The Effect of Ethanol on Gelation, Nanoscopic, and Macroscopic Properties of Serum Albumin Hydrogels
Abstract
:1. Introduction
2. Results
2.1. General Gelation Properties
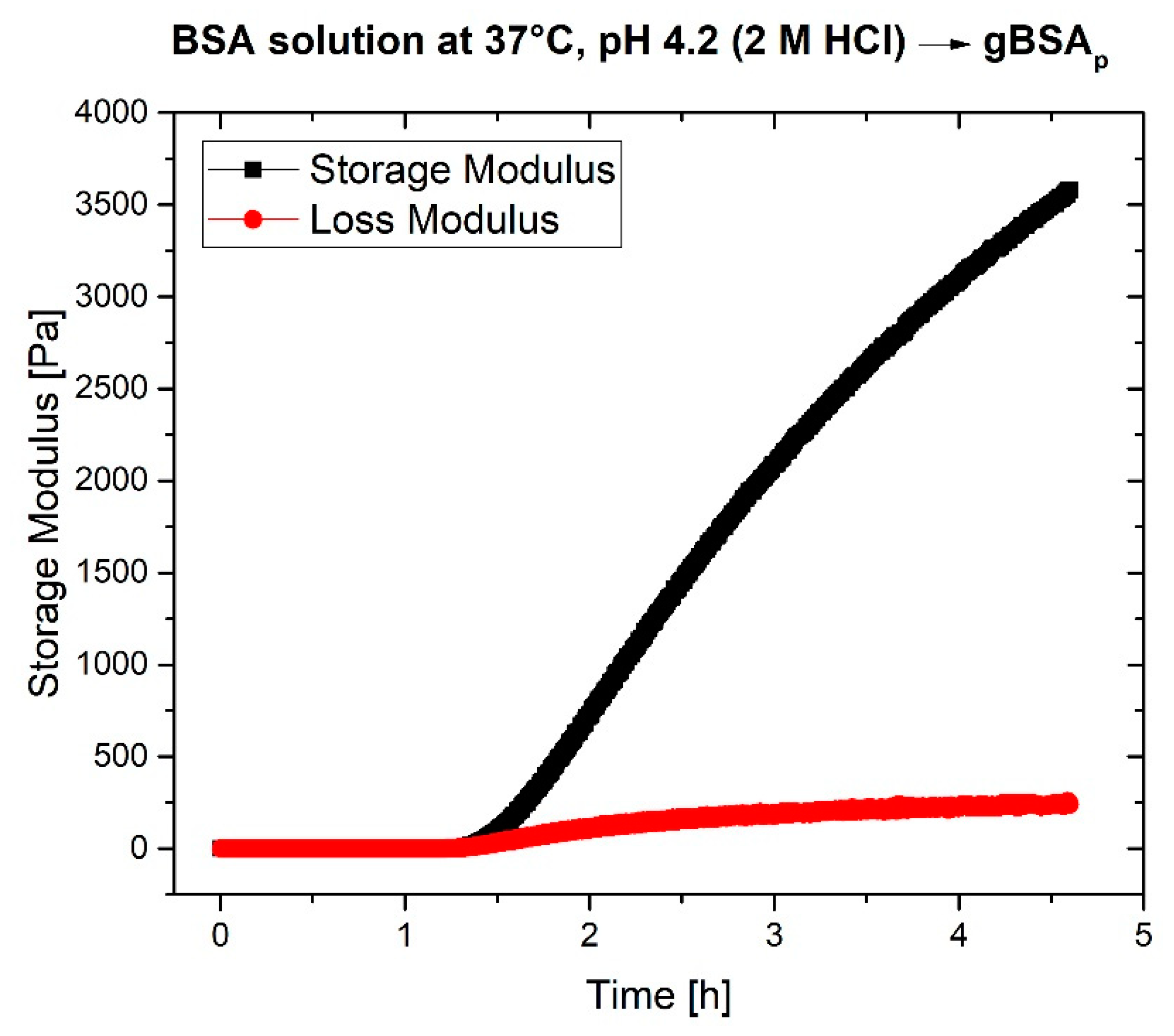
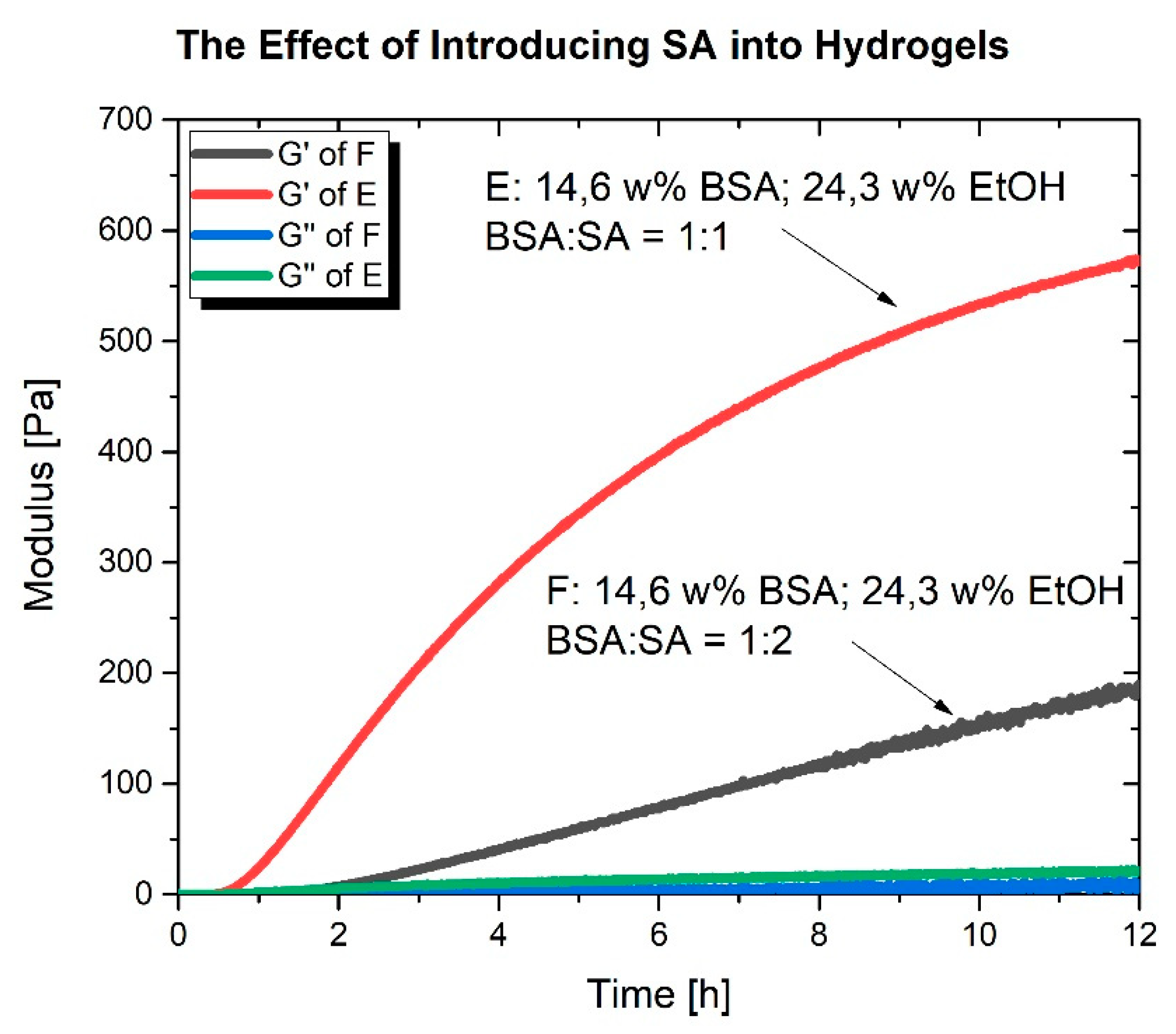
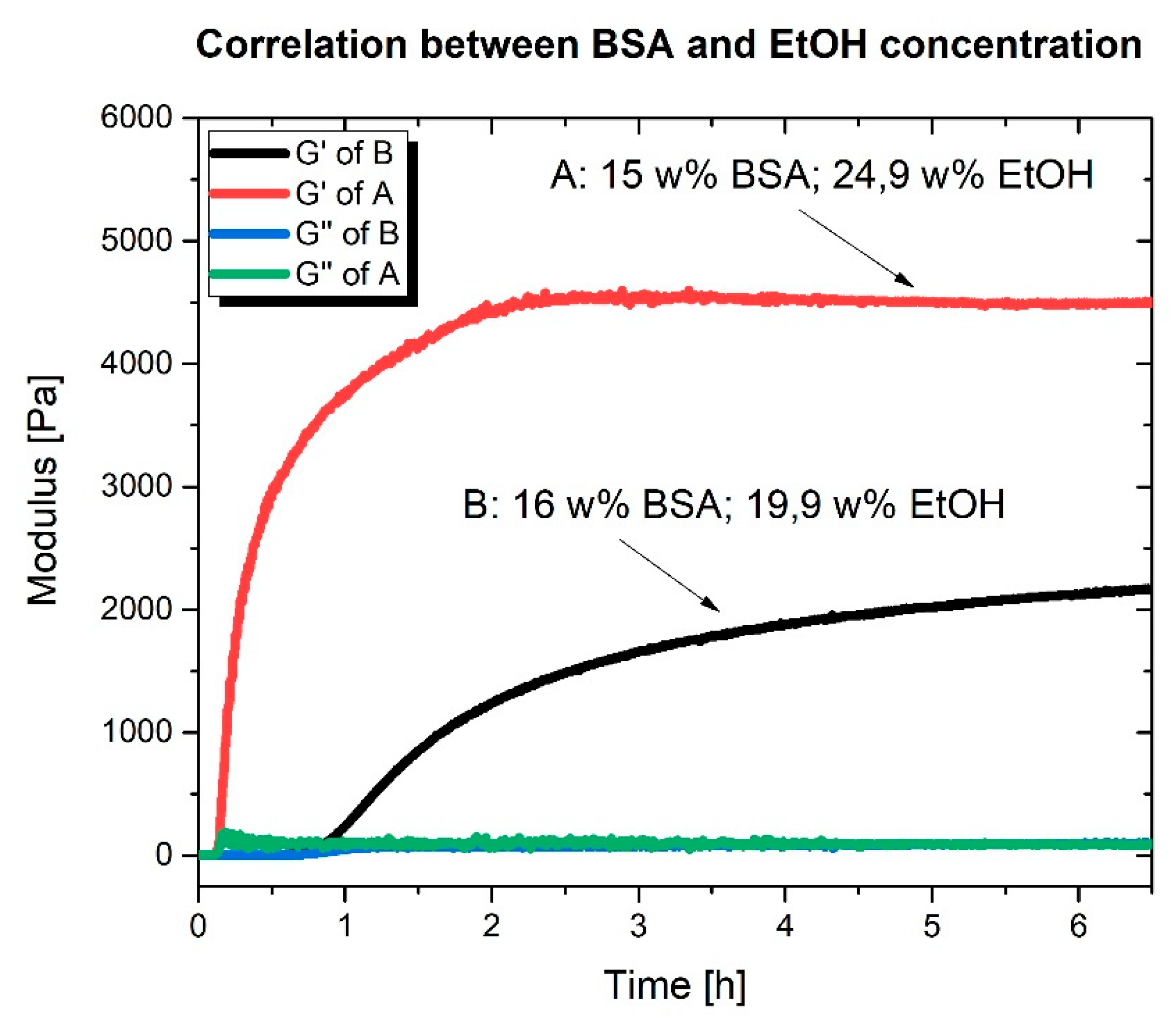
2.2. Rheological Characterization
2.3. Infrared Spectroscopy (IR)
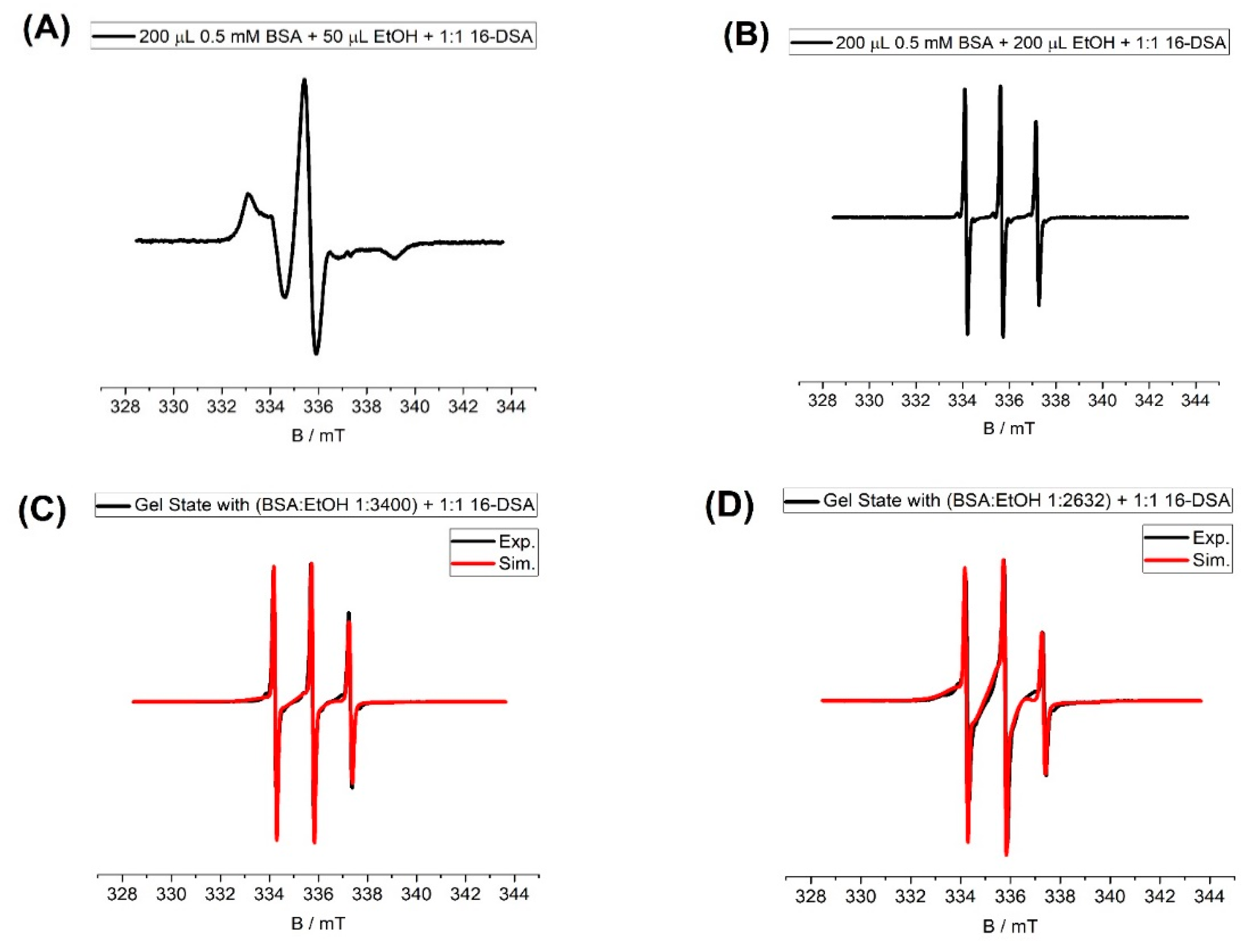
2.4. Electron Paramagnetic Resonance (EPR) Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Materials and Gel Preparation
4.2. Gel Formation
4.3. Loading Stearic Acid (SA) into Gels
4.4. Rheology
4.5. Infrared Spectroscopy (IR)
4.6. Electron Paramagnetic Resonance (EPR) Spectroscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| 20 wt% BSA (μL) | EtOH (μL) | H2O (μL) | w% BSA | w% EtOH | x BSA 10−5 | x EtOH | EtOH AA | Result |
|---|---|---|---|---|---|---|---|---|
| 200 | 1 | 0 | 19.92 | 0.39 | 6.8 | 0.002 | 0.05 | - |
| 200 | 2 | 0 | 19.84 | 0.78 | 6.79 | 0.004 | 0.1 | - |
| 200 | 5 | 0 | 19.61 | 1.93 | 6.75 | 0.01 | 0.24 | - |
| 200 | 10 | 0 | 19.24 | 3.8 | 6.69 | 0.02 | 0.48 | - |
| 200 | 15 | 0 | 18.88 | 5.59 | 6.63 | 0.03 | 0.73 | - |
| 200 | 20 | 0 | 18.54 | 7.31 | 6.56 | 0.04 | 0.97 | - |
| 200 | 25 | 0 | 18.2 | 8.98 | 6.5 | 0.05 | 1.21 | - |
| 200 | 32 | 0 | 17.76 | 11.21 | 6.42 | 0.06 | 1.55 | - |
| 200 | 33 | 0 | 17.7 | 11.52 | 6.41 | 0.06 | 1.6 | - |
| 200 | 34 | 0 | 17.63 | 11.83 | 6.4 | 0.06 | 1.65 | - |
| 200 | 35 | 0 | 17.57 | 12.13 | 6.39 | 0.06 | 1.7 | - |
| 200 | 36 | 0 | 17.51 | 12.44 | 6.38 | 0.06 | 1.74 | - |
| 200 | 37 | 0 | 17.45 | 12.74 | 6.36 | 0.07 | 1.79 | h. v. |
| 200 | 38 | 0 | 17.39 | 13.04 | 6.35 | 0.07 | 1.84 | gel |
| 200 | 39 | 0 | 17.33 | 13.33 | 6.34 | 0.07 | 1.89 | gel |
| 200 | 40 | 0 | 17.27 | 13.63 | 6.33 | 0.07 | 1.94 | gel |
| 200 | 45 | 0 | 16.98 | 15.08 | 6.27 | 0.08 | 2.18 | gel |
| 200 | 50 | 0 | 16.7 | 16.48 | 6.22 | 0.09 | 2.42 | gel |
| 200 | 134.8 | 6.2 | 13.06 | 34.03 | 5.25 | 0.2 | 6.53 | gel |
| 200 | 96.5 | 8.6 | 14.49 | 26.74 | 5.5 | 0.15 | 4.68 | gel |
| 200 | 101.1 | 16.6 | 14.3 | 26.92 | 5.25 | 0.15 | 4.9 | gel |
| 200 | 61.5 | 10.7 | 16.1 | 18.72 | 5.75 | 0.1 | 2.98 | gel |
| 200 | 64.3 | 18.5 | 15.95 | 18.84 | 5.5 | 0.1 | 3.12 | gel |
| 200 | 67.4 | 27 | 15.8 | 18.98 | 5.25 | 0.1 | 3.27 | gel |
| 200 | 28.3 | 5.8 | 17.99 | 9.79 | 6.25 | 0.05 | 1.37 | - |
| 200 | 29.4 | 12.7 | 17.92 | 9.83 | 6 | 0.05 | 1.43 | - |
| 200 | 30.7 | 20.2 | 17.84 | 9.91 | 5.75 | 0.05 | 1.49 | - |
| 200 | 32.2 | 28.4 | 17.75 | 10.01 | 5.5 | 0.05 | 1.56 | - |
| 200 | 33.7 | 37.4 | 17.65 | 10.07 | 5.25 | 0.05 | 1.63 | - |
| 200 | 40.2 | 2.1 | 17.26 | 13.57 | 6.25 | 0.07 | 1.95 | gel |
| 200 | 41.9 | 8.89 | 17.16 | 13.66 | 6 | 0.07 | 2.03 | gel |
| 200 | 43.7 | 16.24 | 17.06 | 13.75 | 5.75 | 0.07 | 2.12 | h. v. |
| 200 | 45.7 | 24.3 | 16.95 | 13.85 | 5.5 | 0.07 | 2.22 | h. v. |
| 200 | 47.9 | 33 | 16.82 | 13.96 | 5.25 | 0.07 | 2.32 | h. v. |
| 200 | 38.5 | 2.66 | 17.36 | 13.04 | 6.25 | 0.07 | 1.87 | gel |
| 200 | 40.1 | 9.44 | 17.27 | 13.12 | 6 | 0.07 | 1.94 | gel |
| 200 | 41.9 | 16.8 | 17.16 | 13.23 | 5.75 | 0.07 | 2.03 | gel |
| 200 | 43.8 | 24.8 | 17.05 | 13.32 | 5.5 | 0.07 | 2.12 | h. v. |
| 200 | 45.8 | 33.7 | 16.94 | 13.39 | 5.25 | 0.07 | 2.22 | - |
| 200 | 36.8 | 3.19 | 17.46 | 12.5 | 6.25 | 0.06 | 1.78 | gel |
| 200 | 38.3 | 9.98 | 17.37 | 12.58 | 6 | 0.06 | 1.86 | h. v. |
| 200 | 40 | 17.4 | 17.27 | 12.68 | 5.75 | 0.06 | 1.94 | - |
| 200 | 41.8 | 25.4 | 17.17 | 12.76 | 5.5 | 0.06 | 2.03 | - |
| 200 | 43.8 | 34.3 | 17.05 | 12.85 | 5.25 | 0.06 | 2.12 | - |
| 200 | 40.6 | 17.2 | 17.24 | 12.85 | 5.75 | 0.07 | 1.97 | gel |
| 200 | 41.2 | 17 | 17.2 | 13.03 | 5.75 | 0.07 | 2 | gel |
| 200 | 50.3 | 42.7 | 16.69 | 14.05 | 5 | 0.07 | 2.44 | gel |
| 200 | 47.2 | 33.2 | 16.86 | 13.77 | 5.25 | 0.07 | 2.29 | gel |
| 200 | 46.5 | 33.4 | 16.9 | 13.58 | 5.25 | 0.07 | 2.25 | - |
| 200 * | 28.5 | 0 | 3.48 | 10.11 | 4.58 | 0.05 | 1.95 | - |
| 200 * | 29.3 | 0 | 13.45 | 10.36 | 4.57 | 0.05 | 2.01 | - |
| 200 * | 30 | 0 | 13.41 | 10.58 | 4.56 | 0.05 | 2.05 | - |
| 200 * | 33.5 | 0 | 13.25 | 11.67 | 4.54 | 0.06 | 2.29 | - |
| 200 ** | 136.1 | 17.8 | 15.38 | 33.02 | 6.5 | 0.19 | 5.04 | gel |
| 200 ** | 141.6 | 24.5 | 14.87 | 33.23 | 6.25 | 0.19 | 5.25 | gel |
| 200 ** | 147.5 | 31.8 | 14.36 | 33.42 | 6 | 0.19 | 5.48 | gel |
| 200 ** | 153.9 | 39.7 | 13.85 | 33.62 | 5.75 | 0.19 | 5.73 | gel |
| 200 ** | 160.9 | 48.3 | 13.32 | 33.83 | 5.5 | 0.19 | 6 | gel |
| 200 ** | 102 | 28.3 | 16.19 | 26.06 | 6.5 | 0.14 | 3.78 | gel |
| 200 ** | 106.2 | 35.4 | 15.66 | 26.25 | 6.25 | 0.14 | 3.94 | gel |
| 200 ** | 110.6 | 43.1 | 15.13 | 26.41 | 6 | 0.14 | 4.11 | gel |
| 200 ** | 115.4 | 51.6 | 14.59 | 26.57 | 5.75 | 0.14 | 4.29 | gel |
| 200 ** | 68 | 38.8 | 17.1 | 18.35 | 6.5 | 0.1 | 2.52 | gel |
| 200 ** | 70.8 | 46.4 | 16.54 | 18.48 | 6.25 | 0.1 | 2.63 | gel |
| 200 ** | 73.7 | 54.5 | 15.99 | 18.6 | 6 | 0.1 | 2.74 | gel |
| 200 ** | 34 | 49.3 | 18.11 | 9.72 | 6.5 | 0.05 | 1.26 | - |
| 200 ** | 46.3 | 45.5 | 17.73 | 12.95 | 6.5 | 0.06 | 1.71 | gel |
| 200 ** | 44.2 | 46.1 | 17.8 | 12.41 | 6.5 | 0.06 | 1.64 | gel |
| 90 | 84 | 110 | 6.76 | 24.89 | 2.36 | 0.12 | 9.05 | h. v. |
| 80 | 84 | 120 | 6.01 | 24.89 | 2.08 | 0.12 | 10.18 | - |
| 70 | 84 | 130 | 5.26 | 24.89 | 1.8 | 0.12 | 11.63 | - |
| 100 | 100 | 85 | 7.58 | 29.9 | 2.79 | 0.16 | 9.69 | gel |
| 100 | 115 | 70 | 7.67 | 34.8 | 2.94 | 0.19 | 11.15 | gel |
| 100 | 130 | 55 | 7.76 | 39.82 | 3.12 | 0.23 | 12.6 | gel |
| 100 | 162.2 | 51.8 | 7.15 | 45.74 | 3 | 0.28 | 15.72 | gel |
| 100 | 191.6 | 42.7 | 6.81 | 51.44 | 3 | 0.32 | 18.57 | t. g. |
| 100 | 206 | 38.2 | 6.65 | 54.05 | 3 | 0.35 | 19.97 | t. g. |
| 100 | 221 | 33.6 | 6.49 | 56.62 | 3 | 0.37 | 21.42 | t. g. |
| 100 | 235 | 29.1 | 6.36 | 58.95 | 3 | 0.4 | 22.78 | t. l. |
| 100 | 250 | 24.5 | 6.22 | 61.31 | 3 | 0.42 | 24.24 | t. l. |
| 100 | 265 | 20 | 6.08 | 63.54 | 3 | 0.45 | 25.69 | t. l. |
| 100 | 280 | 15.4 | 5.95 | 65.69 | 3 | 0.47 | 27.14 | t. l. |
| 100 | 294 | 10.9 | 5.83 | 67.66 | 3 | 0.5 | 28.5 | t. g. |
| 100 | 79 | 90.4 | 7.91 | 24.66 | 2.8 | 0.13 | 7.66 | gel |
| 100 | 85.1 | 103.6 | 7.39 | 24.8 | 2.6 | 0.13 | 8.25 | gel |
| 100 | 92.2 | 118.9 | 6.86 | 24.94 | 2.4 | 0.13 | 8.94 | - |
| 100 | 295 | 14.04 | 5.77 | 67.12 | 2.95 | 0.49 | 28.6 | t. g. |
| 100 | 305 | 10.9 | 5.69 | 68.45 | 2.95 | 0.51 | 29.57 | t. g. |
| 100 | 285 | 9.79 | 5.98 | 67.19 | 3.07 | 0.49 | 27.63 | t. g. |
| 100 | 289 | 12.72 | 5.87 | 66.92 | 3 | 0.49 | 28.02 | t. g. |
| 100 | 300 | 9.09 | 5.78 | 68.45 | 3 | 0.51 | 29.08 | t. g. |
| 100 | 182 | 0 | 8.21 | 58.95 | 4.00 | 0.413 | 17.67 | - |
| 100 | 173 | 3 | 8.35 | 56.99 | 4.00 | 0.392 | 16.80 | - |
| 100 | 160 | 7 | 8.57 | 54.12 | 4.00 | 0.362 | 15.53 | - |
| 100 | 150 | 10 | 8.76 | 51.83 | 4.00 | 0.340 | 14.56 | - |
| 100 | 140 | 13 | 8.95 | 49.43 | 4.00 | 0.317 | 13.59 | t. g. |
| 100 | 121 | 19 | 9.33 | 44.51 | 4.00 | 0.274 | 11.75 | gel |
| 100 | 92 | 28 | 9.97 | 36.19 | 4.00 | 0.208 | 8.93 | gel |
| 100 | 76 | 33 | 10.36 | 31.08 | 4.00 | 0.172 | 7.38 | gel |
| 100 | 50 | 41 | 11.08 | 21.86 | 4.00 | 0.113 | 4.85 | gel |
| 100 | 30 | 47 | 11.72 | 13.87 | 4.00 | 0.068 | 2.91 | - |
| 100 | 94 | 0 | 11.48 | 42.58 | 5.00 | 0.266 | 9.13 | gel |
| 100 | 81 | 4 | 11.91 | 38.06 | 5.00 | 0.229 | 7.86 | gel |
| 100 | 68 | 8 | 12.37 | 33.19 | 5.00 | 0.193 | 6.60 | gel |
| 100 | 49 | 14 | 13.10 | 25.32 | 5.00 | 0.139 | 4.76 | gel |
| 100 | 36 | 18 | 13.66 | 19.40 | 5.00 | 0.102 | 3.50 | gel |
| 100 | 360 | 20 | 4.95 | 70.30 | 2.58 | 0.526 | 34.95 | - |
| 100 | 270 | 14 | 6.12 | 65.14 | 3.08 | 0.470 | 26.21 | - |
| 100 | 240 | 0 | 6.91 | 65.44 | 3.54 | 0.481 | 23.30 | t. g. |
| 100 | 200 | 12 | 7.41 | 58.49 | 3.55 | 0.402 | 19.42 | - |
| 100 | 170 | 21 | 7.84 | 52.57 | 3.55 | 0.342 | 16.50 | - |
| 100 | 140 | 31 | 8.28 | 45.75 | 3.54 | 0.280 | 13.59 | t. g. |
| 100 | 100 | 43 | 9.01 | 35.56 | 3.54 | 0.201 | 9.71 | gel |
| 100 | 70 | 52 | 9.65 | 26.65 | 3.55 | 0.141 | 6.80 | gel |
| 100 | 40 | 62 | 10.33 | 16.31 | 3.53 | 0.080 | 3.88 | gel |
| 100 | 200 | 131 | 5.14 | 40.59 | 2.00 | 0.226 | 19.42 | - |
| 100 | 169 | 141 | 5.34 | 35.62 | 2.00 | 0.191 | 16.41 | - |
| 100 | 140 | 150 | 5.55 | 30.64 | 2.00 | 0.158 | 13.59 | - |
| 100 | 100 | 162 | 5.87 | 23.14 | 2.00 | 0.113 | 9.71 | - |
| 100 | 60 | 100 | 8.09 | 19.14 | 2.75 | 0.093 | 5.83 | gel |
| 100 | 400 | 100 | 3.88 | 61.21 | 1.80 | 0.407 | 38.84 | - |
| 100 | 400 | 50 | 4.30 | 67.78 | 2.15 | 0.487 | 38.84 | - |
| 100 | 50 | 400 | 3.71 | 7.31 | 1.10 | 0.031 | 4.85 | - |
| 150 | 200 | 10 | 9.44 | 49.65 | 4.27 | 0.322 | 12.95 | t. g. |
| 150 | 180 | 16 | 9.74 | 46.11 | 4.27 | 0.290 | 11.65 | t. g. |
| 150 | 160 | 22 | 10.06 | 42.33 | 4.27 | 0.258 | 10.36 | gel |
| 150 | 140 | 28 | 10.40 | 38.29 | 4.28 | 0.226 | 9.06 | gel |
| 150 | 100 | 41 | 11.12 | 29.23 | 4.26 | 0.161 | 6.47 | gel |
| 150 | 83 | 46 | 11.47 | 25.04 | 4.27 | 0.134 | 5.37 | gel |
| 150 | 70 | 50 | 11.75 | 21.64 | 4.27 | 0.113 | 4.53 | gel |
| 150 | 60 | 53 | 11.98 | 18.91 | 4.27 | 0.097 | 3.88 | gel |
| 150 | 50 | 56 | 12.22 | 16.07 | 4.27 | 0.081 | 3.24 | gel |
| 150 | 38 | 60 | 12.50 | 12.49 | 4.27 | 0.061 | 2.46 | - |
| Sample | 20 wt% BSA (μL) | EtOH (μL) | H2O (μL) | 26 mM SA (μL) | w% BSA | w% EtOH | x BSA 10−5 | x EtOH | Ratio BSA:16 DSA |
|---|---|---|---|---|---|---|---|---|---|
| C | 300 | 105 | 44.1 | 35 | 24.3 | 16.48 | 5 | 0.13 | 1:1 |
| D | 300 | 105 | 44.1 | 2*35 | 24.3 | 44.10 | 5 | 0.13 | 1:2 |
| E | 300 | 70 | 44.1 | 70 | 24.3 | 0 | 5 | 0.13 | 1:2 |
| F | 0 | 105 | 344 | 35 | 24.3 | 16.48 | 0 | 0.13 | 1:1 |
References
- Minchiotti, L.; Galliano, M.; Kragh-Hansen, U.; Peters, T., Jr. Mutations and polymorphisms of the gene of the major human blood protein, serum albumin. Human Mutation 2008, 29, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Akashi, S.; Furuya, M.; Fukuhara, K. Rapid confirmation and revision of the primary structure of bovine serum albumin by ESIMS and Frit-FAB LC/MS. Biochem. Biophys. Res. Commun. 1990, 173, 639–646. [Google Scholar] [CrossRef]
- Peters, T., Jr. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Ferrer, M.L.; Duchowicz, R.; Carrasco, B.; de la Torre, J.G.; Acuna, A.U. The conformation of serum albumin in solution: A combined phosphorescence depolarization-hydrodynamic modeling study. Biophys. J. 2001, 80, 2422–2430. [Google Scholar] [CrossRef] [Green Version]
- Baler, K.; Michael, R.; Szleifer, I.; Ameer, G.A. Albumin Hydrogels Formed by Electrostatically Triggered Self-Assembly and Their Drug Delivery Capability. Biomacromolecules 2014, 15, 3625–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, S.H.; Aghelnejad, B.; Schwieger, C.; Meister, A.; Kerth, A.; Hinderberger, D. Serum albumin hydrogels in broad pH and temperature ranges: Characterization of their self-assembled structures and nanoscopic and macroscopic properties. Biomat. Sci. 2018, 6, 478–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichenwallner, J.; Hinderberger, D. Using bound fatty acids to disclose the functional structure of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5382–5393. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, Y.; Reichenwallner, J.; Hinderberger, D. Evidence for Water-Tuned Structural Differences in Proteins: An Approach Emphasizing Variations in Local Hydrophilicity. PLoS ONE 2012, 7, e45681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boye, J.I.; Alli, I.; Ismail, A.A. Interactions Involved in the Gelation of Bovine Serum Albumin. J. Agric. Food Chem. 1996, 44, 996–1004. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Computat. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Prot. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.J.; Freed, J.H. Calculating Slow Motional Magnetic Resonance Spectra. In Spin Labeling: Theory and Applications; Berliner, L.J., Reuben, J., Eds.; Springer US: Boston, MA, USA, 1989; pp. 1–76. [Google Scholar]
Sample Availability: Samples of the compounds showed in phase diagram (Figure 1) are available from the authors. |



| Type | BSA Concentration | G′ (Pa) * | G′′ (Pa) * | Gelation Point |
|---|---|---|---|---|
| EtOH (25 wt%) | 15 wt% (2.2 mM) | 5000 | 150 | 8 min |
| pH induced 1 | 20 wt% (3 mM) | 900 | 130 | 45 min |
| Temp. induced | 20 wt% (3 mM) | 16000 | 300 | 1 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabi, S.H.; Haselberger, D.; Hinderberger, D. The Effect of Ethanol on Gelation, Nanoscopic, and Macroscopic Properties of Serum Albumin Hydrogels. Molecules 2020, 25, 1927. https://doi.org/10.3390/molecules25081927
Arabi SH, Haselberger D, Hinderberger D. The Effect of Ethanol on Gelation, Nanoscopic, and Macroscopic Properties of Serum Albumin Hydrogels. Molecules. 2020; 25(8):1927. https://doi.org/10.3390/molecules25081927
Chicago/Turabian StyleArabi, Seyed Hamidreza, David Haselberger, and Dariush Hinderberger. 2020. "The Effect of Ethanol on Gelation, Nanoscopic, and Macroscopic Properties of Serum Albumin Hydrogels" Molecules 25, no. 8: 1927. https://doi.org/10.3390/molecules25081927







