | F |
|---|---|---|---|---|---|---|---|
| Sticking mass percentage/% | K1 | 97.16 | 115.67 | 115.67 | 118.03 | 96.07 | 152.02 |
| K2 | 106.46 | 111.71 | 118.47 | 112.45 | 122.45 | 112.57 | |
| K3 | 150.91 | 126.99 | 120.39 | 124.05 | 136.01 | 89.94 | |
| k1 | 10.80 | 12.85 | 12.85 | 13.11 | 10.67 | 16.89 | |
| k2 | 11.83 | 12.41 | 13.16 | 12.49 | 13.61 | 12.51 | |
| k3 | 16.77 | 14.11 | 13.38 | 13.78 | 15.11 | 9.99 | |
| R | 5.97 | 1.70 | 0.52 | 1.29 | 4.44 | 6.90 | |
| Primary and secondary factors FAEBDC | |||||||
| Optimization scheme F3A1E1B2D2C1 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Li, Z.; Gu, Z. Experimental Investigation of the Fluidization Reduction Characteristics of Iron Particles Coated with Carbon Powder under Pressurized Conditions. Molecules 2020, 25, 1810. https://doi.org/10.3390/molecules25081810
Xu Q, Li Z, Gu Z. Experimental Investigation of the Fluidization Reduction Characteristics of Iron Particles Coated with Carbon Powder under Pressurized Conditions. Molecules. 2020; 25(8):1810. https://doi.org/10.3390/molecules25081810
Chicago/Turabian StyleXu, Qiyan, Zhiping Li, and Zhanghan Gu. 2020. "Experimental Investigation of the Fluidization Reduction Characteristics of Iron Particles Coated with Carbon Powder under Pressurized Conditions" Molecules 25, no. 8: 1810. https://doi.org/10.3390/molecules25081810





